Impact of Diagnostic Confidence, Perceived Difficulty, and Clinical Experience in Facial Melanoma Detection: Results from a European Multicentric Teledermoscopic Study
Simple Summary
Abstract
1. Introduction
1.1. Facial Melanoma
1.2. Melanoma Dermoscopic Diagnosis
1.3. Differential Dermoscopic Diagnosis of Facial Melanoma
1.4. Objectives
2. Materials and Methods
2.1. Study Design
2.2. Study Dataset
2.3. Teledermoscopic Test
2.4. Statistical Analysis
3. Results
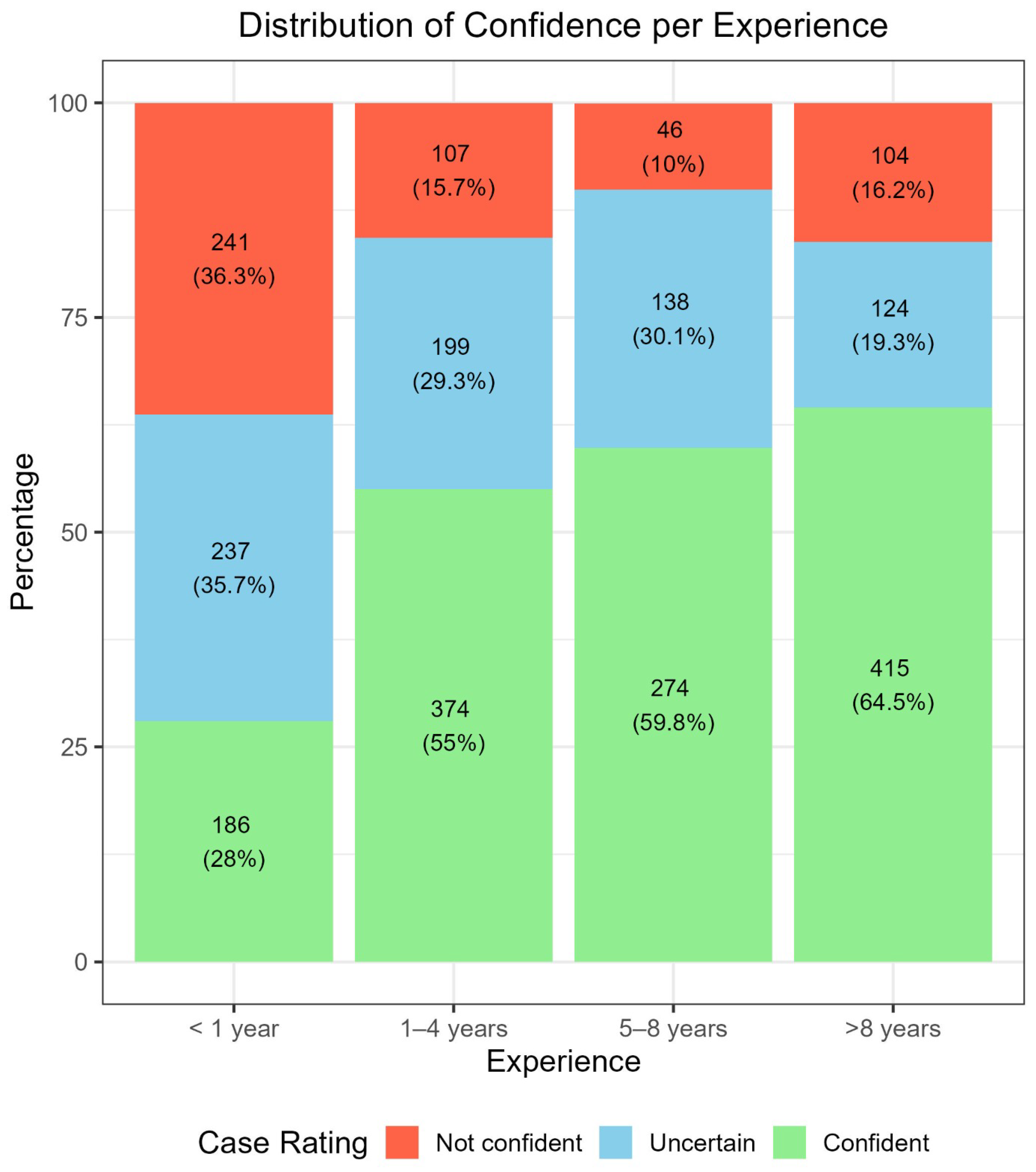
3.1. Correlation Between Confidence Level, Perceived Difficulty, and Experience in Dermoscopy
3.2. Diagnostic Accuracy over 3 Testing Sets: Comparison of Relative Impact of Experience, Confidence, and Difficulty Rating
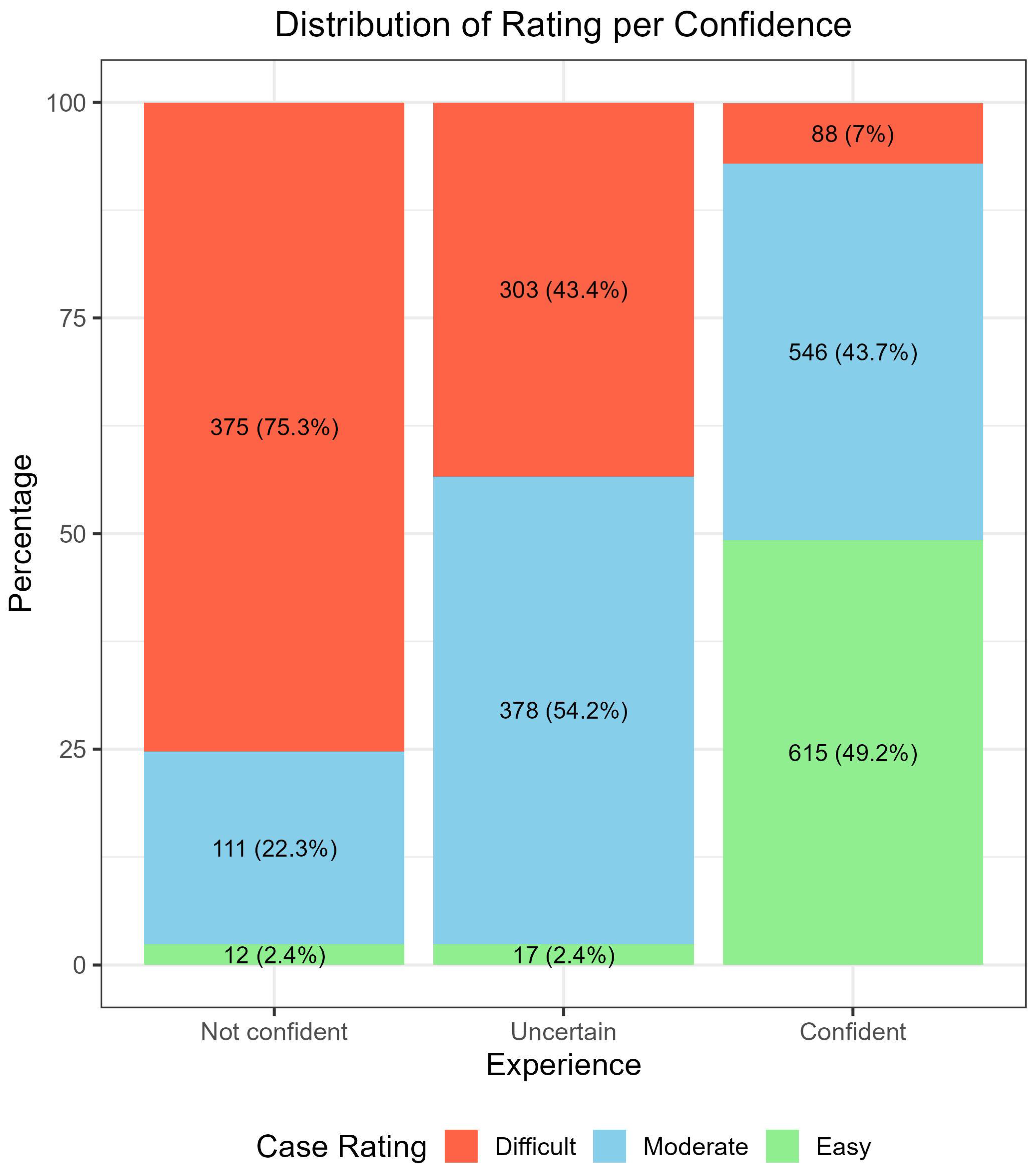
3.3. Specific Impact of Confidence Level on the Diagnostic Performance, Based on the Personal Experience
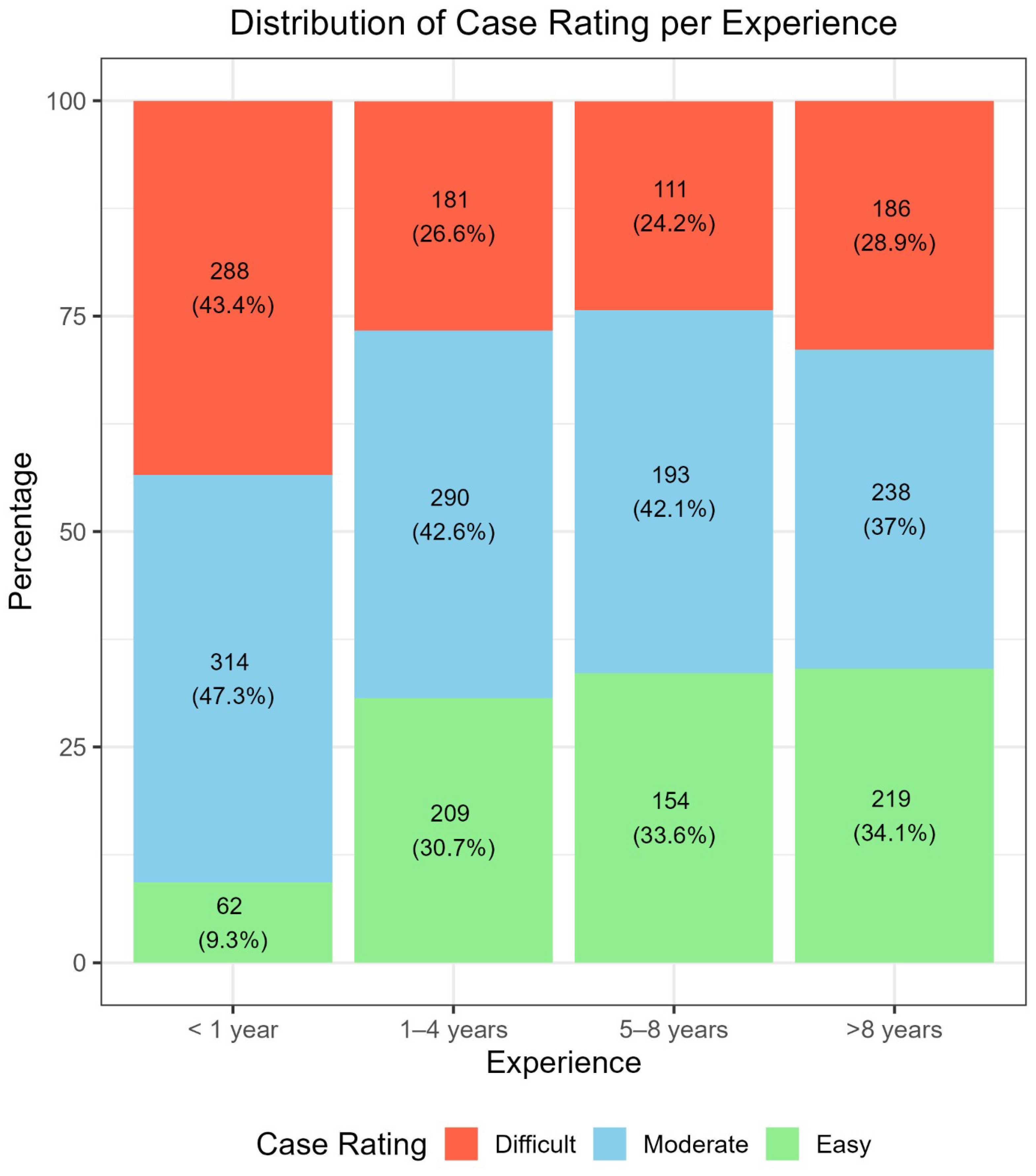
3.4. Specific Impact of Perceived Difficulty on the Diagnostic Performance, Based on the Personal Experience
3.5. Impact of Confidence Level Plus Perceived Case Difficulty on the Diagnostic Performance
3.6. Impact of Dermoscopic Experience, Confidence Level, and Difficulty Rating on aPFLs Management Strategies
3.7. Impact of Dermoscopic Experience, Confidence Level, and Difficulty Rating on Management Strategies of Malignant and Benign aPFLs
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Skill Level | Confidence | Rating | N | Accuracy Setting 1 1 | Accuracy Setting 2 2 | Accuracy Setting 3 3 | Sensitivity |
|---|---|---|---|---|---|---|---|
| <1 year | Not confident | Easy | 2 | 50.0% | 50.0% | 50.0% | N.A. |
| Moderate | 53 | 45.3% | 54.7% | 69.8% | 57.9% | ||
| Difficult | 186 | 43.5% | 52.2% | 67.7% | 55.4% | ||
| Uncertain | Easy | 6 | 66.7% | 83.3% | 100.0% | N.A. | |
| Moderate | 144 | 49.3% | 62.5% | 72.9% | 56.4% | ||
| Difficult | 87 | 55.2% | 60.9% | 65.5% | 62.5% | ||
| Confident | Easy | 54 | 44.4% | 63.0% | 77.8% | 46.2% | |
| Moderate | 117 | 47.0% | 65.0% | 74.4% | 48.6% | ||
| Difficult | 15 | 60.0% | 66.7% | 86.7% | 83.3% | ||
| 1–4 years | Not confident | Moderate | 20 | 30.0% | 50.0% | 55.0% | 33.3% |
| Difficult | 87 | 33.3% | 39.1% | 50.6% | 39.5% | ||
| Uncertain | Easy | 9 | 44.4% | 55.6% | 77.8% | 33.3% | |
| Moderate | 111 | 48.6% | 53.2% | 64.9% | 44.0% | ||
| Difficult | 79 | 48.1% | 59.5% | 67.1% | 61.8% | ||
| Confident | Easy | 200 | 60.0 | 75.0% | 86.0% | 66.7% | |
| Moderate | 159 | 50.3% | 61.6% | 72.3% | 56.3% | ||
| Difficult | 15 | 53.3% | 60.0% | 80.0% | 57.1% | ||
| 5–8 years | Not confident | Easy | 1 | 100.0% | 100.0% | 100.0% | N.A. |
| Moderate | 15 | 33.3% | 40.0% | 53.3% | 44.4% | ||
| Difficult | 30 | 50.0% | 53.3% | 60.0% | 55.6% | ||
| Uncertain | Moderate | 71 | 43.7% | 50.7% | 64.8% | 41.9% | |
| Difficult | 67 | 49.3% | 56.7% | 65.7% | 65.4% | ||
| Confident | Easy | 153 | 44.4% | 58.8% | 78.4% | 26.2% | |
| Moderate | 107 | 55.1% | 65.4% | 74.8% | 38.7% | ||
| Difficult | 14 | 50.0% | 50.0% | 57.1% | 25.0% | ||
| >8 years | Not confident | Easy | 9 | 55.6% | 66.7% | 66.7% | 50.0% |
| Moderate | 23 | 56.5% | 60.9% | 65.2% | 45.5% | ||
| Difficult | 72 | 27.8% | 40.3% | 54.2% | 50.0% | ||
| Uncertain | Easy | 2 | 50.0% | 100.0% | 100.0% | 100.0% | |
| Moderate | 52 | 38.5% | 48.1% | 57.7% | 33.3% | ||
| Difficult | 70 | 38.6% | 47.1% | 54.3% | 51.4% | ||
| Confident | Easy | 208 | 60.1% | 73.6% | 83.2% | 65.2% | |
| Moderate | 163 | 51.5% | 65.6% | 76.1% | 62.9% | ||
| Difficult | 44 | 47.7% | 59.1% | 68.2% | 63.6% |
References
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11, e2021161S. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Tognetti, L.; Cartocci, A.; Żychowska, M.; Savarese, I.; Cinotti, E.; Pizzichetta, M.A.; Moscarella, E.; Longo, C.; Farnetani, F.; Guida, S.; et al. A risk-scoring model for the differential diagnosis of lentigo maligna and other atypical pigmented facial lesions of the face: The facial iDScore. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2301–2310. [Google Scholar] [CrossRef]
- Tognetti, L.; Cartocci, A.; Cinotti, E.; D’Onghia, M.; Żychowska, M.; Moscarella, E.; Dika, E.; Farnetani, F.; Guida, S.; Paoli, J.; et al. Dermoscopy of atypical pigmented lesions of the face: Variation according to facial areas. Exp. Dermatol. 2023, 32, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Sgouros, D.; Theofili, M.; Zafeiropoulou, T.; Lallas, A.; Apalla, Z.; Zaras, A.; Liopyris, K.; Pappa, G.; Polychronaki, E.; Kousta, F.; et al. Dermoscopy of Actinic Keratosis: Is There a True Differentiation between Non-Pigmented and Pigmented Lesions? J. Clin. Med. 2023, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.A.; Hosny, K.M.; Damaševičius, R.; Eltoukhy, M.M. Machine Learning and Deep Learning Methods for Skin Lesion Classification and Diagnosis: A Systematic Review. Diagnostics 2021, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Giuffrida, R.; Vezzoni, R.; Resende, F.S.; di Meo, N.; Zalaudek, I. Dermoscopy and the experienced clinicians. Int. J. Dermatol. 2020, 59, 16–22. [Google Scholar] [CrossRef]
- Wang, D.M.; Petitt, C.E.; Goel, N.S.; Ash, M.M.; Mervak, J.E. Confidence and competency in the use of dermoscopy among new first-year dermatology residents: A repeated-pairs pre-/postassessment study of an online learning module. J. Am. Acad. Dermatol. 2021, 85, 1585–1587. [Google Scholar] [CrossRef]
- Vestergaard, T.; Prasad, S.; Schuster, A.; Laurinaviciene, R.; Andersen, M.; Bygum, A. Diagnostic accuracy and interobserver concordance: Teledermoscopy of 600 suspicious skin lesions in Southern Denmark. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1601–1608. [Google Scholar] [CrossRef]
- Rogers, T.; McCrary, M.R.; Yeung, H.; Krueger, L.; Chen, S. Dermoscopic Photographs Impact Confidence and Management of Remotely Triaged Skin Lesions. Dermatol. Pract. Concept. 2022, 12, e2022129. [Google Scholar] [CrossRef]
- Benvenuto-Andrade, C.; Dusza, S.W.; Hay, J.L.; Agero, A.L.C.; Halpern, A.C.; Kopf, A.W.; Marghoob, A.A. Level of Confidence in Diagnosis: Clinical Examination Versus Dermoscopy Examination. Dermatol. Surg. 2006, 32, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Dusza, S.W.; Scope, A.; Braun, R.P.; Kopf, A.W.; Marghoob, A.A. Differences in Dermoscopic Images from Nonpolarized Dermoscope and Polarized Dermoscope Influence the Diagnostic Accuracy and Confidence Level: A Pilot Study. Dermatol. Surg. 2008, 34, 1389–1395. [Google Scholar] [CrossRef][Green Version]
- Lallas, A.; Lallas, K.; Tschandl, P.; Kittler, H.; Apalla, Z.; Longo, C.; Argenziano, G. The dermoscopic inverse approach significantly improves the accuracy of human readers for lentigo maligna diagnosis. J. Am. Acad. Dermatol. 2021, 84, 381–389. [Google Scholar] [CrossRef]
- Cinotti, E.; Labeille, B.; Debarbieux, S.; Carrera, C.; Lacarrubba, F.; Witkowski, A.; Moscarella, E.; Arzberger, E.; Kittler, H.; Bahadoran, P.; et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1284–1291. [Google Scholar] [CrossRef]
- Nie, T.; Jiang, X.; Zheng, B.; Zhang, Y. Effect of reflectance confocal microscopy compared to dermoscopy in the diagnostic accuracy of lentigo maligna: A meta-analysis. Int. J. Clin. Pract. 2021, 75, e14346. [Google Scholar] [CrossRef] [PubMed]
- Luschi, A.; Tognetti, L.; Cartocci, A.; Cinotti, E.; Rubegni, G.; Calabrese, L.; D’onghia, M.; Dragotto, M.; Moscarella, E.; Brancaccio, G.; et al. Design and development of a systematic validation protocol for synthetic melanoma images for responsible use in medical artificial intelligence. Biocybern. Biomed. Eng. 2025, 45, 608–616. [Google Scholar] [CrossRef]
- Tognetti, L.; Cinotti, E.; Farnetani, F.; Lallas, A.; Paoli, J.; Longo, C.; Pampena, R.; Moscarella, E.; Argenziano, G.; Tiodorovic, D.; et al. Development and Implementation of a Web-Based International Registry Dedicated to Atypical Pigmented Skin Lesions of the Face: Teledermatologic Investigation on Epidemiology and Risk Factors. Telemed. e-Health 2023, 29, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Akay, B.; Kocyigit, P.; Heper, A.; Erdem, C. Dermatoscopy of flat pigmented facial lesions: Diagnostic challenge between pigmented actinic keratosis and lentigo maligna. Br. J. Dermatol. 2010, 163, 1212–1217. [Google Scholar] [CrossRef]
- Tschandl, P.; Rosendahl, C.; Kittler, H. Dermatoscopy of flat pigmented facial lesions. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 120–127. [Google Scholar] [CrossRef]
- Ozbagcivan, O.; Akarsu, S.; Ikiz, N.; Semiz, F.; Fetil, E. Dermoscopic Differentiation of Facial Lentigo Maligna from Pigmented Actinic Keratosis and Solar Lentigines. Acta Dermatovenerol. Croat. ADC 2019, 27, 146–152. [Google Scholar]
- Farnetani, F.; Manfredini, M.; Chester, J.; Ciardo, S.; Gonzalez, S.; Pellacani, G. Reflectance confocal microscopy in the diagnosis of pigmented macules of the face: Differential diagnosis and margin definition. Photochem. Photobiol. Sci. 2019, 18, 963–969. [Google Scholar] [CrossRef]
- Star, P.; Guitera, P. Lentigo Maligna, Macules of the Face, and Lesions on Sun-Damaged Skin: Confocal Makes the Difference. Dermatol. Clin. 2016, 34, 421–429. [Google Scholar] [CrossRef]
- Guida, S.; Alma, A.; Fiorito, F.; Megna, A.; Chester, J.; Kaleci, S.; Ciardo, S.; Manfredini, M.; Rongioletti, F.; Perrot, J.L.; et al. Lentigo maligna and lentigo maligna melanoma in vivo differentiation with dermoscopy and reflectance confocal microscopy: A retrospective, multicentre study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2293–2300. [Google Scholar] [CrossRef]
- Tognetti, L.; Cevenini, G.; Moscarella, E.; Cinotti, E.; Farnetani, F.; Lallas, A.; Tiodorovic, D.; Carrera, C.; Puig, S.; Perrot, J.; et al. Validation of an integrated dermoscopic scoring method in an European teledermoscopy web platform: The iDScore project for early detection of melanoma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 640–647. [Google Scholar] [CrossRef]
- Van Der Rhee, J.; Bergman, W.; Kukutsch, N. The impact of dermoscopy on the management of pigmented lesions in everyday clinical practice of general dermatologists: A prospective study. Br. J. Dermatol. 2010, 162, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Fazil Jaber, N.; Jerkovic Gulin, S.; Seifert, O. Analysis of Teledermoscopy and Face-to-Face Examination of Atypical Pigmented Lesions: A Cross-Sectional, Retrospective Study. Dermatol. Pract. Concept. 2023, 13, e2023212. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, L.; Lo Conte, S.; Leonardelli, S.; Lallas, A.; Moscarella, E.; Giuffrida, R.; Paoli, J.; Dika, E.; Stanganelli, I.; Magi, S.; et al. A clinical-dermoscopic risk scoring model for early melanoma of the soles: The iDScore_plantar. J. Eur. Acad. Dermatol. Venereol. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bandic, J.; Kovacevic, S.; Karabeg, R.; Bandic, M.; Lazarov, A.; Opric, D. Teledermoscopy for Skin Cancer Prevention: A Comparative Study of Clinical and Teledermoscopic Diagnosis. Acta Inform. Med. 2020, 28, 37. [Google Scholar] [CrossRef]
- DeWane, M.E.; Kelsey, A.; Oliviero, M.; Rabinovitz, H.; Grant-Kels, J.M. Melanoma on chronically sun-damaged skin: Lentigo maligna and desmoplastic melanoma. J. Am. Acad. Dermatol. 2019, 81, 823–833. [Google Scholar] [CrossRef]
- Reed, J.A.; Shea, C.R. Lentigo Maligna: Melanoma In Situ on Chronically Sun-Damaged Skin. Arch. Pathol. Lab. Med. 2011, 135, 838–841. [Google Scholar] [CrossRef]
- Avila, A.; Thakur, V.; Vincent, N.; Valencia, P.; Möller, M.; Khurana, R.; Yan, G.; Tang, J.C.; Bedogni, B.; Jaimes, N. Melanoma on Chronically Sun-Damaged Skin: Deciphering Gene Expression Signatures. Dermatol. Pract. Concept. 2025, 15, 4952. [Google Scholar] [CrossRef]
- Naik, P.P. Diagnosis and Management of Lentigo Maligna: Clinical Presentation and Comprehensive Review. J. Ski. Cancer 2021, 2021, 7178305. [Google Scholar] [CrossRef]
- Thamm, J.R.; Schuh, S.; Welzel, J. Epidemiology and Risk Factors of Actinic Keratosis. What Is New for the Management for Sun-Damaged Skin. Dermatol. Pract. Concept. 2024, 14, e2024146S. [Google Scholar] [CrossRef]
- Weber, P.; Sinz, C.; Rinner, C.; Kittler, H.; Tschandl, P. Perilesional sun damage as a diagnostic clue for pigmented actinic keratosis and Bowen’s disease. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2022–2026. [Google Scholar] [CrossRef]
- Trivedi, M.; Mehta, R.D.; Ghiya, B.C.; Soni, P. Cutis Rhomboidalis Nuchae and Solar Lentigo with Squamous and Basal Cell Carcinoma—A Quartet of Chronic Sun Exposure. J. Indian Acad. Geriatr. 2024, 20, 161–163. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, E.J.; Kwon, I.H.; Kim, K.H.; Kim, K.J. Clinical and Histopathologic Study of Benign Lichenoid Keratosis on the Face. Am. J. Dermatopathol. 2013, 35, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Raptoulis, G.; Spencer, R.; Einstein, B.; Oliviero, M.; Braun, R.; Rabinovitz, H. Lichen Planus–like Keratosis of the Face: A Simulator of Melanoma In Situ. Dermatol. Surg. 2007, 33, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.P.; Rabinovitz, H.S.; Krischer, J.; Kreusch, J.; Oliviero, M.; Naldi, L.; Kopf, A.W.; Saurat, J.H. Dermoscopy of Pigmented Seborrheic Keratosis: A Morphological Study. Arch. Dermatol. 2002, 138, 1556–1560. [Google Scholar] [CrossRef]
- Gamo, R.; Malvehy, J.; Puig, S.; Fuentes, M.E.; Naz, E.; Gómez de la Fuente, E.; Calzado, L.; Sanchez-Gilo, A.; Vicente, F.J.; Lopez-Estebaranz, J.L. Dermosopic Features of Melanocytic Nevi in Seven Different Anatomical Locations in Patients with Atypical Nevi Syndrome. Dermatol. Surg. 2013, 39, 864–871. [Google Scholar] [CrossRef] [PubMed]

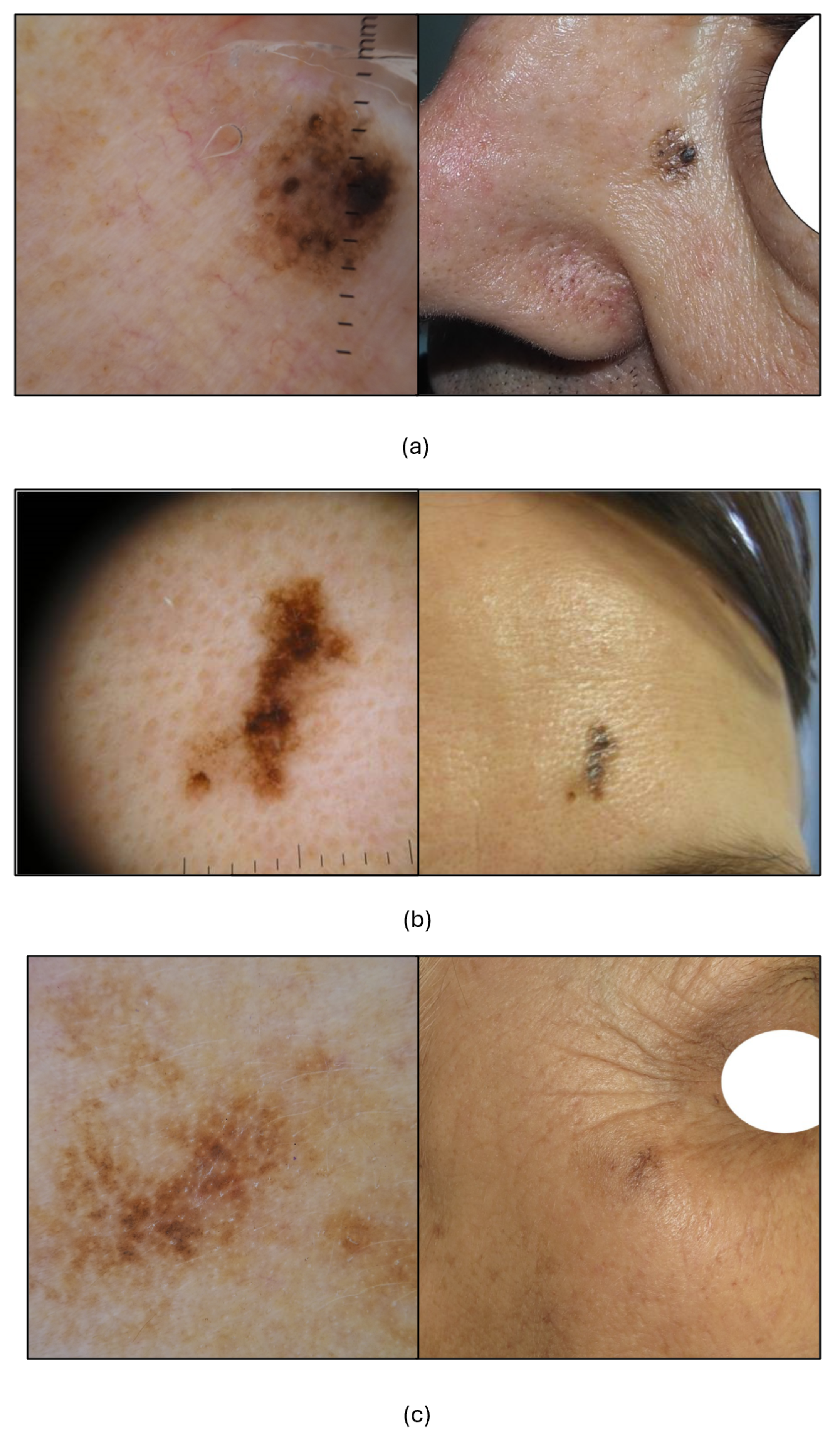
| N | Setting 1 1 (%) | Setting 2 2 (%) | Setting 3 3 (%) | ||
|---|---|---|---|---|---|
| Overall | 2445 | 48.7 (46.7, 50.7) | 60.0 (58.0, 61.9) | 71.2 (69.4, 73.0) | |
| Experience | <1 year | 664 | 47.7 (43.9, 51.6) | 59.5 (55.6, 63.2) | 71.4 (67.8, 74.8) |
| 1–4 years | 680 | 49.9 (46.0, 53.7) | 60.6 (56.8, 64.3) | 71.5 (67.9, 74.8) | |
| 5–8 years | 458 | 47.8 (43.2, 52.5) | 57.6 (53.0, 62.2) | 71.0 (66.6, 75.1) | |
| >8 years | 643 | 49.1 (45.2, 53.1) | 61.4 (57.6, 65.2) | 71.1 (67.4, 74.6) | |
| Confidence | Not confident | 498 | 40.2 (35.8, 44.6) | 48.8 (44.3, 53.3) | 61.4 (57.0, 65.7) |
| Uncertain | 698 | 47.4 (43.7, 51.2) | 56.3 (52.5, 60.0) | 65.9 (62.2, 69.4) | |
| Confident | 1249 | 52.8 (50.0, 55.6) | 66.4 (63.8, 69.1) | 78.1 (75.7, 80.4) | |
| Rating | Difficult | 766 | 43.9 (40.3, 47.5) | 52.1 (48.5, 55.7) | 62.9 (59.4, 66.4) |
| Moderate | 1035 | 48.5 (45.4, 51.6) | 59.9 (56.8, 62.9) | 70.5 (67.6, 73.3) | |
| Easy | 644 | 54.8 (50.9, 58.7) | 69.4 (65.7, 72.9) | 82.3 (79.1, 85.2) |
| Skill Level | Confidence Level | N | Accuracy Setting 1 1 (%) | Accuracy Setting 2 2 (%) | Accuracy Setting 3 3 (%) | Sensitivity (%) |
|---|---|---|---|---|---|---|
| <1 year | Not confident | 241 | 44.0 (37.6, 50.5) | 52.7 (46.2, 59.1) | 68.0 (61.8, 73.9) | 55.9 (45.2, 66.2) |
| Uncertain | 237 | 51.9 (45.3, 58.4) | 62.4 (56.0, 68.6) | 70.9 (64.6, 76.6) | 59.5 (49.7, 68.7) | |
| Confident | 186 | 47.3 (40.0, 54.7) | 64.5 (57.2, 71.4) | 76.3 (69.6, 82.3) | 51.9 (37.8, 65.6) | |
| 1–4 years | Not confident | 107 | 32.7 (24.0, 42.5) | 41.1 (31.7, 51.0) | 51.4 (41.5, 61.2) | 38.3 (24.5, 53.6) |
| Uncertain | 199 | 48.2 (41.1, 55.4) | 55.8 (48.6, 62.8) | 66.3 (59.3, 72.9) | 50.6 (39.6, 61.5) | |
| Confident | 374 | 55.6 (50.4, 60.7) | 68.7 (63.8, 73.4) | 79.9 (75.5, 83.9) | 61.3 (52.6, 69.5) | |
| 5–8 years | Not confident | 46 | 45.7 (30.9, 61.0) | 50.0 (34.9, 65.1) | 58.7 (43.2, 73.0) | 51.9 (31.9, 71.3) |
| Uncertain | 138 | 46.4 (37.9, 55.1) | 53.6 (44.9, 62.1) | 65.2 (56.6, 73.1) | 52.6 (38.9, 66.0) | |
| Confident | 274 | 48.9 (42.4, 55.0) | 60.9 (54.9, 66.8) | 75.9 (70.4, 80.8) | 31.2 (21.0, 42.7) | |
| >8 years | Not confident | 104 | 36.5 (27.3, 46.5) | 47.1 (37.2, 57.1) | 57.7 (47.6, 67.3) | 48.8 (33.3, 64.5) |
| Uncertain | 124 | 38.7 (30.1, 47.9) | 48.4 (39.3, 57.5) | 56.5 (47.3, 65.3) | 45.2 (32.5, 58.3) | |
| Confident | 415 | 55.4 (50.5, 60.3) | 68.9 (64.2, 73.3) | 78.8 (74.5, 82.6) | 63.9 (55.9, 71.4) |
| Skill Level | Perceived Difficulty | N | Accuracy Setting 1 1 (%) | Accuracy Setting 2 2 (%) | Accuracy Setting 3 3 (%) | Sensitivity (%) |
|---|---|---|---|---|---|---|
| <1 year | Easy | 62 | 46.8 (34.0, 59.9) | 64.5 (51.3, 76.3) | 79.0 (66.8, 88.3) | 46.2 (19.2, 74.9) |
| Moderate | 314 | 47.8 (42.1, 53.5) | 62.1 (56.5, 67.5) | 72.9 (67.6, 77.8) | 54.1 (44.3, 63.7) | |
| Difficult | 288 | 47.9 (42.0, 53.9) | 55.6 (49.6, 61.4) | 68.1 (62.3, 73.4) | 59.6 (50.8, 67.9) | |
| 1–4 years | Easy | 209 | 59.3 (52.3, 66.0) | 74.2 (67.7, 80.0) | 85.6 (80.1, 90.1) | 65.2 (52.8, 76.3) |
| Moderate | 290 | 48.3 (42.4, 54.2) | 57.6 (51.7, 63.3) | 68.3 (62.6, 73.6) | 49.6 (40.4, 58.8) | |
| Difficult | 181 | 41.4 (34.2, 49.0) | 49.7 (42.2, 57.2) | 60.2 (52.7, 67.4) | 50.6 (39.1, 62.1) | |
| 5–8 years | Easy | 154 | 44.8 (36.8, 53.0) | 59.1 (50.9, 66.9) | 78.6 (71.2, 84.8) | 26.2 (13.9, 42.0) |
| Moderate | 193 | 49.2 (42.0, 56.5) | 58.0 (50.7, 65.1) | 69.4 (62.4, 75.8) | 40.8 (29.3, 53.1) | |
| Difficult | 111 | 49.5 (39.9, 59.2) | 55.0 (45.2, 64.4) | 63.1 (53.4, 72.0) | 58.3 (43.2, 72.4) | |
| >8 years | Easy | 219 | 59.8 (53.0, 66.4) | 73.5 (67.1, 79.2) | 82.6 (77.0, 87.4) | 64.8 (52.5, 75.7) |
| Moderate | 238 | 49.2 (42.6, 55.7) | 61.3 (54.8, 67.6) | 71.0 (64.8, 76.7) | 54.3 (44.3, 64.0) | |
| Difficult | 186 | 36.6 (29.6, 43.9) | 47.3 (40.0, 54.7) | 57.5 (50.0, 64.7) | 54.0 (43.0, 64.8) |
| Close Dermoscopic Follow-Up | Reflectance Confocal Microscopy | Skin Biopsy | ||
|---|---|---|---|---|
| Experience | <1 year | 276 (41.6%) | 153 (23.0%) | 235 (35.4%) |
| 1–4 years | 298 (42.4%) | 152 (22.4%) | 230 (32.8%) | |
| 5–8 years | 209 (45.6%) | 126 (27.5%) | 123 (26.9%) | |
| >8 years | 221 (34.4%) | 166 (25.9%) | 256 (39.8%) | |
| Confidence | Not confident | 120 (24.1%) | 120 (36.1%) | 198 (39.8%) |
| Uncertain | 170 (24.4%) | 232 (33.2%) | 296 (42.4%) | |
| Confident | 714 (57.2%) | 180 (14.8%) | 350 (28.0%) | |
| Rating | Easy | 446 (69.3%) | 49 (7.6%) | 149 (23.1%) |
| Moderate | 424 (41.0%) | 256 (24.7%) | 355 (34.3%) | |
| Difficult | 134 (17.5%) | 292 (38.1%) | 340 (44.4%) |
| Factor | Level | Histology | Close Dermoscopic Follow-Up | Reflectance Confocal Microscopy | Skin Biopsy |
|---|---|---|---|---|---|
| Skill Level | <1 year | Benign | 216 (53.2%) | 94 (23.2%) | 96 (23.6%) |
| LM + LMM | 60 (23.3%) | 59 (22.9%) | 139 (53.9%) | ||
| 1–4 years | Benign | 233 (53.8%) | 94 (21.7%) | 82 (18.9%) | |
| LM + LMM | 65 (22.8%) | 58 (20.3%) | 148 (54.6%) | ||
| 5–8 years | Benign | 158 (45.8%) | 78 (22.6%) | 109 (31.6%) | |
| LM + LMM | 51 (31.7%) | 48 (29.8%) | 62 (38.5%) | ||
| >8 years | Benign | 174 (52.3%) | 102 (30.6%) | 104 (31.2%) | |
| LM + LMM | 47 (19.7%) | 64 (26.8%) | 152 (63.6%) | ||
| Confidence | Not confident | Benign | 90 (36.0%) | 106 (42.4%) | 54 (21.6%) |
| LM + LMM | 30 (14.3%) | 74 (35.2%) | 106 (50.5%) | ||
| Uncertain | Benign | 115 (30.2%) | 136 (35.7%) | 130 (34.1%) | |
| LM + LMM | 55 (17.4%) | 96 (30.3%) | 166 (52.4%) | ||
| Confident | Benign | 576 (57.6%) | 124 (12.4%) | 121 (12.1%) | |
| LM + LMM | 138 (32.4%) | 59 (13.8%) | 229 (53.8%) | ||
| Rating | Difficult | Benign | 94 (22.6%) | 176 (42.3%) | 146 (35.1%) |
| LM + LMM | 40 (11.4%) | 116 (33.1%) | 194 (55.4%) | ||
| Moderate | Benign | 321 (51.2%) | 153 (24.4%) | 153 (24.4%) | |
| LM + LMM | 103 (25.2%) | 103 (25.2%) | 202 (49.6%) | ||
| Easy | Benign | 366 (80.0%) | 39 (8.5%) | 54 (11.8%) | |
| LM + LMM | 80 (41.0%) | 24 (12.3%) | 105 (53.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartocci, A.; Luschi, A.; Lo Conte, S.; Cinotti, E.; Farnetani, F.; Lallas, A.; Paoli, J.; Longo, C.; Moscarella, E.; Tiodorovic, D.; et al. Impact of Diagnostic Confidence, Perceived Difficulty, and Clinical Experience in Facial Melanoma Detection: Results from a European Multicentric Teledermoscopic Study. Cancers 2025, 17, 3388. https://doi.org/10.3390/cancers17203388
Cartocci A, Luschi A, Lo Conte S, Cinotti E, Farnetani F, Lallas A, Paoli J, Longo C, Moscarella E, Tiodorovic D, et al. Impact of Diagnostic Confidence, Perceived Difficulty, and Clinical Experience in Facial Melanoma Detection: Results from a European Multicentric Teledermoscopic Study. Cancers. 2025; 17(20):3388. https://doi.org/10.3390/cancers17203388
Chicago/Turabian StyleCartocci, Alessandra, Alessio Luschi, Sofia Lo Conte, Elisa Cinotti, Francesca Farnetani, Aimilios Lallas, John Paoli, Caterina Longo, Elvira Moscarella, Danica Tiodorovic, and et al. 2025. "Impact of Diagnostic Confidence, Perceived Difficulty, and Clinical Experience in Facial Melanoma Detection: Results from a European Multicentric Teledermoscopic Study" Cancers 17, no. 20: 3388. https://doi.org/10.3390/cancers17203388
APA StyleCartocci, A., Luschi, A., Lo Conte, S., Cinotti, E., Farnetani, F., Lallas, A., Paoli, J., Longo, C., Moscarella, E., Tiodorovic, D., Stanganelli, I., Suppa, M., Dika, E., Zalaudek, I., Pizzichetta, M. A., Perrot, J. L., Savarese, I., Żychowska, M., Rubegni, G., ... Tognetti, L. (2025). Impact of Diagnostic Confidence, Perceived Difficulty, and Clinical Experience in Facial Melanoma Detection: Results from a European Multicentric Teledermoscopic Study. Cancers, 17(20), 3388. https://doi.org/10.3390/cancers17203388












