Bing–Neel Syndrome in Waldenström Macroglobulinemia: Updates on Clinical Management and BTK Inhibitor Efficacy
Simple Summary
Abstract
1. Introduction
2. Epidemiology and Pathobiology
3. Clinical Presentation and Differential Diagnosis
3.1. Neurological and Clinical Manifestations
3.2. Distinguishing BNS from IgM-Associated Neuropathies
3.3. Other Mimics
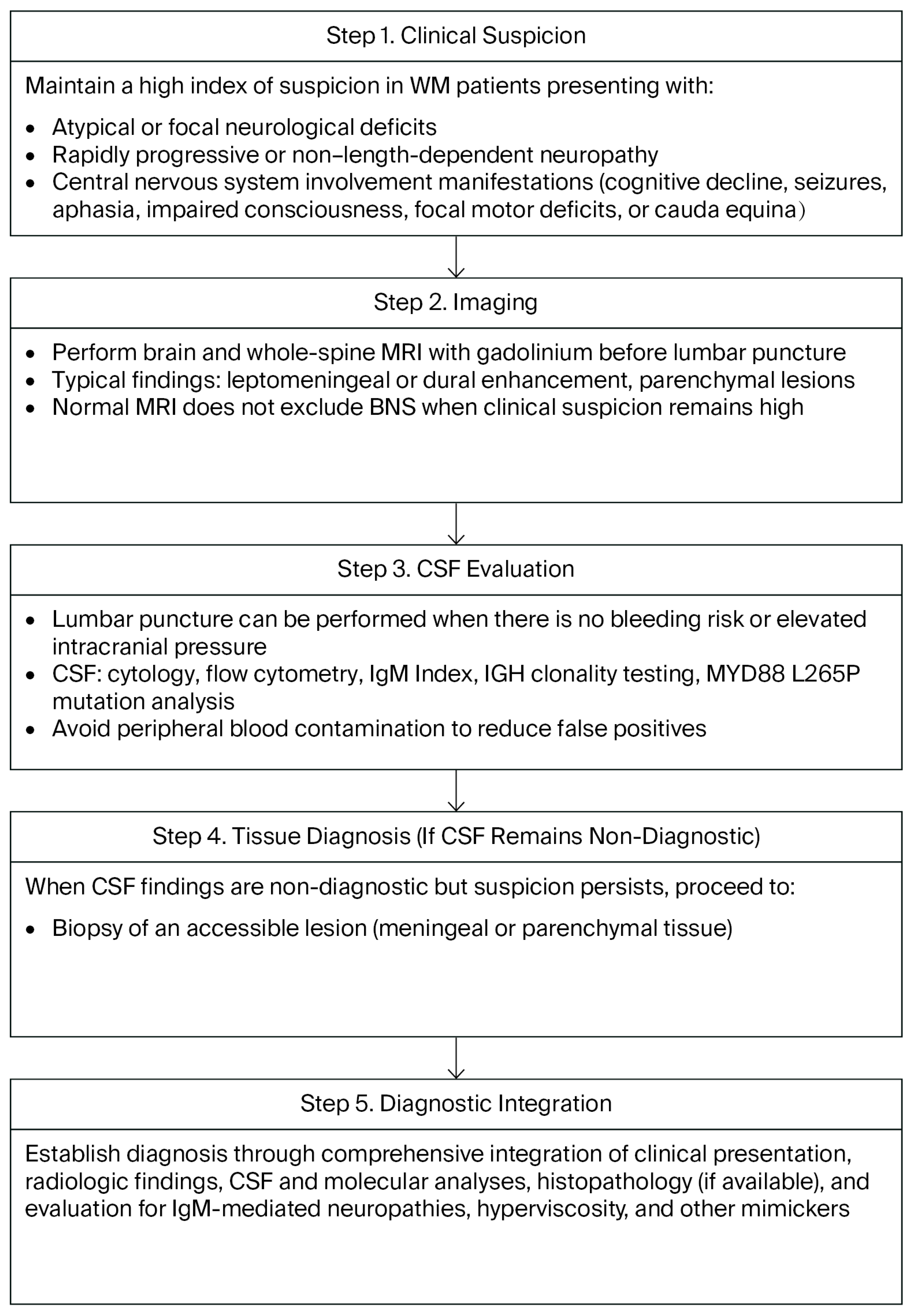
4. Diagnostic Work-Up
4.1. Imaging
4.2. CSF Studies
4.3. Tissue Diagnosis
4.4. Laboratory Adjuncts
4.5. Proposed Diagnostic Algorithm
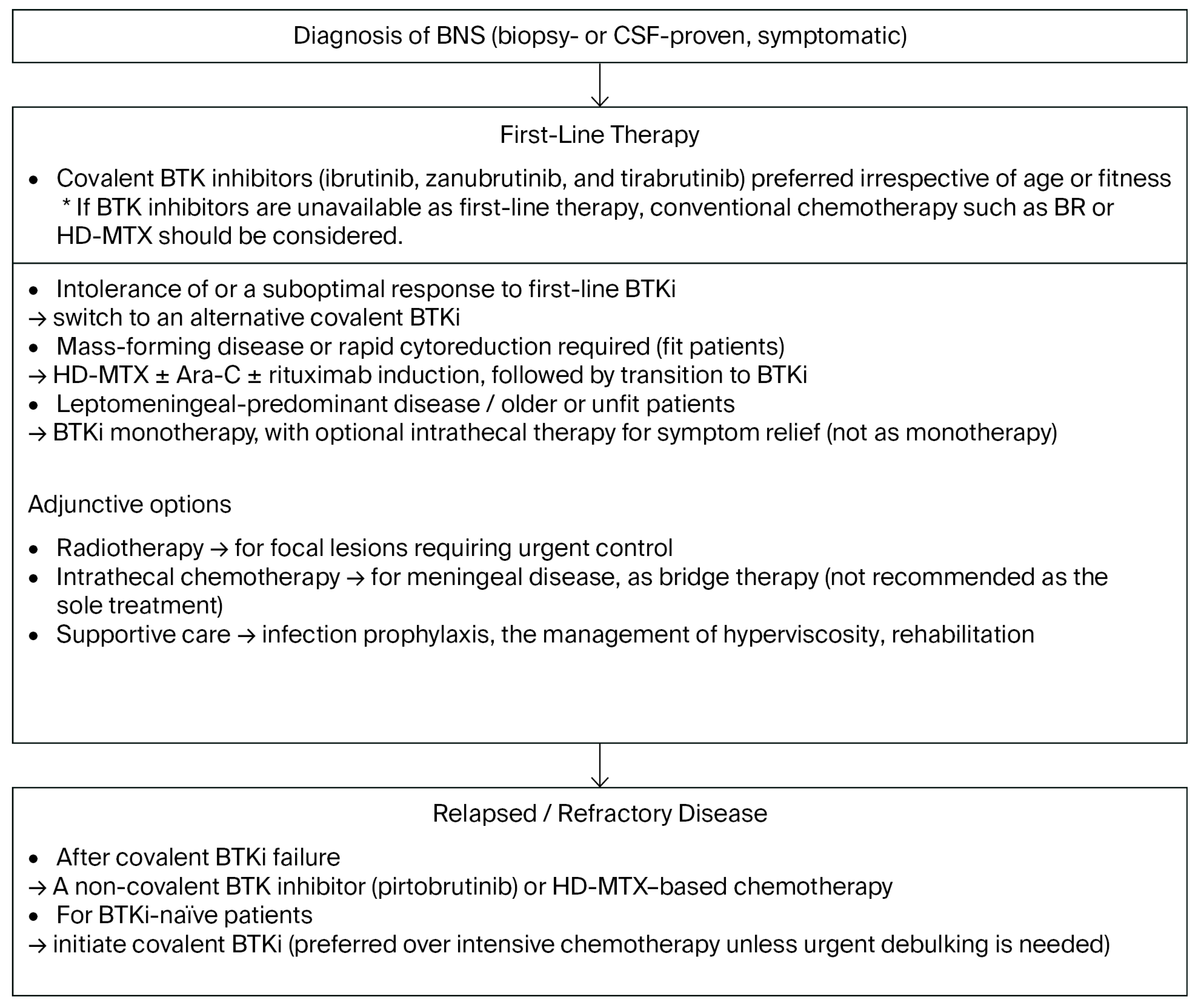
5. Treatment Landscape
5.1. General Principles and Goals
5.2. Conventional Chemotherapy and Radiotherapy
5.3. Covalent BTK Inhibitors
5.4. Non-Covalent BTK Inhibitor
5.5. Combining a BTK Inhibitor with Rituximab
5.6. Supportive and Preventive Care
6. Practical Treatment Algorithm
6.1. Initial Therapy
6.2. Relapsed/Refractory BNS
6.3. Role of Genotypes
7. Unmet Needs and Future Directions
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Sarosiek, S.; Becking, A.L.; Branagan, A.; Ferrero, S.; Khwaja, J.; Kimby, E.; Roos-Weil, D.; Sekiguchi, N.; Trneny, M.; Yi, S.; et al. Report of Consensus Panel 2 from the 12th International Workshop on the management of Bing-Neel syndrome in patients with Waldenstrom’s Macroglobulinemia. Semin. Hematol. 2025, 62, 85–89. [Google Scholar] [CrossRef]
- Minnema, M.C.; Kimby, E.; D’Sa, S.; Fornecker, L.M.; Poulain, S.; Snijders, T.J.; Kastritis, E.; Kremer, S.; Fitsiori, A.; Simon, L.; et al. Guideline for the diagnosis, treatment and response criteria for Bing-Neel syndrome. Haematologica 2017, 102, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Treon, S.P. How we manage Bing-Neel syndrome. Br. J. Haematol. 2019, 187, 277–285. [Google Scholar] [CrossRef]
- Treon, S.P.; Sarosiek, S.; Castillo, J.J. How I use genomics and BTK inhibitors in the treatment of Waldenström macroglobulinemia. Blood 2024, 143, 1702–1714. [Google Scholar] [CrossRef] [PubMed]
- Nanah, A.; Al Hadidi, S. Bing-Neel syndrome: Update on the diagnosis and treatment. Clin. Lymphoma Myeloma Leuk. 2022, 22, e213–e219. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Waldenström macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2021, 96, 258–269. [Google Scholar] [CrossRef]
- Frustaci, A.M.; Zappaterra, A.; Turri, G.; Rossi, V.; Deodato, M.; Gambacorti-Passerini, C.; Cairoli, R.; Danesi, R.; Tedeschi, A. Pirtobrutinib is an effective salvage treatment after ibrutinib in Bing-Neel syndrome. Am. J. Hematol. 2025, 100, 1878–1881. [Google Scholar] [CrossRef]
- Østergaard, S.; Munksgaard, L.; Hammer, T.; Nielsen, T.H.; Pedersen, M.Ø.; Gjerdrum, L.M.R. Central nervous system involvement in Waldenström macroglobulinemia: A comparative population-based study of Bing-Neel syndrome and histological transformation. Ann. Hematol. 2025, 104, 1007–1014. [Google Scholar] [CrossRef]
- Castillo, J.J.; D’Sa, S.; Lunn, M.P.; Minnema, M.C.; Tedeschi, A.; Lansigan, F.; Palomba, M.L.; Varettoni, M.; Garcia-Sanz, R.; Nayak, L.; et al. Central nervous system involvement by Waldenström macroglobulinaemia (Bing-Neel syndrome): A multi-institutional retrospective study. Br. J. Haematol. 2016, 172, 709–715. [Google Scholar] [CrossRef]
- Simon, L.; Fitsiori, A.; Lemal, R.; Dupuis, J.; Carpentier, B.; Boudin, L.; Corby, A.; Aurran-Schleinitz, T.; Gastaud, L.; Talbot, A.; et al. Bing-Neel syndrome, a rare complication of Waldenström macroglobulinemia: Analysis of 44 cases and review of the literature. Haematologica 2015, 100, 1587–1594. [Google Scholar] [CrossRef]
- Tomkins, O.; Khwaja, J.; Koay, S.; Japzon, N.; Hoskote, C.; Gupta, R.; Baker, R.; Lindsay, J.; Kyriakou, C.; Lunn, M.P.; et al. Bing-Neel syndrome—A case series of 46 patients from the United Kingdom. Blood Adv. 2025, 9, 4614–4617. [Google Scholar] [CrossRef]
- Treon, S.P.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Sheehy, P.; Manning, R.J.; Patterson, C.J.; Tripsas, C.; et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N. Engl. J. Med. 2012, 367, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Venet-Caillault, A.; Figeac, M.; Herbaux, C.; Marot, G.; Doye, E.; Bertrand, E.; Geffroy, S.; Lepretre, F.; et al. Genomic landscape of CXCR4 mutations in Waldenström macroglobulinemia. Clin. Cancer Res. 2016, 22, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Bertrand, E.; Renneville, A.; Caillault-Venet, A.; Doye, E.; Geffroy, S.; Sebda, S.; Nibourel, O.; Nudel, M.; et al. TP53 mutation and its prognostic significance in Waldenström’s macroglobulinemia. Clin. Cancer Res. 2017, 23, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, S.; Al Ustwani, O.; Pokuri, V.; Hatoum, H.; Bhat, S. Bing-Neel syndrome in a patient with Waldenström’s macroglobulinemia: A challenging diagnosis in the face of normal brain imaging. CNS Neurosci. Ther. 2014, 20, 945–946. [Google Scholar] [CrossRef]
- Sekiguchi, N. The Impact of Tirabrutinib Monotherapy for Bing-Neel Syndrome in Waldenström’s Macroglobulinemia. Intern. Med. 2022, 61, 3473–3474. [Google Scholar] [CrossRef]
- Poulain, S.; Boyle, E.M.; Roumier, C.; Demarquette, H.; Wemeau, M.; Geffroy, S.; Herbaux, C.; Bertrand, E.; Hivert, B.; Terriou, L.; et al. MYD88 L265P mutation contributes to the diagnosis of Bing-Neel syndrome. Br. J. Haematol. 2014, 167, 506–513. [Google Scholar] [CrossRef]
- Montesinos-Rongen, M.; Godlewska, E.; Brunn, A.; Wiestler, O.D.; Siebert, R.; Deckert, M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011, 122, 791–792. [Google Scholar] [CrossRef]
- Nakamura, T.; Tateishi, K.; Niwa, T.; Matsushita, Y.; Tamura, K.; Kinoshita, M.; Tanaka, K.; Fukushima, S.; Takami, H.; Arita, H.; et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol. Appl. Neurobiol. 2016, 42, 279–290. [Google Scholar] [CrossRef]
- Ueba, T.; Okawa, M.; Abe, H.; Inoue, T.; Takano, K.; Hayashi, H.; Nabeshima, K.; Oshima, K. Central nervous system marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type involving the brain and spinal cord parenchyma. Neuropathology 2013, 33, 306–311. [Google Scholar] [CrossRef]
- Jesionek-Kupnicka, D.; Smolewski, P.; Kupnicki, P.; Płuciennik, E.; Zawlik, I.; Papierz, W.; Kordek, R. Primary extranodal marginal zone B-cell lymphoma of the central nervous system presented as traumatic subdural hematoma and subarachnoid bleeding: Case report. Clin. Neuropathol. 2013, 32, 384–392. [Google Scholar] [CrossRef]
- Ayanambakkam, A.; Ibrahimi, S.; Bilal, K.; Cherry, M.A. Extra-nodal marginal zone lymphoma of the central nervous system. Clin. Lymphoma Myeloma Leuk. 2017, 17, e71–e76. [Google Scholar]
- Wanquet, A.; Birsen, R.; Lemal, R.; Hunault, M.; Leblond, V.; Aurran-Schleinitz, T. Ibrutinib responsive central nervous system involvement in chronic lymphocytic leukemia. Blood 2016, 127, 2356–2358. [Google Scholar] [CrossRef][Green Version]
- Wanquet, A.; Birsen, R.; Bonnet, C.; Boubaya, M.; Choquet, S.; Dupuis, J.; Lepretre, S.; Re, D.; Fahri, J.; Michallet, A.-S.; et al. Management of central nervous system involvement in chronic lymphocytic leukaemia: A retrospective cohort of 30 patients. Br. J. Haematol. 2016, 176, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Hunter, Z.R.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Manning, R.J.; Tripsas, C.; Patterson, C.J.; Sheehy, P.; et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014, 123, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Jimenez, C.; Maiso, P.; Moschetta, M.; Mishima, Y.; Aljawai, Y.; Sahin, I.; Kuhne, M.; Cardarelli, P.; et al. C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma. Blood 2014, 123, 4120–4131. [Google Scholar] [CrossRef]
- Becking, A.-M.L.; van de Mortel, J.P.M.; Tomkins, O.; Flinsenberg, T.W.H.; Japzon, N.; Kersten, M.J.; Khwaja, J.; Kuipers, S.; Levenga, H.; McKeague, S.; et al. Zanubrutinib in Bing-Neel syndrome: Efficacy and tolerability. Leukemia 2025, 39, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hunter, Z.R.; Tsakmaklis, N.; Cao, Y.; Yang, G.; Chen, J.; Liu, X.; Kanan, S.; Castillo, J.J.; Tai, Y.-T.; et al. Clonal architecture of CXCR4 WHIM-like mutations in Waldenström macroglobulinaemia. Br. J. Haematol. 2016, 172, 735–744. [Google Scholar] [CrossRef]
- Simon, L.; Lemal, R.; Fornecker, L.M.; Tournilhac, O.; Leblond, V. High-dose therapy with autologous stem cells transplantation in Bing-Neel syndrome: A retrospective analysis of 14 cases. Am. J. Hematol. 2019, 94, E227–E229. [Google Scholar] [CrossRef]
- Khwaja, J.; Japzon, N.; Rismani, A.; Carr, A.; Lunn, M.; Kyriakou, C.; D’Sa, S. IBCL-099 Intensive Chemotherapy and Autologous Stem Cell (ASCT) Consolidation for Bing Neel Syndrome. Clin. Lymphoma Myeloma Leuk. 2023, 23, S448. [Google Scholar] [CrossRef]
- Malkani, R.G.; Tallman, M.; Gottardi-Littell, N.; Karpus, W.; Marszalek, L.; Variakojis, D.; Kaden, B.; Walker, M.; Levy, R.M.; Raizer, J.J. Bing-Neel syndrome: An illustrative case and a comprehensive review of the published literature. J. Neurooncol. 2010, 96, 301–312. [Google Scholar] [CrossRef]
- Minnema, M.C.; Kimby, E.; D’Sa, S.; Fornecker, L.-M.; Poulain, S.; Snijders, T.J.; Kastritis, E.; Kremer, S.; Fitsiori, A.; Simon, L.; et al. Effective treatment of Bing-Neel syndrome with oral fludarabine: A case series of four consecutive patients. Br. J. Haematol. 2016, 172, 461–467. [Google Scholar]
- Richards, A.I. Response of meningeal Waldenström’s macroglobulinemia to 2-chlorodeoxyadenosine. J. Clin. Oncol. 1995, 13, 2424–2425. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Canales, M.A.; Garcia, B.; Alvarez-Ferreira, J.; Garcia-Grande, A.; Hernandez-Navarro, F. Radiation therapy and combination of cladribine, cyclophosphamide, and prednisone as treatment of Bing-Neel syndrome: Case report and review of the literature. Am. J. Hematol. 2002, 70, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Marchioni, E.; Bonfichi, M.; Picchiecchio, A.; Arcaini, L.; Arbasino, C.; Gotti, M.; Da Via, M.; Delmonte, M.; Sciarra, R.; et al. Successful treatment with rituximab and bendamustine in a patient with newly diagnosed Waldenström’s macroglobulinemia complicated by Bing-Neel syndrome. Am. J. Hematol. 2015, 90, E152–E153. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberge, M.G.; Depreter, B.; Dumoulin, E.N.; Emmerechts, J.; Nollet, F.; Vanopdenbosch, L.J. Bing-Neel syndrome: Two unexpected cases and a review of the literature. J. Neurol. Sci. 2015, 356, 19–26. [Google Scholar] [CrossRef]
- Bhatti, M.T. Optic neuropathy from Bing-Neel syndrome. Neurology 2005, 64, 574. [Google Scholar]
- Sánchez-Guerrero, S.; Castillo, J.J. Bing-Neel syndrome: A rare complication of Waldenström macroglobulinemia. Blood 2015, 126, 1390. [Google Scholar] [CrossRef]
- Saburi, M.; Sakata, M.; Okuhiro, K.; Kawano, K.; Uesugi, S.; Wada, J.; Urabe, S.; Saburi, Y.; Ohtsuka, E. Successful treatment with tirabrutinib for relapsed Bing-Neel syndrome following high-dose methotrexate and craniospinal irradiation. J. Clin. Exp. Hematop. 2022, 62, 181–186. [Google Scholar] [CrossRef]
- Cabannes-Hamy, A.; Lemal, R.; Goldwirt, L.; Poulain, S.; Amorim, S.; Quessada, J.; Mercier, M.; Dartigeas, C.; Maerevoet, M.; Verhoef, G.; et al. Efficacy of ibrutinib in the treatment of Bing-Neel syndrome. Am. J. Hematol. 2016, 91, E17–E19. [Google Scholar] [CrossRef]
- Mason, C.; Savona, S.; Castillo, J.J. Ibrutinib penetrates the blood brain barrier and shows efficacy in the therapy of Bing-Neel syndrome. Br. J. Haematol. 2017, 179, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Itchaki, G.; Paludo, J.; Varettoni, M.; Buske, C.; Eyre, T.A.; Chavez, J.C.; Shain, K.H.; Issa, S.; Palomba, M.L.; et al. Ibrutinib for the treatment of Bing-Neel syndrome: A multicenter study. Blood 2019, 133, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Saburi, M.; Masunari, T.; Fukuhara, N.; Inagaki, Y.; Shimura, A.; Imoto, N.; Hasegawa, Y.; Hagihara, M.; Kobayashi, N.; Kumekawa, H.; et al. The impact of tirabrutinib monotherapy for the treatment of Bing-Neel syndrome: A multicenter retrospective study. Am. J. Hematol. 2025, 100, 1912. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, N.; Rai, S.; Munakata, W.; Suzuki, K.; Handa, H.; Shibayama, H.; Endo, T.; Terui, Y.; Iwaki, N.; Fukuhara, N.; et al. A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenström’s macroglobulinemia. Cancer Sci. 2020, 111, 3327–3337. [Google Scholar] [CrossRef]
- Narita, Y.; Nagane, M.; Mishima, K.; Terui, Y.; Arakawa, Y.; Yonezawa, H.; Asai, K.; Fukuhara, N.; Sugiyama, K.; Shinojima, N.; et al. Phase I/II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2021, 23, 122–133. [Google Scholar] [CrossRef]
- Sarosiek, S.; Ramirez-Gamero, A.; Flynn, C.A.; Treon, S.P.; Castillo, J.J. Zanubrutinib for the treatment of Bing-Neel syndrome. Br. J. Haematol. 2025, 206, 1136–1140. [Google Scholar] [CrossRef]
- Seymour, J.F.; Tam, C.S.; Cheah, C.Y.; Parrondo, R.D.; Allan, J.N.; Trotman, J.; Advani, R.H.; Eradat, H.A.; Zinzani, P.L.; Lasica, M.; et al. Preliminary efficacy and safety of the Bruton tyrosine kinase degrader BGB-16673 in patients with relapsed or refractory Waldenström macroglobulinemia: Results from the Phase 1 CaDAnCe-101 study. Blood 2024, 144 (Suppl. 1), 860–862. [Google Scholar] [CrossRef]
- Castillo, J.J.; Allan, J.N.; Siddiqi, T.; Advani, R.H.; Meid, K.; Leventoff, C.; White, T.P.; Flynn, C.A.; Sarosiek, S.; Branagan, A.R.; et al. Venetoclax in previously treated Waldenström macroglobulinemia. J. Clin. Oncol. 2021, 39, 63–71. [Google Scholar] [CrossRef]
- Reda, G.; Cassin, R.; Dovrtelova, G.; Matteo, C.; Giannotta, J.; D’Incalci, M.; Cortelezzi, A.; Zucchetti, M. Venetoclax penetrates in cerebrospinal fluid and may be effective in chronic lymphocytic leukemia with central nervous system involvement. Haematologica 2019, 104, e222–e224. [Google Scholar] [CrossRef]
- Palomba, M.L.; Qualls, D.; Monette, S.; Sethi, S.; Dogan, A.; Roshal, M.; Senechal, B.; Wang, X.; Rivière, I.; Sadelain, M.; et al. CD19-directed chimeric antigen receptor T cell therapy in Waldenström macroglobulinemia: A preclinical model and initial clinical experience. J. Immunother. Cancer 2022, 10, e004128. [Google Scholar] [CrossRef]
- Cook, M.R.; Dorris, C.S.; Makambi, K.H.; Luo, Y.; Munshi, P.N.; Donato, M.; Rowley, S.; Saad, A.; Goy, A.; Dunleavy, K.; et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: A meta-analysis of 128 patients. Blood Adv. 2023, 7, 175–185. [Google Scholar] [CrossRef]
- Yang, G.; Liu, X.; Chen, J.; Xu, L.; Tsakmaklis, N.; Chen, J.; Patterson, C.J.; Castillo, J.J.; Cohen, P.; Tan, L.; et al. Targeting IRAK1/IRAK4 signaling in Waldenström’s macroglobulinemia. Blood 2015, 126, 4004. [Google Scholar] [CrossRef]
- Von Roemeling, C.A.; Doonan, B.P.; Klippel, K.; Schultz, D.; Hoang-Minh, L.; Trivedi, V.; Li, C.; Russell, R.A.; Kanumuri, R.S.; Sharma, A.; et al. Oral IRAK-4 inhibitor CA-4948 is blood-brain barrier penetrant and has single-agent activity against CNS lymphoma and melanoma brain metastases. Clin. Cancer Res. 2023, 29, 1751–1762. [Google Scholar] [CrossRef]
| Response Category | Previous Criteria (Haematologica 2017) [2] | Updated Criteria (IWWM-12, 2025) [1] | Key Changes from Previous to Updated Criteria |
|---|---|---|---|
| Complete Response (CR) | Resolution of all neurological symptoms with the normalization of CSF and MRI findings | Resolution of all reversible neurological symptoms, with the normalization of CSF (cytology, flow cytometry, and MYD88 PCR) and MRI findings, and the absence of new neurological symptoms or MRI findings | Addition of MYD88 PCR in the CSF evaluation; explicit requirement of “no new symptoms/findings” |
| Clinical Complete Response (CCR) | Not defined | Resolution of all reversible neurological symptoms and MRI abnormalities attributed to BNS | New category introduced |
| Partial Response (PR) | Improvement in neurological symptoms, but with persistent radiological abnormalities and negative CSF | Improvement, but not complete resolution, of reversible neurological symptoms | Removed the requirement for negative CSF and persistent imaging abnormalities; simplified to a symptom-based definition |
| No Response (NR) | Persistence or progression of neurological symptoms, radiological findings, or CSF findings | No improvement in neurological symptoms related to BNS | Removed imaging/CSF requirements; defined solely on clinical symptoms |
| Progressive Disease (PD) | Defined only as “relapse”: reappearance of new signs/symptoms or progression/new MRI findings | Appearance of new or progressive neurological symptoms, or worsening of MRI findings attributed to BNS | Expanded from the relapse-only definition to include progressive disease |
| Ibrutinib (Blood 2019) [42] | Zanubrutinib (Leukemia 2025) [27] | Tirabrutinib (Am J Hematol 2025) [43] | |
|---|---|---|---|
| Number of patients | 28 | 30 | 21 |
| Follow-up Duration | Median: 1.9 yrs from BNS diagnosis; 1.0 year from ibrutinib initiation | Median: 13 mo (range: 1–87 mo) | Median: 30.9 mo (range: 4.5–49.5 mo); from BNS diagnosis: 39.3 mo |
| Prior Therapies | Most had prior WM therapy; mix of chemoimmunotherapy and HD-MTX; some untreated at BNS diagnosis | 67% prior WM therapy; 40% prior BNS therapy; 2 had prior ibrutinib | 52.4% prior BNS therapy (IT chemo, HD-MTX, Ara-C, RT, F/C, BR); no prior BTKi |
| Time to Response | 84% symptomatic, 57% radiological within 3 mo | 92% of symptomatic patients improved within 3 mo | median time to best response: 5 mo |
| Response Rate | ORR 85% (CR 6%) | ORR 55% (CR 27%, PR 27%, NR 45%); clinical/radiological > 90% | ORR 100% (CR 55.5%) |
| Survival | 2 yrs EFS: 80%; 2 yrs OS: 81%; 5 yrs OS: 86% | Median EFS not reached; OS not reached (no relapses observed) | 30 mo EFS: 90.5%; 30 mo OS: 90.2% |
| Adverse Events | 45% any AE; grade ≥ 3: pneumonia, arrhythmia, bleeding, neutropenia; 2 discontinuations | 47% any AE; grade ≥ 3 in 20% (HTN, SCC, infection, FN); 3 discontinuations | 76% any AE; grade ≥ 3 in 33% (neutropenia, lymphopenia, pneumonia, thrombocytopenia, appendicitis); 7 dose reductions, 8 interruptions, no discontinuations |
| Genetic Mutations (MYD88/CXCR4) | MYD88 L265P in 96% (CSF/BM); CXCR4 not reported | MYD88 L265P: 100% in CSF; CXCR4: 1/7 BM (14%) positive, 0/2 CSF tested | MYD88 L265P: 7/8 CSF (87.5%), 4/7 BM (57.1%); CXCR4 not tested |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saburi, M.; Sekiguchi, N. Bing–Neel Syndrome in Waldenström Macroglobulinemia: Updates on Clinical Management and BTK Inhibitor Efficacy. Cancers 2025, 17, 3358. https://doi.org/10.3390/cancers17203358
Saburi M, Sekiguchi N. Bing–Neel Syndrome in Waldenström Macroglobulinemia: Updates on Clinical Management and BTK Inhibitor Efficacy. Cancers. 2025; 17(20):3358. https://doi.org/10.3390/cancers17203358
Chicago/Turabian StyleSaburi, Masuho, and Naohiro Sekiguchi. 2025. "Bing–Neel Syndrome in Waldenström Macroglobulinemia: Updates on Clinical Management and BTK Inhibitor Efficacy" Cancers 17, no. 20: 3358. https://doi.org/10.3390/cancers17203358
APA StyleSaburi, M., & Sekiguchi, N. (2025). Bing–Neel Syndrome in Waldenström Macroglobulinemia: Updates on Clinical Management and BTK Inhibitor Efficacy. Cancers, 17(20), 3358. https://doi.org/10.3390/cancers17203358






