Repurposing the Tyrosine Kinase Inhibitors Targeting FGFR and VEGFR Pathways for Cancer Therapy: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Cancer Stem Cells (CSCs) and Chemoresistance
3. DNA Damage Repair (DDR) and Sensitivity of Cancer to Chemotherapy
4. Epithelial-to-Mesenchymal Transition (EMT) and Cancer Resistance to Chemotherapies
5. ABC Transporters and Chemoresistance
6. The Inhibitors of ABC Transporters
7. P-Glycoprotein/ABCB1 Inhibitors
8. MRP1 (ABCC1) Inhibitors
9. BCRP/ABCG2 Inhibitors
10. “Off-Target” Effects of Tyrosine Kinase Inhibitors (TKIs) and Cancer Resistance to Chemotherapy
11. Fibroblast Growth Factor Receptor (FGFR) Inhibitors
12. FGFR Inhibitors and DNA Damage Repair
13. FGFR Inhibitors and ABC Transporters
14. FGFR Inhibitors and Epithelial-to-Mesenchymal Transition (EMT)
15. FGFR Inhibitors and Cancer Stem Cells (CSCs)
16. Non-Selective FGFR Inhibitors
17. Vascular Endothelial Growth Factor Receptor (VEGFR) Inhibitors
18. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Richiardone, E.; Jorge, J.; Polónia, B.; Xavier, C.P.R.; Salaroglio, I.C.; Riganti, C.; Vasconcelos, M.H.; Corbet, C.; Sarmento-Ribeiro, A.B. Impact of cancer metabolism on therapy resistance—Clinical implications. Drug Resist. Updates 2021, 59, 100797. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: Molecular mechanisms and future perspective. Signal Transduct. Target. Ther. 2022, 7, 329. [Google Scholar] [CrossRef]
- Juan-Carlos, P.M.; Perla-Lidia, P.P.; Stephanie-Talia, M.M.; Mónica-Griselda, A.M.; Luz-María, T.E. ABC transporter superfamily. An updated overview, relevance in cancer multidrug resistance and perspectives with personalized medicine. Mol. Biol. Rep. 2021, 48, 1883–1901. [Google Scholar] [CrossRef]
- Boichuk, S.V.; Ivoilova, T.V. The role of ABC-transporters in homeostasis, cancer pathogenesis and therapy. Usp. Mol. onkol. 2024, 11, 8–21. [Google Scholar] [CrossRef]
- Yu, W.K.; Wang, Z.; Fong, C.C.; Liu, D.; Yip, T.C.; Au, S.K.; Zhu, G.; Yang, M. Chemoresistant lung cancer stem cells display high DNA repair capability to remove cisplatin-induced DNA damage. Br. J. Pharmacol. 2017, 174, 302–313. [Google Scholar] [CrossRef]
- Abad, E.; Civit, L.; Potesil, D.; Zdrahal, Z.; Lyakhovich, A. Enhanced DNA damage response through RAD50 in triple negative breast cancer resistant and cancer stem-like cells contributes to chemoresistance. FEBS J. 2021, 288, 2184–2202. [Google Scholar] [CrossRef]
- Meyer, F.; Engel, A.M.; Krause, A.K.; Wagner, T.; Poole, L.; Dubrovska, A.; Peitzsch, C.; Rothkamm, K.; Petersen, C.; Borgmann, K. Efficient DNA Repair Mitigates Replication Stress Resulting in Less Immunogenic Cytosolic DNA in Radioresistant Breast Cancer Stem Cells. Front. Immunol. 2022, 13, 765284. [Google Scholar] [CrossRef]
- Azzoni, V.; Wicinski, J.; Macario, M.; Castagné, M.; Finetti, P.; Ambrosova, K.; Rouault, C.D.; Sergé, A.; Farina, A.; Agavnian, E.; et al. BMI1 nuclear location is critical for RAD51-dependent response to replication stress and drives chemoresistance in breast cancer stem cells. Cell Death Dis. 2022, 13, 96. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Schulz, A.; Meyer, F.; Dubrovska, A.; Borgmann, K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers 2019, 11, 862. [Google Scholar] [CrossRef]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Seike, M.; Noro, R.; Soeno, C.; Chiba, M.; Zou, F.; Nakamichi, S.; Nishijima, N.; Matsumoto, M.; Miyanaga, A.; et al. Inhibition of ABCB1 Overcomes Cancer Stem Cell-like Properties and Acquired Resistance to MET Inhibitors in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2015, 14, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Fu, S.J.; Fan, W.Z.; Wang, Z.H.; Chen, Z.B.; Guo, S.J.; Chen, J.X.; Qiu, S.P. PKCε inhibits isolation and stemness of side population cells via the suppression of ABCB1 transporter and PI3K/Akt, MAPK/ERK signaling in renal cell carcinoma cell line 769P. Cancer Lett. 2016, 376, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Schwan, J.V.; Fujita, M.; Norris, D.A.; Shellman, Y.G. Alternative Treatments For Melanoma: Targeting BCL-2 Family Members to De-Bulk and Kill Cancer Stem Cells. J. Investig. Dermatol. 2015, 135, 2155–2161. [Google Scholar] [CrossRef]
- Safa, A.R. Resistance to Cell Death and Its Modulation in Cancer Stem Cells. Crit. Rev. Oncog. 2016, 21, 203–219. [Google Scholar] [CrossRef]
- Safa, A.R. Drug and apoptosis resistance in cancer stem cells: A puzzle with many pieces. Cancer Drug Resist. 2022, 5, 850–872. [Google Scholar] [CrossRef]
- Androutsellis-Theotokis, A.; Leker, R.R.; Soldner, F.; Hoeppner, D.J.; Ravin, R.; Poser, S.W.; Rueger, M.A.; Bae, S.K.; Kittappa, R.; McKay, R.D. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006, 442, 823–826. [Google Scholar] [CrossRef]
- Chiba, S. Notch signaling in stem cell systems. Stem Cells 2006, 24, 2437–2447. [Google Scholar] [CrossRef]
- Carnero, A.; Garcia-Mayea, Y.; Mir, C.; Lorente, J.; Rubio, I.T.; LLeonart, M.E. The cancer stem-cell signaling network and resistance to therapy. Cancer Treat. Rev. 2016, 49, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.P.; Spinola, M.; Dodge, M.; Raso, M.G.; Behrens, C.; Gao, B.; Schuster, K.; Shao, C.; Larsen, J.E.; Sullivan, L.A.; et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010, 70, 9937–9948. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Sun, S.; Qi, X.; Ji, P. Aldehyde dehydrogenase activity is a cancer stem cell marker of tongue squamous cell carcinoma. Mol. Med. Rep. 2012, 5, 1116–1120. [Google Scholar] [CrossRef]
- Li, L.; Xie, T. Stem cell niche: Structure and function. Annu. Rev. Cell Dev. Biol. 2005, 21, 605–631. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Jia, Y.; Yu, Q.; Grau, G.E.; Peng, L.; Ran, Y.; Yang, Z.; Deng, H.; Lou, J. Single-cell clones of liver cancer stem cells have the potential of differentiating into different types of tumor cells. Cell Death Dis. 2013, 4, e857. [Google Scholar] [CrossRef]
- Ishimoto, T.; Sawayama, H.; Sugihara, H.; Baba, H. Interaction between gastric cancer stem cells and the tumor microenvironment. J. Gastroenterol. 2014, 49, 1111–1120. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Takahashi, R.-u.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Sundaram, S.M.; Varier, L.; Fathima, K.Z.; Dharmarajan, A.; Warrier, S. Short peptide domains of the Wnt inhibitor sFRP4 target ovarian cancer stem cells by neutralizing the Wnt β-catenin pathway, disrupting the interaction between β-catenin and CD24 and suppressing autophagy. Life Sci. 2023, 316, 121384. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zeng, B.; Zhao, Q.; Chen, H.; Zhang, Y.; Chen, Y.; Wang, J.; Xing, H.R. Exosomal microRNA-4535 of Melanoma Stem Cells Promotes Metastasis by Inhibiting Autophagy Pathway. Stem Cell Rev. Rep. 2023, 19, 155–169. [Google Scholar] [CrossRef]
- Li, D.; Peng, X.; He, G.; Liu, J.; Li, X.; Lin, W.; Fang, J.; Li, X.; Yang, S.; Yang, L.; et al. Crosstalk between autophagy and CSCs: Molecular mechanisms and translational implications. Cell Death Dis. 2023, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Eun, K.; Ham, S.W.; Kim, H. Cancer stem cell heterogeneity: Origin and new perspectives on CSC targeting. BMB Rep. 2017, 50, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hollier, B.G.; Tinnirello, A.A.; Werden, S.J.; Evans, K.W.; Taube, J.H.; Sarkar, T.R.; Sphyris, N.; Shariati, M.; Kumar, S.V.; Battula, V.L.; et al. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013, 73, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.S.; Ward, C.M.; Davies, C.C. DNA Repair and Therapeutic Strategies in Cancer Stem Cells. Cancers 2023, 15, 1897. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Lheureux, S.; Bruce, J.P.; Burnier, J.V.; Karakasis, K.; Shaw, P.A.; Clarke, B.A.; Yang, S.Y.; Quevedo, R.; Li, T.; Dowar, M.; et al. Somatic BRCA1/2 Recovery as a Resistance Mechanism After Exceptional Response to Poly (ADP-ribose) Polymerase Inhibition. J. Clin. Oncol. 2017, 35, 1240–1249. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Zhai, L.; Zhang, T.; Yin, H.; Gao, H.; Zhao, F.; Wang, Z.; Yang, X.; Jin, M.; et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 2024, 187, 294–311.e21. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, H.; Chen, X.; Fu, H.; Mao, D.; Chen, W.; Lan, L.; Wang, C.; Hu, K.; et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature 2024, 631, 663–669. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Cheng, S.C.; Wahner Hendrickson, A.E.; Penson, R.T.; Schumer, S.T.; Doyle, L.A.; Lee, E.K.; Kohn, E.C.; Duska, L.R.; Crispens, M.A.; et al. Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 957–968. [Google Scholar] [CrossRef]
- Gallo, D.; Young, J.T.F.; Fourtounis, J.; Martino, G.; Álvarez-Quilón, A.; Bernier, C.; Duffy, N.M.; Papp, R.; Roulston, A.; Stocco, R.; et al. CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature 2022, 604, 749–756. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.A.; Lin, Y.C.; Ma, J.W.; Huang, L.F.; Yang, N.S.; Ho, C.T.; Kuo, S.C.; Way, T.D. Curcumin suppresses doxorubicin-induced epithelial-mesenchymal transition via the inhibition of TGF-β and PI3K/AKT signaling pathways in triple-negative breast cancer cells. J. Agric. Food Chem. 2013, 61, 11817–11824. [Google Scholar] [CrossRef] [PubMed]
- Anandi, L.; Chakravarty, V.; Ashiq, K.A.; Bodakuntla, S.; Lahiri, M. DNA-dependent protein kinase plays a central role in transformation of breast epithelial cells following alkylation damage. J. Cell Sci. 2017, 130, 3749–3763. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ozawa, Y.; Kimura, T.; Sato, Y.; Kuznetsov, G.; Xu, S.; Uesugi, M.; Agoulnik, S.; Taylor, N.; Funahashi, Y.; et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br. J. Cancer 2014, 110, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Weyemi, U.; Redon, C.E.; Choudhuri, R.; Aziz, T.; Maeda, D.; Boufraqech, M.; Parekh, P.R.; Sethi, T.K.; Kasoji, M.; Abrams, N.; et al. The histone variant H2A.X is a regulator of the epithelial-mesenchymal transition. Nat. Commun. 2016, 7, 10711. [Google Scholar] [CrossRef]
- Sengodan, S.K.; Sreelatha, K.H.; Nadhan, R.; Srinivas, P. Regulation of Epithelial to Mesenchymal Transition by BRCA1 in Breast Cancer. Crit. Rev. Oncol. Hematol. 2018, 123, 74–82. [Google Scholar] [CrossRef]
- Park, S.Y.; Korm, S.; Chung, H.J.; Choi, S.J.; Jang, J.J.; Cho, S.; Lim, Y.T.; Kim, H.; Lee, J.Y. RAP80 regulates epithelial-mesenchymal transition related with metastasis and malignancy of cancer. Cancer Sci. 2016, 107, 267–273. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Li, X.Y.; Hu, C.Y.; Ford, M.; Kleer, C.G.; Weiss, S.J. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc. Natl. Acad. Sci. USA 2012, 109, 16654–16659. [Google Scholar] [CrossRef]
- Saxena, M.; Stephens, M.A.; Pathak, H.; Rangarajan, A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011, 2, e179. [Google Scholar] [CrossRef]
- Yu, C.C.; Lo, W.L.; Chen, Y.W.; Huang, P.I.; Hsu, H.S.; Tseng, L.M.; Hung, S.C.; Kao, S.Y.; Chang, C.J.; Chiou, S.H. Bmi-1 Regulates Snail Expression and Promotes Metastasis Ability in Head and Neck Squamous Cancer-Derived ALDH1 Positive Cells. J. Oncol. 2011, 2011, 609259. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, H.; Zhang, Z.; Zhang, G.; Wang, H.; Zhang, Q.; Sun, P.; Xiang, R.; Yang, S. ZEB1 confers stem cell-like properties in breast cancer by targeting neurogenin-3. Oncotarget 2017, 8, 54388–54401. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B.P. Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer 2011, 11, 49. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial–Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef] [PubMed]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Brabletz, T.; Ceppi, P. Targeting EMT in Cancer with Repurposed Metabolic Inhibitors. Trends Cancer 2020, 6, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.P.; Kaplan, E.; Crow, A.; Koronakis, V. Antibiotic Resistance Mediated by the MacB ABC Transporter Family: A Structural and Functional Perspective. Front. Microbiol. 2018, 9, 950. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Thomas, C.; Tampé, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Moore, J.M.; Bell, E.L.; Hughes, R.O.; Garfield, A.S. ABC transporters: Human disease and pharmacotherapeutic potential. Trends Mol. Med. 2023, 29, 152–172. [Google Scholar] [CrossRef]
- Choudhuri, S.; Klaassen, C.D. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006, 25, 231–259. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wu, Z.X.; Yang, Y.; Teng, Q.X.; Li, Y.D.; Lei, Z.N.; Jani, K.A.; Kaushal, N.; Chen, Z.S. ATP-binding cassette (ABC) transporters in cancer: A review of recent updates. J. Evid. Based Med. 2021, 14, 232–256. [Google Scholar] [CrossRef]
- Domenichini, A.; Adamska, A.; Falasca, M. ABC transporters as cancer drivers: Potential functions in cancer development. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; To, K.K.W.; Chen, Z.S.; Fu, L. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist. Updates 2023, 66, 100905. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updates 2020, 50, 100682. [Google Scholar] [CrossRef] [PubMed]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981, 41, 1967–1972. [Google Scholar]
- Dalton, W.S.; Grogan, T.M.; Meltzer, P.S.; Scheper, R.J.; Durie, B.G.; Taylor, C.W.; Miller, T.P.; Salmon, S.E. Drug-resistance in multiple myeloma and non-Hodgkin’s lymphoma: Detection of P-glycoprotein and potential circumvention by addition of verapamil to chemotherapy. J. Clin. Oncol. 1989, 7, 415–424. [Google Scholar] [CrossRef]
- Qi, D.; Dou, Y.; Zhang, W.; Wang, M.; Li, Y.; Zhang, M.; Qin, J.; Cao, J.; Fang, D.; Ma, J.; et al. The influence of verapamil on the pharmacokinetics of the pan-HER tyrosine kinase inhibitor neratinib in rats: The role of P-glycoprotein-mediated efflux. Investig. New Drugs 2023, 41, 13–24. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y. Efflux mechanism and pathway of verapamil pumping by human P-glycoprotein. Arch. Biochem. Biophys. 2020, 696, 108675. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Chen, T.; Wang, W.; Liu, Q.; Li, H.; Yi, J.; Wang, J. Mechanisms of verapamil-enhanced chemosensitivity of gallbladder cancer cells to platinum drugs: Glutathione reduction and MRP1 downregulation. Oncol. Rep. 2013, 29, 676–684. [Google Scholar] [CrossRef]
- Henrich, C.J.; Bokesch, H.R.; Dean, M.; Bates, S.E.; Robey, R.W.; Goncharova, E.I.; Wilson, J.A.; McMahon, J.B. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J. Biomol. Screen. 2006, 11, 176–183. [Google Scholar] [CrossRef]
- Demeule, M.; Laplante, A.; Sepehr-Araé, A.; Beaulieu, E.; Averill-Bates, D.; Wenger, R.M.; Béliveau, R. Inhibition of P-glycoprotein by cyclosporin A analogues and metabolites. Biochem. Cell Biol. 1999, 77, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Safa, A.R. Competitive interaction of cyclosporins with the Vinca alkaloid-binding site of P-glycoprotein in multidrug-resistant cells. J. Biol. Chem. 1990, 265, 16509–16513. [Google Scholar] [PubMed]
- Qadir, M.; O’Loughlin, K.L.; Fricke, S.M.; Williamson, N.A.; Greco, W.R.; Minderman, H.; Baer, M.R. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin. Cancer Res. 2005, 11, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Pawarode, A.; Shukla, S.; Minderman, H.; Fricke, S.M.; Pinder, E.M.; O’Loughlin, K.L.; Ambudkar, S.V.; Baer, M.R. Differential effects of the immunosuppressive agents cyclosporin A, tacrolimus and sirolimus on drug transport by multidrug resistance proteins. Cancer Chemother. Pharmacol. 2007, 60, 179–188. [Google Scholar] [CrossRef]
- Ejendal, K.F.; Hrycyna, C.A. Differential sensitivities of the human ATP-binding cassette transporters ABCG2 and P-glycoprotein to cyclosporin A. Mol. Pharmacol. 2005, 67, 902–911. [Google Scholar] [CrossRef]
- Xia, C.Q.; Liu, N.; Miwa, G.T.; Gan, L.S. Interactions of cyclosporin a with breast cancer resistance protein. Drug Metab. Dispos. 2007, 35, 576–582. [Google Scholar] [CrossRef]
- Daenen, S.; van der Holt, B.; Verhoef, G.E.; Löwenberg, B.; Wijermans, P.W.; Huijgens, P.C.; van Marwijk Kooy, R.; Schouten, H.C.; Kramer, M.H.; Ferrant, A.; et al. Addition of cyclosporin A to the combination of mitoxantrone and etoposide to overcome resistance to chemotherapy in refractory or relapsing acute myeloid leukaemia: A randomised phase II trial from HOVON, the Dutch-Belgian Haemato-Oncology Working Group for adults. Leuk. Res. 2004, 28, 1057–1067. [Google Scholar] [CrossRef]
- Becton, D.; Dahl, G.V.; Ravindranath, Y.; Chang, M.N.; Behm, F.G.; Raimondi, S.C.; Head, D.R.; Stine, K.C.; Lacayo, N.J.; Sikic, B.I.; et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood 2006, 107, 1315–1324. [Google Scholar] [CrossRef]
- Cui, W.; Zhao, H.; Wang, C.; Chen, Y.; Luo, C.; Zhang, S.; Sun, B.; He, Z. Co-encapsulation of docetaxel and cyclosporin A into SNEDDS to promote oral cancer chemotherapy. Drug Deliv. 2019, 26, 542–550. [Google Scholar] [CrossRef]
- Gao, W.; Lin, Z.; Chen, M.; Yang, X.; Cui, Z.; Zhang, X.; Yuan, L.; Zhang, Q. The co-delivery of a low-dose P-glycoprotein inhibitor with doxorubicin sterically stabilized liposomes against breast cancer with low P-glycoprotein expression. Int. J. Nanomed. 2014, 9, 3425–3437. [Google Scholar] [CrossRef]
- Ling, G.; Zhang, T.; Zhang, P.; Sun, J.; He, Z. Synergistic and complete reversal of the multidrug resistance of mitoxantrone hydrochloride by three-in-one multifunctional lipid-sodium glycocholate nanocarriers based on simultaneous BCRP and Bcl-2 inhibition. Int. J. Nanomed. 2016, 11, 4077–4091. [Google Scholar] [CrossRef]
- Wilson, W.H.; Jamis-Dow, C.; Bryant, G.; Balis, F.M.; Klecker, R.W.; Bates, S.E.; Chabner, B.A.; Steinberg, S.M.; Kohler, D.R.; Wittes, R.E. Phase I and pharmacokinetic study of the multidrug resistance modulator dexverapamil with EPOCH chemotherapy. J. Clin. Oncol. 1995, 13, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.; Hedley, D.; Andrulis, I.; Myers, R.; Trudeau, M.; Warr, D.; Pritchard, K.I.; Blackstein, M.; Goss, P.E.; Franssen, E.; et al. Phase II study of dexverapamil plus anthracycline in patients with metastatic breast cancer who have progressed on the same anthracycline regimen. Clin. Cancer Res. 1998, 4, 1451–1457. [Google Scholar] [PubMed]
- Chico, I.; Kang, M.H.; Bergan, R.; Abraham, J.; Bakke, S.; Meadows, B.; Rutt, A.; Robey, R.; Choyke, P.; Merino, M.; et al. Phase I study of infusional paclitaxel in combination with the P-glycoprotein antagonist PSC 833. J. Clin. Oncol. 2001, 19, 832–842. [Google Scholar] [CrossRef]
- Lhommé, C.; Joly, F.; Walker, J.L.; Lissoni, A.A.; Nicoletto, M.O.; Manikhas, G.M.; Baekelandt, M.M.; Gordon, A.N.; Fracasso, P.M.; Mietlowski, W.L.; et al. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J. Clin. Oncol. 2008, 26, 2674–2682. [Google Scholar] [CrossRef]
- Friedenberg, W.R.; Rue, M.; Blood, E.A.; Dalton, W.S.; Shustik, C.; Larson, R.A.; Sonneveld, P.; Greipp, P.R. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): A trial of the Eastern Cooperative Oncology Group. Cancer 2006, 106, 830–838. [Google Scholar] [CrossRef]
- Fracasso, P.M.; Fisher, G.A., Jr.; Goodner, S.A.; Beumer, J.H.; Egorin, M.J.; Fears, C.L.; Wildi, J.D.; Jones, G.J.; Pearce, T.E.; Sikic, B.I. A Phase I Trial of the ABCB1 Inhibitor, Oral Valspodar, in Combination with Paclitaxel in Patients with Advanced Solid Tumors. Am. J. Clin. Oncol. 2023, 46, 353–359. [Google Scholar] [CrossRef]
- Bankstahl, J.P.; Bankstahl, M.; Römermann, K.; Wanek, T.; Stanek, J.; Windhorst, A.D.; Fedrowitz, M.; Erker, T.; Müller, M.; Löscher, W.; et al. Tariquidar and elacridar are dose-dependently transported by P-glycoprotein and Bcrp at the blood-brain barrier: A small-animal positron emission tomography and in vitro study. Drug Metab. Dispos. 2013, 41, 754–762. [Google Scholar] [CrossRef]
- Kannan, P.; Telu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Halldin, C.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The “specific” P-glycoprotein inhibitor Tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2). ACS Chem. Neurosci. 2011, 2, 82–89. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, C.; Luo, L.; Tang, Y.; Yu, Y.; Li, Y.; Xing, J.; Pan, X. Membrane-assisted tariquidar access and binding mechanisms of human ATP-binding cassette transporter P-glycoprotein. Front. Mol. Biosci. 2024, 11, 1364494. [Google Scholar] [CrossRef]
- Hamaguchi-Suzuki, N.; Adachi, N.; Moriya, T.; Yasuda, S.; Kawasaki, M.; Suzuki, K.; Ogasawara, S.; Anzai, N.; Senda, T.; Murata, T. Cryo-EM structure of P-glycoprotein bound to triple elacridar inhibitor molecules. Biochem. Biophys. Res. Commun. 2024, 709, 149855. [Google Scholar] [CrossRef]
- Packeiser, E.M.; Engels, L.; Nolte, I.; Goericke-Pesch, S.; Murua Escobar, H. MDR1 Inhibition Reverses Doxorubicin-Resistance in Six Doxorubicin-Resistant Canine Prostate and Bladder Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 8136. [Google Scholar] [CrossRef] [PubMed]
- Braconi, L.; Dei, S.; Contino, M.; Riganti, C.; Bartolucci, G.; Manetti, D.; Romanelli, M.N.; Perrone, M.G.; Colabufo, N.A.; Guglielmo, S.; et al. Tetrazole and oxadiazole derivatives as bioisosteres of tariquidar and elacridar: New potent P-gp modulators acting as MDR reversers. Eur. J. Med. Chem. 2023, 259, 115716. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, P.; Sopel, J.; Lipowicz, J.M.; Rawłuszko-Wieczorek, A.A.; Korbecki, J.; Januchowski, R. The Role of Elacridar, a P-gp Inhibitor, in the Re-Sensitization of PAC-Resistant Ovarian Cancer Cell Lines to Cytotoxic Drugs in 2D and 3D Cell Culture Models. Int. J. Mol. Sci. 2025, 26, 1124. [Google Scholar] [CrossRef]
- Chen, H.; Shien, K.; Suzawa, K.; Tsukuda, K.; Tomida, S.; Sato, H.; Torigoe, H.; Watanabe, M.; Namba, K.; Yamamoto, H.; et al. Elacridar, a third-generation ABCB1 inhibitor, overcomes resistance to docetaxel in non-small cell lung cancer. Oncol. Lett. 2017, 14, 4349–4354. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Jorge, J.; Almeida, A.M.; Sarmento-Ribeiro, A.B. Combination of Elacridar with Imatinib Modulates Resistance Associated with Drug Efflux Transporters in Chronic Myeloid Leukemia. Biomedicines 2022, 10, 1158. [Google Scholar] [CrossRef]
- Sato, H.; Siddig, S.; Uzu, M.; Suzuki, S.; Nomura, Y.; Kashiba, T.; Gushimiyagi, K.; Sekine, Y.; Uehara, T.; Arano, Y.; et al. Elacridar enhances the cytotoxic effects of sunitinib and prevents multidrug resistance in renal carcinoma cells. Eur. J. Pharmacol. 2015, 746, 258–266. [Google Scholar] [CrossRef]
- Stewart, A.; Steiner, J.; Mellows, G.; Laguda, B.; Norris, D.; Bevan, P. Phase I trial of XR9576 in healthy volunteers demonstrates modulation of P-glycoprotein in CD56+ lymphocytes after oral and intravenous administration. Clin. Cancer Res. 2000, 6, 4186–4191. [Google Scholar]
- Sparreboom, A.; Planting, A.S.; Jewell, R.C.; van der Burg, M.E.; van der Gaast, A.; de Bruijn, P.; Loos, W.J.; Nooter, K.; Chandler, L.H.; Paul, E.M.; et al. Clinical pharmacokinetics of doxorubicin in combination with GF120918, a potent inhibitor of MDR1 P-glycoprotein. Anticancer Drugs 1999, 10, 719–728. [Google Scholar] [CrossRef]
- Malingré, M.M.; Beijnen, J.H.; Rosing, H.; Koopman, F.J.; Jewell, R.C.; Paul, E.M.; Ten Bokkel Huinink, W.W.; Schellens, J.H. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br. J. Cancer 2001, 84, 42–47. [Google Scholar] [CrossRef]
- Kruijtzer, C.M.; Beijnen, J.H.; Rosing, H.; ten Bokkel Huinink, W.W.; Schot, M.; Jewell, R.C.; Paul, E.M.; Schellens, J.H. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J. Clin. Oncol. 2002, 20, 2943–2950. [Google Scholar] [CrossRef]
- Planting, A.S.; Sonneveld, P.; van der Gaast, A.; Sparreboom, A.; van der Burg, M.E.; Luyten, G.P.; de Leeuw, K.; de Boer-Dennert, M.; Wissel, P.S.; Jewell, R.C.; et al. A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2005, 55, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Edgerly, M.; Wilson, R.; Chen, C.; Rutt, A.; Bakke, S.; Robey, R.; Dwyer, A.; Goldspiel, B.; Balis, F.; et al. A phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbine. Clin. Cancer Res. 2009, 15, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Widemann, B.C.; Pastakia, D.; Chen, C.C.; Yang, S.X.; Cole, D.; Balis, F.M. Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors. Cancer Chemother. Pharmacol. 2015, 76, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Draper, D.; Chen, C.C.; Robey, R.W.; Figg, W.D.; Piekarz, R.L.; Chen, X.; Gardner, E.R.; Balis, F.M.; Venkatesan, A.M.; et al. A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clin. Cancer Res. 2011, 17, 569–580. [Google Scholar] [CrossRef]
- Bauer, F.; Kuntner, C.; Bankstahl, J.P.; Wanek, T.; Bankstahl, M.; Stanek, J.; Mairinger, S.; Dörner, B.; Löscher, W.; Müller, M.; et al. Synthesis and in vivo evaluation of [11C]tariquidar, a positron emission tomography radiotracer based on a third-generation P-glycoprotein inhibitor. Bioorg. Med. Chem. 2010, 18, 5489–5497. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hoti, S.L. Development of Fourth Generation ABC Inhibitors from Natural Products: A Novel Approach to Overcome Cancer Multidrug Resistance. Anticancer Agents Med. Chem. 2015, 15, 605–615. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W.; Qu, L.; Wu, J.; Si, J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and P-glycoprotein expression. Mol. Med. Rep. 2013, 8, 1883–1887. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Ling, V. Effect of quercetin on Hoechst 33342 transport by purified and reconstituted P-glycoprotein. Biochem. Pharmacol. 1997, 53, 587–596. [Google Scholar] [CrossRef]
- Nabekura, T.; Yamaki, T.; Kitagawa, S. Effects of chemopreventive citrus phytochemicals on human P-glycoprotein and multidrug resistance protein 1. Eur. J. Pharmacol. 2008, 600, 45–49. [Google Scholar] [CrossRef]
- Borska, S.; Chmielewska, M.; Wysocka, T.; Drag-Zalesinska, M.; Zabel, M.; Dziegiel, P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem. Toxicol. 2012, 50, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Patel, S.K.; Kumar, P.; Das, K.C.; Verma, D.; Sharma, R.; Tripathi, T.; Giri, R.; Martins, N.; Garg, N. Quercetin acts as a P-gp modulator via impeding signal transduction from nucleotide-binding domain to transmembrane domain. J. Biomol. Struct. Dyn. 2022, 40, 4507–4515. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Q.; Wang, B.; Yuan, S.; Wang, X.; Li, K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother. Res. 2018, 32, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Mao, Q.; Oleschuk, C.J.; Deeley, R.G.; Cole, S.P. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and atpase activities by interaction with dietary flavonoids. Mol. Pharmacol. 2001, 59, 1171–1180. [Google Scholar] [CrossRef]
- Nguyen, H.; Zhang, S.; Morris, M.E. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J. Pharm. Sci. 2003, 92, 250–257. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Ikegami, Y.; Sano, K.; Yoshida, H.; Mitomo, H.; Sawada, S.; Ishikawa, T. Transport of SN-38 by the wild type of human ABC transporter ABCG2 and its inhibition by quercetin, a natural flavonoid. J. Exp. Ther. Oncol. 2004, 4, 25–35. [Google Scholar]
- Martínez-Esquivias, F.; Guzmán-Flores, J.M.; Pech-Santiago, E.O.; Guerrero-Barrera, A.L.; Delgadillo-Aguirre, C.K.; Anaya-Esparza, L.M. Therapeutic Role of Quercetin in Prostate Cancer: A Study of Network Pharmacology, Molecular Docking, and Dynamics Simulation. Cell Biochem. Biophys. 2025, 83, 3153–3164. [Google Scholar] [CrossRef]
- Yin, H.; Dong, J.; Cai, Y.; Shi, X.; Wang, H.; Liu, G.; Tang, Y.; Liu, J.; Ma, L. Design, synthesis and biological evaluation of chalcones as reversers of P-glycoprotein-mediated multidrug resistance. Eur. J. Med. Chem. 2019, 180, 350–366. [Google Scholar] [CrossRef]
- Huang, H.Y.; Niu, J.L.; Lu, Y.H. Multidrug resistance reversal effect of DMC derived from buds of Cleistocalyx operculatus in human hepatocellular tumor xenograft model. J. Sci. Food Agric. 2012, 92, 135–140. [Google Scholar] [CrossRef]
- Pires, M.M.; Emmert, D.; Hrycyna, C.A.; Chmielewski, J. Inhibition of P-glycoprotein-mediated paclitaxel resistance by reversibly linked quinine homodimers. Mol. Pharmacol. 2009, 75, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.D.; Wang, J.S.; Kong, L.Y. Reversal effects of components from the fruits of Illicium simonsii on human Adriamycin-resistant MCF-7 and 5-fluorouracil-resistant Bel7402 cells. Phytother. Res. 2012, 26, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Pokharel, D.; Bebawy, M. MRP1 and its role in anticancer drug resistance. Drug Metab. Rev. 2015, 47, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Lorendeau, D.; Dury, L.; Nasr, R.; Boumendjel, A.; Teodori, E.; Gutschow, M.; Falson, P.; Di Pietro, A.; Baubichon-Cortay, H. MRP1-dependent Collateral Sensitivity of Multidrug-resistant Cancer Cells: Identifying Selective Modulators Inducing Cellular Glutathione Depletion. Curr. Med. Chem. 2017, 24, 1186–1213. [Google Scholar] [CrossRef]
- Tan, K.W.; Sampson, A.; Osa-Andrews, B.; Iram, S.H. Calcitriol and Calcipotriol Modulate Transport Activity of ABC Transporters and Exhibit Selective Cytotoxicity in MRP1-overexpressing Cells. Drug Metab. Dispos. 2018, 46, 1856–1866. [Google Scholar] [CrossRef]
- Gana, C.C.; Hanssen, K.M.; Yu, D.M.T.; Flemming, C.L.; Wheatley, M.S.; Conseil, G.; Cole, S.P.C.; Norris, M.D.; Haber, M.; Fletcher, J.I. MRP1 modulators synergize with buthionine sulfoximine to exploit collateral sensitivity and selectively kill MRP1-expressing cancer cells. Biochem. Pharmacol. 2019, 168, 237–248. [Google Scholar] [CrossRef]
- Riganti, C.; Giampietro, R.; Kopecka, J.; Costamagna, C.; Abatematteo, F.S.; Contino, M.; Abate, C. MRP1-Collateral Sensitizers as a Novel Therapeutic Approach in Resistant Cancer Therapy: An In Vitro and In Vivo Study in Lung Resistant Tumor. Int. J. Mol. Sci. 2020, 21, 3333. [Google Scholar] [CrossRef]
- Jones, T.R.; Zamboni, R.; Belley, M.; Champion, E.; Charette, L.; Ford-Hutchinson, A.W.; Frenette, R.; Gauthier, J.Y.; Leger, S.; Masson, P. Pharmacology of L-660,711 (MK-571): A novel potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1989, 67, 17–28. [Google Scholar] [CrossRef]
- Leier, I.; Jedlitschky, G.; Buchholz, U.; Cole, S.P.; Deeley, R.G.; Keppler, D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994, 269, 27807–27810. [Google Scholar] [CrossRef]
- Gekeler, V.; Ise, W.; Sanders, K.H.; Ulrich, W.R.; Beck, J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995, 208, 345–352. [Google Scholar] [CrossRef]
- Benyahia, B.; Huguet, S.; Declèves, X.; Mokhtari, K.; Crinière, E.; Bernaudin, J.F.; Scherrmann, J.M.; Delattre, J.Y. Multidrug resistance-associated protein MRP1 expression in human gliomas: Chemosensitization to vincristine and etoposide by indomethacin in human glioma cell lines overexpressing MRP1. J. Neurooncol. 2004, 66, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Peigñan, L.; Garrido, W.; Segura, R.; Melo, R.; Rojas, D.; Cárcamo, J.G.; San Martín, R.; Quezada, C. Combined use of anticancer drugs and an inhibitor of multiple drug resistance-associated protein-1 increases sensitivity and decreases survival of glioblastoma multiforme cells in vitro. Neurochem. Res. 2011, 36, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Tivnan, A.; Zakaria, Z.; O’Leary, C.; Kögel, D.; Pokorny, J.L.; Sarkaria, J.N.; Prehn, J.H. Inhibition of multidrug resistance protein 1 (MRP1) improves chemotherapy drug response in primary and recurrent glioblastoma multiforme. Front. Neurosci. 2015, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Veringa, S.J.; Biesmans, D.; van Vuurden, D.G.; Jansen, M.H.; Wedekind, L.E.; Horsman, I.; Wesseling, P.; Vandertop, W.P.; Noske, D.P.; Kaspers, G.J.; et al. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS ONE 2013, 8, e61512. [Google Scholar] [CrossRef]
- Kopanitsa, L.; Kopanitsa, M.V.; Safitri, D.; Ladds, G.; Bailey, D.S. Suppression of Proliferation of Human Glioblastoma Cells by Combined Phosphodiesterase and Multidrug Resistance-Associated Protein 1 Inhibition. Int. J. Mol. Sci. 2021, 22, 9665. [Google Scholar] [CrossRef]
- Mease, K.; Sane, R.; Podila, L.; Taub, M.E. Differential selectivity of efflux transporter inhibitors in Caco-2 and MDCK-MDR1 monolayers: A strategy to assess the interaction of a new chemical entity with P-gp, BCRP, and MRP2. J. Pharm. Sci. 2012, 101, 1888–1897. [Google Scholar] [CrossRef]
- Rabindran, S.K.; He, H.; Singh, M.; Brown, E.; Collins, K.I.; Annable, T.; Greenberger, L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998, 58, 5850–5858. [Google Scholar]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.; Koomen, G.J.; Schinkel, A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002, 1, 417–425. [Google Scholar]
- Weidner, L.D.; Zoghbi, S.S.; Lu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Mulder, J.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The Inhibitor Ko143 Is Not Specific for ABCG2. J. Pharmacol. Exp. Ther. 2015, 354, 384–393. [Google Scholar] [CrossRef]
- Lustig, S.D.; Kodali, S.K.; Longo, S.L.; Kundu, S.; Viapiano, M.S. Ko143 Reverses MDR in Glioblastoma via Deactivating P-Glycoprotein, Sensitizing a Resistant Phenotype to TMZ Treatment. Anticancer Res. 2022, 42, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Peña-Solórzano, D.; Stark, S.A.; König, B.; Sierra, C.A.; Ochoa-Puentes, C. ABCG2/BCRP: Specific and Nonspecific Modulators. Med. Res. Rev. 2017, 37, 987–1050. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, K.; Shah, C.P.; Sahu, N.U.; Juvale, K.; Stefan, S.M.; Kharkar, P.S.; Wiese, M. Novel chalcone and flavone derivatives as selective and dual inhibitors of the transport proteins ABCB1 and ABCG2. Eur. J. Med. Chem. 2019, 164, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Cassinelli, G.; Pennati, M.; Zuco, V.; Gatti, L. Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur. J. Med. Chem. 2017, 142, 271–289. [Google Scholar] [CrossRef]
- Wu, S.; Fu, L. Tyrosine kinase inhibitors enhanced the efficacy of conventional chemotherapeutic agent in multidrug resistant cancer cells. Mol. Cancer 2018, 17, 25. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Fardi, E.; Ghaderi, H.; Palizdar, S.; Khorram, R.; Vafadar, R.; Ghanaatian, M.; Rezaei-Tazangi, F.; Baziyar, P.; Ahmadi, A.; et al. Receptor tyrosine kinase inhibitors in cancer. Cell. Mol. Life Sci. 2023, 80, 104. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Tiong, K.H.; Mah, L.Y.; Leong, C.O. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis 2013, 18, 1447–1468. [Google Scholar] [CrossRef]
- Babina, I.S.; Turner, N.C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 2017, 17, 318–332. [Google Scholar] [CrossRef]
- Shi, G.M.; Huang, X.Y.; Wen, T.F.; Song, T.Q.; Kuang, M.; Mou, H.B.; Bao, L.Q.; Zhao, H.T.; Zhao, H.; Feng, X.L.; et al. Pemigatinib in previously treated Chinese patients with locally advanced or metastatic cholangiocarcinoma carrying FGFR2 fusions or rearrangements: A phase II study. Cancer Med. 2023, 12, 4137–4146. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Gotlib, J.; Kiladjian, J.-J.; Vannucchi, A.; Rambaldi, A.; Reiter, A.; Shomali, W.; George, T.I.; Patel, J.L.; Colucci, P.; Walker, C.; et al. A Phase 2 Study of Pemigatinib (FIGHT-203; INCB054828) in Patients with Myeloid/Lymphoid Neoplasms (MLNs) with Fibroblast Growth Factor Receptor 1 (FGFR1) Rearrangement (MLN FGFR1). Blood 2021, 138, 385. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, H.A.; Sawers, L.; Clarke, R.G.; Hiom, K.J.; Ferguson, M.J.; Smith, G. Fibroblast growth factor signalling influences homologous recombination-mediated DNA damage repair to promote drug resistance in ovarian cancer. Br. J. Cancer 2022, 127, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, S.; Dunaev, P.; Galembikova, A.; Bikinieva, F.; Nurgatina, I.; Mustafin, I.; Aukhadieva, A.; Kurtasanov, R.; Andriutsa, N.; Shagimardanova, E.; et al. Inhibition of FGFR2-Signaling Attenuates a Homology-Mediated DNA Repair in GIST and Sensitizes Them to DNA-Topoisomerase II Inhibitors. Int. J. Mol. Sci. 2020, 21, 352. [Google Scholar] [CrossRef]
- Chen, M.K.; Yamaguchi, H.; Gao, Y.; Xia, W.; Chang, J.T.; Hsiao, Y.C.; Shegute, T.W.; Lin, Z.S.; Wu, C.S.; Wang, Y.H.; et al. FGFR3-induced Y158 PARP1 phosphorylation promotes PARP inhibitor resistance via BRG1/MRE11-mediated DNA repair in breast cancer models. J. Clin. Investig. 2025, 135, e173757. [Google Scholar] [CrossRef]
- Ma, J.; Benitez, J.A.; Li, J.; Miki, S.; Ponte de Albuquerque, C.; Galatro, T.; Orellana, L.; Zanca, C.; Reed, R.; Boyer, A.; et al. Inhibition of Nuclear PTEN Tyrosine Phosphorylation Enhances Glioma Radiation Sensitivity through Attenuated DNA Repair. Cancer Cell 2019, 35, 504–518.e7. [Google Scholar] [CrossRef]
- Deshors, P.; Kheil, Z.; Ligat, L.; Gouazé-Andersson, V.; Cohen-Jonathan Moyal, E. FGFR inhibition as a new therapeutic strategy to sensitize glioblastoma stem cells to tumor treating fields. Cell Death Discov. 2025, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, L.; Hao, Y.; Xu, C.; Wang, X.; Jia, Z.; Xie, X.; Huang, Z.; Gao, X.; Chen, Y.; et al. Erdafitinib inhibits the tumorigenicity of MDA-MB-231 triple-negative breast cancer cells by inducing TRIM25/ubiquitin-dependent degradation of FGFR4. Breast Cancer Res. 2025, 27, 128. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J.H.; Valderrama, B.P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1961–1971. [Google Scholar] [CrossRef]
- Wu, C.-P.; Hung, T.-H.; Hsiao, S.-H.; Huang, Y.-H.; Hung, L.-C.; Yu, Y.-J.; Chang, Y.-T.; Wang, S.-P.; Wu, Y.-S. Erdafitinib Resensitizes ABCB1-Overexpressing Multidrug-Resistant Cancer Cells to Cytotoxic Anticancer Drugs. Cancers 2020, 12, 1366. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, M.; Wu, Z.X.; Wang, J.Q.; Dong, X.D.; Yang, Y.; Teng, Q.X.; Chen, X.Y.; Cui, Q.; Yang, D.H. Erdafitinib Antagonizes ABCB1-Mediated Multidrug Resistance in Cancer Cells. Front. Oncol. 2020, 10, 955. [Google Scholar] [CrossRef]
- Boichuk, S.; Dunaev, P.; Mustafin, I.; Mani, S.; Syuzov, K.; Valeeva, E.; Bikinieva, F.; Galembikova, A. Infigratinib (BGJ 398), a Pan-FGFR Inhibitor, Targets P-Glycoprotein and Increases Chemotherapeutic-Induced Mortality of Multidrug-Resistant Tumor Cells. Biomedicines 2022, 10, 601. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Pemigatinib: Hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat. Res. Commun. 2021, 27, 100337. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, M.N.; Shan, J.Q.; Hu, Z.J.; Li, Z.W.; Liu, J.Y. Pemigatinib, a selective FGFR inhibitor overcomes ABCB1-mediated multidrug resistance in cancer cells. Biochem. Biophys. Res. Commun. 2024, 691, 149314. [Google Scholar] [CrossRef]
- Tomlinson, D.C.; Baxter, E.W.; Loadman, P.M.; Hull, M.A.; Knowles, M.A. FGFR1-induced epithelial to mesenchymal transition through MAPK/PLCγ/COX-2-mediated mechanisms. PLoS ONE 2012, 7, e38972. [Google Scholar] [CrossRef]
- Brown, W.S.; Akhand, S.S.; Wendt, M.K. FGFR signaling maintains a drug persistent cell population following epithelial-mesenchymal transition. Oncotarget. 2016, 7, 83424–83436. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Song, W.; Li, L.; Cao, L.; Yu, Y.; Song, C.; Wang, Y.; Zhang, F.; Li, Y.; Zhang, B.; et al. FGF4 induces epithelial-mesenchymal transition by inducing store-operated calcium entry in lung adenocarcinoma. Oncotarget 2016, 7, 74015–74030. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Tsunematsu, T.; Yanagisawa, S.; Kudo, Y.; Miyauchi, M.; Kamata, N.; Takata, T. The FGFR1 inhibitor PD173074 induces mesenchymal-epithelial transition through the transcription factor AP-1. Br. J. Cancer 2013, 109, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Raoof, S.; Mulford, I.J.; Frisco-Cabanos, H.; Nangia, V.; Timonina, D.; Labrot, E.; Hafeez, N.; Bilton, S.J.; Drier, Y.; Ji, F.; et al. Targeting FGFR overcomes EMT-mediated resistance in EGFR mutant non-small cell lung cancer. Oncogene 2019, 38, 6399–6413. [Google Scholar] [CrossRef]
- McDermott, S.C.; Rodriguez-Ramirez, C.; McDermott, S.P.; Wicha, M.S.; Nör, J.E. FGFR signaling regulates resistance of head and neck cancer stem cells to cisplatin. Oncotarget 2018, 9, 25148–25165. [Google Scholar] [CrossRef]
- Ji, W.; Yu, Y.; Li, Z.; Wang, G.; Li, F.; Xia, W.; Lu, S. FGFR1 promotes the stem cell-like phenotype of FGFR1-amplified non-small cell lung cancer cells through the Hedgehog pathway. Oncotarget 2016, 7, 15118–15134. [Google Scholar] [CrossRef]
- Ko, J.; Meyer, A.N.; Haas, M.; Donoghue, D.J. Characterization of FGFR signaling in prostate cancer stem cells and inhibition via TKI treatment. Oncotarget 2021, 12, 22–36. [Google Scholar] [CrossRef]
- Cheng, Q.; Ma, Z.; Shi, Y.; Parris, A.B.; Kong, L.; Yang, X. FGFR1 Overexpression Induces Cancer Cell Stemness and Enhanced Akt/Erk-ER Signaling to Promote Palbociclib Resistance in Luminal A Breast Cancer Cells. Cells 2021, 10, 3008. [Google Scholar] [CrossRef]
- Maehara, O.; Suda, G.; Natsuizaka, M.; Ohnishi, S.; Komatsu, Y.; Sato, F.; Nakai, M.; Sho, T.; Morikawa, K.; Ogawa, K.; et al. Fibroblast growth factor-2-mediated FGFR/Erk signaling supports maintenance of cancer stem-like cells in esophageal squamous cell carcinoma. Carcinogenesis 2017, 38, 1073–1083. [Google Scholar] [CrossRef]
- Boichuk, S.; Dunaev, P.; Galembikova, A.; Valeeva, E. Fibroblast Growth Factor 2 (FGF2) Activates Vascular Endothelial Growth Factor (VEGF) Signaling in Gastrointestinal Stromal Tumors (GIST): An Autocrine Mechanism Contributing to Imatinib Mesylate (IM) Resistance. Cancers 2024, 16, 3103. [Google Scholar] [CrossRef]
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.S.; Xu, Q.; et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 2009, 16, 401–412. [Google Scholar] [CrossRef]
- Kort, A.; van Hoppe, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Brain Accumulation of Ponatinib and Its Active Metabolite, N-Desmethyl Ponatinib, Is Limited by P-Glycoprotein (P-GP/ABCB1) and Breast Cancer Resistance Protein (BCRP/ABCG2). Mol. Pharm. 2017, 14, 3258–3268. [Google Scholar] [CrossRef] [PubMed]
- Laramy, J.K.; Kim, M.; Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Pharmacokinetic Assessment of Cooperative Efflux of the Multitargeted Kinase Inhibitor Ponatinib Across the Blood-Brain Barrier. J. Pharmacol. Exp. Ther. 2018, 365, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, Y.; Akamine, Y.; Abumiya, M.; Tozawa, N.; Yamashita, T.; Nara, M.; Kameoka, Y.; Takahashi, N.; Miura, M. Effects of ABCB1 polymorphisms on the transport of ponatinib into the cerebrospinal fluid in Japanese Philadelphia chromosome-positive acute lymphoblastic leukaemia patients. Br. J. Clin. Pharmacol. 2023, 89, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Natarajan, K.; Bhullar, J.; Shukla, S.; Fang, H.B.; Cai, L.; Chen, Z.S.; Ambudkar, S.V.; Baer, M.R. The novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2. Mol. Cancer Ther. 2012, 11, 2033–2044. [Google Scholar] [CrossRef]
- Sun, Y.L.; Kumar, P.; Sodani, K.; Patel, A.; Pan, Y.; Baer, M.R.; Chen, Z.S.; Jiang, W.Q. Ponatinib enhances anticancer drug sensitivity in MRP7-overexpressing cells. Oncol. Rep. 2014, 31, 1605–1612. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, L.; Kang, Z.; Jiang, W.; Lv, C. Ponatinib ameliorates pulmonary fibrosis by suppressing TGF-β1/Smad3 pathway. Pulm. Pharmacol. Ther. 2015, 34, 1–7. [Google Scholar] [CrossRef]
- Tusa, I.; Cheloni, G.; Poteti, M.; Silvano, A.; Tubita, A.; Lombardi, Z.; Gozzini, A.; Caporale, R.; Scappini, B.; Dello Sbarba, P.; et al. In Vitro Comparison of the Effects of Imatinib and Ponatinib on Chronic Myeloid Leukemia Progenitor/Stem Cell Features. Target. Oncol. 2020, 15, 659–671. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fukushima, T.; Mikami, K.; Adachi, K.; Fukuyama, T.; Goyama, S.; Kitamura, T. Efficacy of tyrosine kinase inhibitors on a mouse chronic myeloid leukemia model and chronic myeloid leukemia stem cells. Exp. Hematol. 2020, 90, 46–51.e2. [Google Scholar] [CrossRef]
- Angevin, E.; Lopez-Martin, J.A.; Lin, C.C.; Gschwend, J.E.; Harzstark, A.; Castellano, D.; Soria, J.C.; Sen, P.; Chang, J.; Shi, M.; et al. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin. Cancer Res. 2013, 19, 1257–1268. [Google Scholar] [CrossRef]
- Weiss, J.; Theile, D.; Dvorak, Z.; Haefeli, W.E. Interaction Potential of the Multitargeted Receptor Tyrosine Kinase Inhibitor Dovitinib with Drug Transporters and Drug Metabolising Enzymes Assessed in Vitro. Pharmaceutics 2014, 6, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Chen, L.; Ann, V.; Lin, W.C.; Wang, Y.; Chang, V.H.; Hsu, N.Y.; Shia, H.S.; Yen, Y. Dovitinib synergizes with oxaliplatin in suppressing cell proliferation and inducing apoptosis in colorectal cancer cells regardless of RAS-RAF mutation status. Mol. Cancer 2014, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Thanasupawat, T.; Natarajan, S.; Rommel, A.; Glogowska, A.; Bergen, H.; Krcek, J.; Pitz, M.; Beiko, J.; Krawitz, S.; Verma, I.M.; et al. Dovitinib enhances temozolomide efficacy in glioblastoma cells. Mol. Oncol. 2017, 11, 1078–1098. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B.; Wu, X.; Nitiss, J.L.; Kanagasabai, R.; Yalowich, J.C. The anticancer multi-kinase inhibitor dovitinib also targets topoisomerase I and topoisomerase II. Biochem. Pharmacol. 2012, 84, 1617–1626. [Google Scholar] [CrossRef]
- Hernandez-Agudo, E.; Soto-Montenegro, M.; Mondejar, T.; Sanchez, J.; Mulero, F.; Desco, M.; Quintela-Fandino, M. 18F-misonidazole positron-emission tomography (FMISO-PET) as an early biomarker of vascular normalization in response to antiangiogenic therapy. Eur. J. Cancer 2013, 49, S155. [Google Scholar]
- Cao, L.L.; Lu, H.; Soutto, M.; Bhat, N.; Chen, Z.; Peng, D.; Gomaa, A.; Wang, J.B.; Xie, J.W.; Li, P.; et al. Multivalent tyrosine kinase inhibition promotes T cell recruitment to immune-desert gastric cancers by restricting epithelial-mesenchymal transition via tumour-intrinsic IFN-γ signalling. Gut 2023, 72, 2038–2050. [Google Scholar] [CrossRef]
- Minocha, M.; Khurana, V.; Qin, B.; Pal, D.; Mitra, A.K. Enhanced brain accumulation of pazopanib by modulating P-gp and Bcrp1 mediated efflux with canertinib or erlotinib. Int. J. Pharm. 2012, 436, 127–134. [Google Scholar] [CrossRef]
- D’Cunha, R.; Bae, S.; Murry, D.J.; An, G. TKI combination therapy: Strategy to enhance dasatinib uptake by inhibiting Pgp- and BCRP-mediated efflux. Biopharm. Drug Dispos. 2016, 37, 397–408. [Google Scholar] [CrossRef]
- Isham, C.R.; Bossou, A.R.; Negron, V.; Fisher, K.E.; Kumar, R.; Marlow, L.; Lingle, W.L.; Smallridge, R.C.; Sherman, E.J.; Suman, V.J.; et al. Pazopanib enhances paclitaxel-induced mitotic catastrophe in anaplastic thyroid cancer. Sci. Transl. Med. 2013, 5, 166ra3. [Google Scholar] [CrossRef]
- Tan, A.R.; Dowlati, A.; Jones, S.F.; Infante, J.R.; Nishioka, J.; Fang, L.; Hodge, J.P.; Gainer, S.D.; Arumugham, T.; Suttle, A.B.; et al. Phase I study of pazopanib in combination with weekly paclitaxel in patients with advanced solid tumors. Oncologist 2010, 15, 1253–1261. [Google Scholar] [CrossRef]
- Narayanan, S.; Lam, A.; Vaishampayan, U.; Harshman, L.; Fan, A.; Pachynski, R.; Poushnejad, S.; Haas, D.; Li, S.; Srinivas, S. Phase II Study of Pazopanib and Paclitaxel in Patients with Refractory Urothelial Cancer. Clin. Genitourin. Cancer 2016, 14, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; El-Masry, M.; Osann, K.; Parmakhtiar, B.; Yamamoto, M.; Jakowatz, J.G. Phase II study of pazopanib in combination with paclitaxel in patients with metastatic melanoma. Cancer Chemother. Pharmacol. 2018, 82, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Pink, D.; Andreou, D.; Bauer, S.; Brodowicz, T.; Kasper, B.; Reichardt, P.; Richter, S.; Lindner, L.H.; Szkandera, J.; Grünwald, V.; et al. Treatment of Angiosarcoma with Pazopanib and Paclitaxel: Results of the EVA (Evaluation of Votrient® in Angiosarcoma) Phase II Trial of the German Interdisciplinary Sarcoma Group (GISG-06). Cancers 2021, 13, 1223. [Google Scholar] [CrossRef]
- Richardson, D.L.; Sill, M.W.; Coleman, R.L.; Sood, A.K.; Pearl, M.L.; Kehoe, S.M.; Carney, M.E.; Hanjani, P.; Van Le, L.; Zhou, X.C.; et al. Paclitaxel with and Without Pazopanib for Persistent or Recurrent Ovarian Cancer: A Randomized Clinical Trial. JAMA Oncol. 2018, 4, 196–202. [Google Scholar] [CrossRef]
- Sherman, E.J.; Harris, J.; Bible, K.C.; Xia, P.; Ghossein, R.A.; Chung, C.H.; Riaz, N.; Gunn, G.B.; Foote, R.L.; Yom, S.S.; et al. Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): A randomised, double-blind, placebo-controlled, multicentre, phase 2 trial. Lancet Oncol. 2023, 24, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Li, D.; Teng, L.; Chen, Y.; Meng, J.; Yang, C.; Yin, Z.; Li, C. Pazopanib stimulates senescence of renal carcinoma cells through targeting nuclear factor E2-related factor 2 (Nrf2). J. Biochem. Mol. Toxicol. 2024, 38, e23689. [Google Scholar] [CrossRef]
- Prasad, C.B.; Singh, D.; Pandey, L.K.; Pradhan, S.; Singh, S.; Narayan, G. VEGFa/VEGFR2 autocrine and paracrine signaling promotes cervical carcinogenesis via β-catenin and snail. Int. J. Biochem. Cell Biol. 2022, 142, 106122. [Google Scholar] [CrossRef]
- Patel, A.; Tiwari, A.K.; Chufan, E.E.; Sodani, K.; Anreddy, N.; Singh, S.; Ambudkar, S.V.; Stephani, R.; Chen, Z.S. PD173074, a selective FGFR inhibitor, reverses ABCB1-mediated drug resistance in cancer cells. Cancer Chemother. Pharmacol. 2013, 72, 189–199. [Google Scholar] [CrossRef]
- Anreddy, N.; Patel, A.; Sodani, K.; Kathawala, R.J.; Chen, E.P.; Wurpel, J.N.; Chen, Z.S. PD173074, a selective FGFR inhibitor, reverses MRP7 (ABCC10)-mediated MDR. Acta Pharm. Sin. B 2014, 4, 202–207. [Google Scholar] [CrossRef]
- Ye, Y.W.; Hu, S.; Shi, Y.Q.; Zhang, X.F.; Zhou, Y.; Zhao, C.L.; Wang, G.J.; Wen, J.G.; Zong, H. Combination of the FGFR4 inhibitor PD173074 and 5-fluorouracil reduces proliferation and promotes apoptosis in gastric cancer. Oncol. Rep. 2013, 30, 2777–2784. [Google Scholar] [CrossRef]
- Byron, S.A.; Loch, D.C.; Pollock, P.M. Fibroblast growth factor receptor inhibition synergizes with Paclitaxel and Doxorubicin in endometrial cancer cells. Int. J. Gynecol. Cancer 2012, 22, 1517–1526. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Ragunathan, P.; Shin, J.; Peter, S.; Kleissle, S.; Neuenschwander, M.; Schäfer, R.; Kries, J.P.V.; Grüber, G.; Dröge, P. The FGFR inhibitor PD173074 binds to the C-terminus of oncofetal HMGA2 and modulates its DNA-binding and transcriptional activation functions. FEBS Lett. 2023, 597, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Dwivedi, S.K.D.; Bhattacharya, R.; Mukherjee, P.; Rao, G. VEGF signaling: Role in angiogenesis and beyond. Biochim. Biophys. Acta. Rev. Cancer 2024, 1879, 189079. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Dvorak, A.M.; Dvorak, H.F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2007, 2, 251–275. [Google Scholar] [CrossRef]
- Rak, J.; Mitsuhashi, Y.; Bayko, L.; Filmus, J.; Shirasawa, S.; Sasazuki, T.; Kerbel, R.S. Mutant ras oncogenes upregulate VEGF/VPF expression: Implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995, 55, 4575–4580. [Google Scholar]
- Khromova, N.V.; Kopnin, P.B.; Stepanova, E.V.; Agapova, L.S.; Kopnin, B.P. p53 hot-spot mutants increase tumor vascularization via ROS-mediated activation of the HIF1/VEGF-A pathway. Cancer Lett. 2009, 276, 143–151. [Google Scholar] [CrossRef]
- Bachelder, R.E.; Crago, A.; Chung, J.; Wendt, M.A.; Shaw, L.M.; Robinson, G.; Mercurio, A.M. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001, 61, 5736–5740. [Google Scholar]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef]
- Ohm, J.E.; Gabrilovich, D.I.; Sempowski, G.D.; Kisseleva, E.; Parman, K.S.; Nadaf, S.; Carbone, D.P. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 2003, 101, 4878–4886. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, M.J.; Kumar, A.; Lee, H.W.; Yang, Y.; Kim, Y. Vascular endothelial growth factor signaling in health and disease: From molecular mechanisms to therapeutic perspectives. Signal Transduct. Target Ther. 2025, 10, 170. [Google Scholar] [CrossRef]
- Javle, M.; Smyth, E.C.; Chau, I. Ramucirumab: Successfully targeting angiogenesis in gastric cancer. Clin. Cancer Res. 2014, 20, 5875–5881. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tabernero, J.; Tomášek, J.; Chau, I.; Melichar, B.; Safran, H.; Tehfe, M.A.; Filip, D.; Topuzov, E.; Schlittler, L.; et al. Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab. Br. J. Cancer 2016, 115, 974–982. [Google Scholar] [CrossRef]
- Goodman, V.L.; Rock, E.P.; Dagher, R.; Ramchandani, R.P.; Abraham, S.; Gobburu, J.V.; Booth, B.P.; Verbois, S.L.; Morse, D.E.; Liang, C.Y.; et al. Approval summary: Sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin. Cancer Res. 2007, 13, 1367–1373. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; François, L.; Quivy, A.; Ravaud, A. Axitinib: A review of its safety and efficacy in the treatment of adults with advanced renal cell carcinoma. Clin. Med. Insights Oncol. 2013, 7, 269–277. [Google Scholar] [CrossRef]
- Hoy, S.M. Cabozantinib: A review of its use in patients with medullary thyroid cancer. Drugs 2014, 74, 1435–1444. [Google Scholar] [CrossRef]
- Scott, L.J. Lenvatinib: First global approval. Drugs 2015, 75, 553–560. [Google Scholar] [CrossRef]
- Heo, Y.A.; Syed, Y.Y. Regorafenib: A Review in Hepatocellular Carcinoma. Drugs 2018, 78, 951–958. [Google Scholar] [CrossRef]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human malignancies. Onco Targets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef]
- Poller, B.; Iusuf, D.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Differential impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on axitinib brain accumulation and oral plasma pharmacokinetics. Drug Metab. Dispos. 2011, 39, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Sassa, N.; Miyazaki, M.; Takeuchi, M.; Asai, M.; Iwai, A.; Noda, Y.; Gotoh, M.; Yamada, K. Association of axitinib plasma exposure and genetic polymorphisms of ABC transporters with axitinib-induced toxicities in patients with renal cell carcinoma. Cancer Chemother. Pharmacol. 2016, 78, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mi, Y.J.; Chen, X.G.; Wu, X.P.; Liu, Z.; Chen, S.P.; Liang, Y.J.; Cheng, C.; To, K.K.; Fu, L.W. Axitinib targeted cancer stemlike cells to enhance efficacy of chemotherapeutic drugs via inhibiting the drug transport function of ABCG2. Mol. Med. 2012, 18, 887–898. [Google Scholar] [CrossRef]
- Mi, Y.J.; Liang, Y.J.; Huang, H.B.; Zhao, H.Y.; Wu, C.P.; Wang, F.; Tao, L.Y.; Zhang, C.Z.; Dai, C.L.; Tiwari, A.K.; et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010, 70, 7981–7991. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.F.; Wang, F.; Su, X.D.; Liang, Y.J.; Zheng, L.S.; Mi, Y.J.; Chen, W.Q.; Fu, L.W. Effect of BIBF 1120 on reversal of ABCB1-mediated multidrug resistance. Cell Oncol. 2011, 34, 33–44. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Kapadia, A.; Zhou, Q.; Harper, M.K.; Schaack, J.; LaBarbera, D.V. 3D models of epithelial-mesenchymal transition in breast cancer metastasis: High-throughput screening assay development, validation, and pilot screen. J. Biomol. Screen. 2011, 16, 141–154. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Solarek, W.; Kornakiewicz, A.; Szczylik, C. Tyrosine kinase inhibitors target cancer stem cells in renal cell cancer. Oncol. Rep. 2016, 35, 1433–1442. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- De la Fouchardière, C. Regorafenib in the treatment of metastatic colorectal cancer. Future Oncol. 2018, 14, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, S.; Rausch, J.; Duensing, A. New developments in management of gastrointestinal stromal tumors: Regorafenib, the new player in the team. Gastrointest. Cancer Targets Ther. 2014, 4, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Wang, Y.J.; Lei, Z.N.; Zhang, G.N.; Zhang, X.Y.; Wang, D.S.; Al-Rihani, S.B.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib antagonizes BCRP-mediated multidrug resistance in colon cancer. Cancer Lett. 2019, 442, 104–112. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.K.; Zhang, G.N.; Al Rihani, S.B.; Wei, M.N.; Gupta, P.; Zhang, X.Y.; Shukla, S.; Ambudkar, S.V.; Kaddoumi, A.; et al. Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: In vitro and in vivo study. Cancer Lett. 2017, 396, 145–154. [Google Scholar] [CrossRef]
- Kort, A.; Durmus, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Brain and Testis Accumulation of Regorafenib is Restricted by Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1). Pharm. Res. 2015, 32, 2205–2216. [Google Scholar] [CrossRef]
- Ohya, H.; Shibayama, Y.; Ogura, J.; Narumi, K.; Kobayashi, M.; Iseki, K. Regorafenib is transported by the organic anion transporter 1B1 and the multidrug resistance protein 2. Biol. Pharm. Bull. 2015, 38, 582–586. [Google Scholar] [CrossRef]
- Mehta, M.; Griffith, J.; Panneerselvam, J.; Babu, A.; Mani, J.; Herman, T.; Ramesh, R.; Munshi, A. Regorafenib sensitizes human breast cancer cells to radiation by inhibiting multiple kinases and inducing DNA damage. Int. J. Radiat. Biol. 2021, 97, 1109–1120. [Google Scholar] [CrossRef]
- Pham, T.D.; Becker, J.H.; Metropulos, A.E.; Mubin, N.; Spaulding, C.; Bentrem, D.J.; Munshi, H.G. Regorafenib induces DNA damage and enhances PARP inhibitor efficacy in pancreatic ductal carcinoma. BMC Cancer 2024, 24, 1562. [Google Scholar] [CrossRef]
- Bi, W.; Sun, X.; Yi, Q.; Jiang, X.; He, H.; Jiang, L.; Fan, Z.; Huang, H.; Wen, W.; Jiang, X. PRMT5 attenuates regorafenib-induced DNA damage in hepatocellular carcinoma cells through symmetric dimethylation of RPL14. J. Gastrointest. Oncol. 2025, 16, 191–208. [Google Scholar] [CrossRef]
- Xuan, X.; Li, Y.; Huang, C.; Zhang, Y. Regorafenib promotes antitumor progression in melanoma by reducing RRM2. iScience 2024, 27, 110993. [Google Scholar] [CrossRef]
- Chang, Y.C.; Li, C.H.; Chan, M.H.; Chen, M.H.; Yeh, C.N.; Hsiao, M. Regorafenib inhibits epithelial-mesenchymal transition and suppresses cholangiocarcinoma metastasis via YAP1-AREG axis. Cell Death Dis. 2022, 13, 391. [Google Scholar] [CrossRef] [PubMed]
- Kehagias, P.; Kindt, N.; Krayem, M.; Najem, A.; Agostini, G.; Acedo Reina, E.; Bregni, G.; Sclafani, F.; Journe, F.; Awada, A.; et al. Regorafenib Induces Senescence and Epithelial-Mesenchymal Transition in Colorectal Cancer to Promote Drug Resistance. Cells 2022, 11, 3663. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.H.; Xu, X.G.; Yan, S.L.; Sun, Z.; Ying, Y.; Wang, B.K.; Tu, Y.X. Regorafenib suppresses colon tumorigenesis and the generation of drug resistant cancer stem-like cells via modulation of miR-34a associated signaling. J. Exp. Clin. Cancer Res. 2018, 37, 151. [Google Scholar] [CrossRef]
- Kurzrock, R.; Sherman, S.I.; Ball, D.W.; Forastiere, A.A.; Cohen, R.B.; Mehra, R.; Pfister, D.G.; Cohen, E.E.; Janisch, L.; Nauling, F.; et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 2011, 29, 2660–2666. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef]
- Zhang, G.N.; Zhang, Y.K.; Wang, Y.J.; Barbuti, A.M.; Zhu, X.J.; Yu, X.Y.; Wen, A.W.; Wurpel, J.N.D.; Chen, Z.S. Modulating the function of ATP-binding cassette subfamily G member 2 (ABCG2) with inhibitor cabozantinib. Pharmacol. Res. 2017, 119, 89–98. [Google Scholar] [CrossRef]
- Nazari, S.; Mosaffa, F.; Poustforoosh, A.; Saso, L.; Firuzi, O.; Moosavi, F. c-MET tyrosine kinase inhibitors reverse multidrug resistance in breast cancer cells by targeting ABCG2 transporter. J. Pharm. Pharmacol. 2025, 77, 685–697. [Google Scholar] [CrossRef]
- Lei, Z.N.; Teng, Q.X.; Gupta, P.; Zhang, W.; Narayanan, S.; Yang, D.H.; Wurpel, J.N.D.; Fan, Y.F.; Chen, Z.S. Cabozantinib Reverses Topotecan Resistance in Human Non-Small Cell Lung Cancer NCI-H460/TPT10 Cell Line and Tumor Xenograft Model. Front. Cell Dev. Biol. 2021, 9, 640957. [Google Scholar] [CrossRef]
- Xiang, Q.F.; Zhang, D.M.; Wang, J.N.; Zhang, H.W.; Zheng, Z.Y.; Yu, D.C.; Li, Y.J.; Xu, J.; Chen, Y.J.; Shang, C.Z. Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int. 2015, 35, 1010–1023. [Google Scholar] [CrossRef]
- Hage, C.; Rausch, V.; Giese, N.; Giese, T.; Schönsiegel, F.; Labsch, S.; Nwaeburu, C.; Mattern, J.; Gladkich, J.; Herr, I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013, 4, e627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhu, X.; Cui, K.; Mancuso, J.; Federley, R.; Fischer, K.; Teng, G.; Mittal, V.; Gao, D.; Zhao, H.; et al. In Vivo Visualization and Characterization of Epithelial-Mesenchymal Transition in Breast Tumors. Cancer Res. 2016, 76, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.L.; Liang, Y.J.; Wang, Y.S.; Tiwari, A.K.; Yan, Y.Y.; Wang, F.; Chen, Z.S.; Tong, X.Z.; Fu, L.W. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009, 279, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Robey, R.W.; Bates, S.E.; Ambudkar, S.V. Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab. Dispos. 2009, 37, 359–365. [Google Scholar] [CrossRef]
- Yan, S.; Liu, L.; Ren, F.; Gao, Q.; Xu, S.; Hou, B.; Wang, Y.; Jiang, X.; Che, Y. Sunitinib induces genomic instability of renal carcinoma cells through affecting the interaction of LC3-II and PARP-1. Cell Death Dis. 2017, 8, e2988. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, L.; Zhang, J.; Hu, X.; Liu, Y.; Yin, H.; Lv, T.; Zhang, H.; Liu, L.; An, H.; et al. Sunitinib induces cellular senescence via p53/Dec1 activation in renal cell carcinoma cells. Cancer Sci. 2013, 104, 1052–1061. [Google Scholar] [CrossRef]
- Xie, R.; Taohuang, Z.; Kieran, R.; Li, Z.; Wang, L.; Dong, C.; Ge, J.; Wang, X.; Li, M. Everolimus and Sunitinib potentially work as therapeutic drugs for infantile hemangiomas. Pediatr. Res. 2025. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Zhu, H.C.; Chen, X.C.; Sun, X.C.; Yang, X.; Qin, Q.; Zhang, H.; Yang, Y.; Yang, Y.H.; Gao, L.; et al. Sunitinib modulates the radiosensitivity of esophageal squamous cell carcinoma cells in vitro. Dis. Esophagus 2016, 29, 1144–1151. [Google Scholar] [CrossRef]
- Tomida, C.; Yamagishi, N.; Nagano, H.; Uchida, T.; Ohno, A.; Hirasaka, K.; Nikawa, T.; Teshima-Kondo, S. Antiangiogenic agent sunitinib induces epithelial to mesenchymal transition and accelerates motility of colorectal cancer cells. J. Med. Investig. 2017, 64, 250–254. [Google Scholar] [CrossRef]
- Boichuk, S.; Galembikova, A.; Mikheeva, E.; Bikinieva, F.; Aukhadieva, A.; Dunaev, P.; Khalikov, D.; Petrov, S.; Kurtasanov, R.; Valeeva, E.; et al. Inhibition of FGF2-Mediated Signaling in GIST—Promising Approach for Overcoming Resistance to Imatinib. Cancers 2020, 12, 1674. [Google Scholar] [CrossRef]
- He, M.; Yang, H.; Shi, H.; Hu, Y.; Chang, C.; Liu, S.; Yeh, S. Sunitinib increases the cancer stem cells and vasculogenic mimicry formation via modulating the lncRNA-ECVSR/ERβ/Hif2-α signaling. Cancer Lett. 2022, 524, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Nguewa, P.A.; Redrado, M.; Manrique, I.; Calvo, A. Sunitinib reduces tumor hypoxia and angiogenesis, and radiosensitizes prostate cancer stem-like cells. Prostate 2015, 75, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Brossa, A.; Grange, C.; Mancuso, L.; Annaratone, L.; Satolli, M.A.; Mazzone, M.; Camussi, G.; Bussolati, B. Sunitinib but not VEGF blockade inhibits cancer stem cell endothelial differentiation. Oncotarget 2015, 6, 11295–11309. [Google Scholar] [CrossRef] [PubMed]
- Jovelet, C.; Bénard, J.; Forestier, F.; Farinotti, R.; Bidart, J.M.; Gil, S. Inhibition of P-glycoprotein functionality by vandetanib may reverse cancer cell resistance to doxorubicin. Eur. J. Pharm. Sci. 2012, 46, 484–491. [Google Scholar] [CrossRef]
- Jovelet, C.; Deroussent, A.; Broutin, S.; Paci, A.; Farinotti, R.; Bidart, J.M.; Gil, S. Influence of the multidrug transporter P-glycoprotein on the intracellular pharmacokinetics of vandetanib. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 149–157. [Google Scholar] [CrossRef]
- To, K.K.; Poon, D.C.; Wei, Y.; Wang, F.; Lin, G.; Fu, L.W. Vatalanib sensitizes ABCB1 and ABCG2-overexpressing multidrug resistant colon cancer cells to chemotherapy under hypoxia. Biochem. Pharmacol. 2015, 97, 27–37. [Google Scholar] [CrossRef]
- Wang, G.; Cao, L.; Jiang, Y.; Zhang, T.; Wang, H.; Wang, Z.; Xu, J.; Mao, M.; Hua, Y.; Cai, Z.; et al. Anlotinib Reverses Multidrug Resistance (MDR) in Osteosarcoma by Inhibiting P-Glycoprotein (PGP1) Function In Vitro and In Vivo. Front. Pharmacol. 2022, 12, 798837. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kathawala, R.J.; Zhang, Y.K.; Patel, A.; Kumar, P.; Shukla, S.; Fung, K.L.; Ambudkar, S.V.; Talele, T.T.; Chen, Z.S. Motesanib (AMG706), a potent multikinase inhibitor, antagonizes multidrug resistance by inhibiting the efflux activity of the ABCB1. Biochem. Pharmacol. 2014, 90, 367–378. [Google Scholar] [CrossRef]
- Sodani, K.; Patel, A.; Anreddy, N.; Singh, S.; Yang, D.H.; Kathawala, R.J.; Kumar, P.; Talele, T.T.; Chen, Z.S. Telatinib reverses chemotherapeutic multidrug resistance mediated by ABCG2 efflux transporter in vitro and in vivo. Biochem. Pharmacol. 2014, 89, 52–61. [Google Scholar] [CrossRef]
- Wu, C.P.; Murakami, M.; Wu, Y.S.; Lin, C.L.; Li, Y.Q.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V. The multi-targeted tyrosine kinase inhibitor SKLB610 resensitizes ABCG2-overexpressing multidrug-resistant cancer cells to chemotherapeutic drugs. Biomed. Pharmacother. 2022, 149, 112922. [Google Scholar] [CrossRef]
- Kim, K.S.; Jiang, C.; Kim, J.Y.; Park, J.H.; Kim, H.R.; Lee, S.H.; Kim, H.S.; Yoon, S. Low-Dose Crizotinib, a Tyrosine Kinase Inhibitor, Highly and Specifically Sensitizes P-Glycoprotein-Overexpressing Chemoresistant Cancer Cells Through Induction of Late Apoptosis in vivo and in vitro. Front. Oncol. 2020, 10, 696. [Google Scholar] [CrossRef]
- Song, B.; Kim, D.; Ho, J.-N.; Le, V.-H.; Lee, S. Crizotinib Inhibits Viability, Migration, and Invasion by Suppressing the c-Met/PI3K/Akt Pathway in the Three-Dimensional Bladder Cancer Spheroid Model. Curr. Oncol. 2025, 32, 236. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Noh, K.H.; Lee, Y.H.; Hong, S.O.; Song, K.H.; Lee, H.J.; Kim, S.; Kim, T.M.; Jeon, J.H.; Seo, J.H.; et al. Targeting stemness is an effective strategy to control EML4-ALK+ non-small cell lung cancer cells. Oncotarget 2015, 6, 40255–40267. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, K.C.; Mehta, R.K.; Kurapati, H.; Thomas, D.G.; Lawrence, T.S.; Nyati, M.K. Enhancing the Radiation Response in KRAS Mutant Colorectal Cancers Using the c-Met Inhibitor Crizotinib. Transl. Oncol. 2019, 12, 209–216. [Google Scholar] [CrossRef]
- Czechowska, A.; Poplawski, T.; Drzewoski, J.; Blasiak, J. Imatinib (STI571) induces DNA damage in BCR/ABL-expressing leukemic cells but not in normal lymphocytes. Chem. Biol. Interact. 2005, 152, 139–150. [Google Scholar] [CrossRef]
- Morii, M.; Fukumoto, Y.; Kubota, S.; Yamaguchi, N.; Nakayama, Y.; Yamaguchi, N. Imatinib inhibits inactivation of the ATM/ATR signaling pathway and recovery from adriamycin/doxorubicin-induced DNA damage checkpoint arrest. Cell Biol. Int. 2015, 39, 923–932. [Google Scholar] [CrossRef]
- Sims, J.T.; Ganguly, S.S.; Bennett, H.; Friend, J.W.; Tepe, J.; Plattner, R. Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-κB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS ONE 2013, 8, e55509. [Google Scholar] [CrossRef]
- Shen, T.; Kuang, Y.H.; Ashby, C.R.; Lei, Y.; Chen, A.; Zhou, Y.; Chen, X.; Tiwari, A.K.; Hopper-Borge, E.; Ouyang, J.; et al. Imatinib and nilotinib reverse multidrug resistance in cancer cells by inhibiting the efflux activity of the MRP7 (ABCC10). PLoS ONE 2009, 4, e7520. [Google Scholar] [CrossRef]
- Sarkar, S.; Kandasamy, T.; Ghosh, S.S. Imatinib Impedes EMT and Notch Signalling by Inhibiting p300 Acetyltransferase in Breast Cancer Cells. Mol. Carcinog. 2025, 64, 344–356. [Google Scholar] [CrossRef]
- Jönsson, S.; Hjorth-Hansen, H.; Olsson, B.; Wadenvik, H.; Sundan, A.; Standal, T. Imatinib inhibits proliferation of human mesenchymal stem cells and promotes early but not late osteoblast differentiation in vitro. J. Bone Miner. Metab. 2012, 30, 119–123. [Google Scholar] [CrossRef]
- Dong, Y.; Han, Q.; Zou, Y.; Deng, Z.; Lu, X.; Wang, X.; Zhang, W.; Jin, H.; Su, J.; Jiang, T.; et al. Long-term exposure to imatinib reduced cancer stem cell ability through induction of cell differentiation via activation of MAPK signaling in glioblastoma cells. Mol. Cell Biochem. 2012, 370, 89–102. [Google Scholar] [CrossRef]
- Jaidee, R.; Kukongviriyapan, V.; Senggunprai, L.; Prawan, A.; Jusakul, A.; Laphanuwat, P.; Kongpetch, S. Inhibition of FGFR2 enhances chemosensitivity to gemcitabine in cholangiocarcinoma through the AKT/mTOR and EMT signaling pathways. Life Sci. 2022, 296, 120427. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Santoni, M.; Soriani, A.; Nabissi, M.; Cardinali, C.; Santoni, A.; Santoni, G. Axitinib induces DNA damage response leading to senescence, mitotic catastrophe, and increased NK cell recognition in human renal carcinoma cells. Oncotarget 2015, 6, 36245–36259. [Google Scholar] [CrossRef] [PubMed]
- Oldani, M.; Cantone, M.C.; Gaudenzi, G.; Carra, S.; Dicitore, A.; Saronni, D.; Borghi, M.O.; Lombardi, A.; Caraglia, M.; Persani, L.; et al. Exploring the multifaceted antitumor activity of axitinib in lung carcinoids. Front. Endocrinol. 2024, 15, 1433707. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.B.; Amantini, C.; Nabissi, M.; Cardinali, C.; Santoni, M.; Bernardini, G.; Santoni, A.; Santoni, G. Axitinib induces senescence-associated cell death and necrosis in glioma cell lines: The proteasome inhibitor, bortezomib, potentiates axitinib-induced cytotoxicity in a p21(Waf/Cip1) dependent manner. Oncotarget 2017, 8, 3380–3395. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, B.; Chen, W.; Chen, J.; Zhang, H.; Bai, M. Multiomic traits reveal that critical irinotecan-related core regulator FSTL3 promotes CRC progression and affects ferroptosis. Cancer Cell Int. 2025, 25, 115. [Google Scholar] [CrossRef]
- Xiang, M.; Yang, X.; Ren, S.; Du, H.; Geng, L.; Yuan, L.; Wen, Y.; Lin, B.; Li, J.; Zhang, Y.; et al. Anlotinib Combined with S-1 in Third- or Later-Line Stage IV Non-Small Cell Lung Cancer Treatment: A Phase II Clinical Trial. Oncologist 2021, 26, e2130–e2135. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chu, J.F.; Zhao, Y.; Tang, H.; Wang, L.L.; Zhou, M.Q.; Yan, Z.; Liu, Y.Y.; Yao, Z.H. A Trial of the Safety and Efficacy of Chemotherapy Plus Anlotinib vs Chemotherapy Alone as Second- or Third-Line Salvage Treatment for Advanced Non-Small Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 3827–3834. [Google Scholar] [CrossRef]
- Hetta, H.F.; Alqifari, S.F.; Alshehri, K.; Alhowiti, A.; Alharbi, S.S.; Mirghani, H.; Alrasheed, T.; Mostafa, M.E.A.; Sheikh, M.; Elodemi, M.; et al. Efficacy of Anlotinib Plus Docetaxel in Advanced NSCLC Previously Treated with Platinum-Based Chemotherapy: A Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 652. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Zheng, Y.; Ma, C.; Zhao, Q.; Wang, Y.; Miao, L.; Ding, J. Efficacy and safety of anlotinib monotherapy or combination therapy in the treatment of patients with advanced non-small cell lung cancer: A retrospective real-world study conducted in East China. BMC Pulm. Med. 2025, 25, 170. [Google Scholar] [CrossRef]
- Song, Y.; Miao, L.; Wang, Z.; Shi, M. Combination of apatinib and docetaxel in treating advanced non-squamous non-small cell lung cancer patients with wild-type EGFR: A multi-center, phase II trial. J. Thorac. Dis. 2020, 12, 2450. [Google Scholar] [CrossRef]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.-Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, A.; Gomez, H.L.; Aziz, Z.; Zvirbule, Z.; Bines, J.; Arbushites, M.C.; Guerrera, S.F.; Koehler, M.; Oliva, C.; Stein, S.H.; et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J. Clin. Oncol. 2008, 26, 5544–5552. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Yuan, J.Q.; Di, M.Y.; Zheng, D.Y.; Chen, J.Z.; Ding, H.; Wu, X.Y.; Huang, Y.F.; Mao, C.; Tang, J.L. Gemcitabine Plus Erlotinib for Advanced Pancreatic Cancer: A Systematic Review with Meta-Analysis. PLoS ONE 2013, 8, e57528. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Yun, J.; Lee, M.-Y.; Kim, H.J.; Kim, K.H.; Kim, S.H.; Lee, S.-C.; Bae, S.B.; Kim, C.K.; Lee, N.; et al. A randomized phase II clinical trial of gemcitabine, oxaliplatin, erlotinib combination chemotherapy versus gemcitabine and erlotinib in previously untreated patients with locally advanced or metastatic pancreatic cancer. J. Clin. Oncol. 2018, 36, 344. [Google Scholar] [CrossRef]

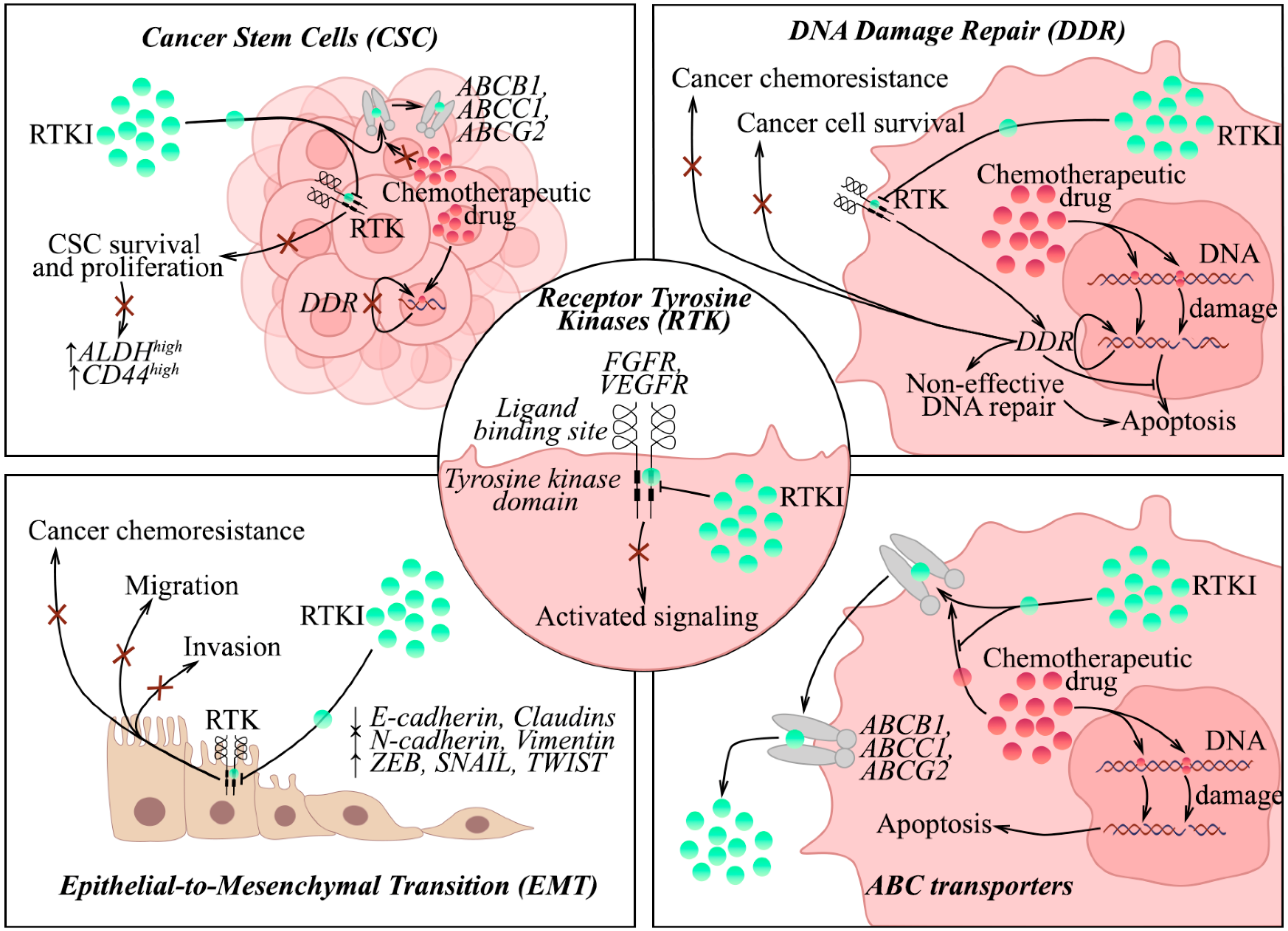
| Drug | Primary Target | Non-Targeted (i.e., “Off-Target”) Effects | |||
|---|---|---|---|---|---|
| DNA Damage Response (DDR) | ABC Transporters | Epithelial-to-Mesenchymal Transition (EMT) or Mesenchymal-to-Epithelial Transition (MET) | Cancer Stem Cells (CSCs) | ||
| Infigratinib (BGJ398) | pan-FGFR | Inhibited HR-mediated DNA repair [159] | ↓ ABCB1 functional activity [167] | Inhibited EMT (↓ Vimentin and Slug) [292] | ↓ ALDHhighCD44high cells [175] ↓ ALDH7A1 and OCT4 [177] |
| Fexagratinib (AZD4547) | FGFR1, FGFR2, and FGFR3 | Inhibited PTEN-mediated DNA repair [161] | ND | Induced MET [179] | ↓ ALDH-positive cells [176] Reduced the number of CSCs [179] |
| Erdafitinib | pan-FGFR | Induced ROS-mediated DNA damage [163] | ↓ ABCB1 functional activity [165,166] | ND | ND |
| Pemigatinib | FGFR1, FGFR2, FGFR3, and FGFR4 | Decreased irradiation-mediated DDR [162] | ↓ ABCB1 functional activity [169] | ND | Decreased CSC survival and proliferation [162] |
| Ponatinib | BCR-ABL, FGFR1, VEGFR2, PDGFRα, Abl, RET, and Src | ND | ↓ ABCB1 and ABCG2 expression and functional activity [185] ↓ MRP7 expression and functional activity [186] | Inhibited EMT (↑ E-cadherin;↓ Vimentin, p-Smad3) [187] | ↑ NANOG and SOX2 [188] ↓ CSCs [189] |
| Dovitinib (TKI-258) | KIT, FGFR1-3, VEGFR1-3, PDGFR A/B/, RET, and FLT-3 | Targeted topoisomer-ase I and topoisomer-ase II [194] Inhibited BER and MGMT [193] | ↓ ABCB1 and ABCG2 functional activity [191] | Inhibited EMT [196] | Inhibited CSC-like protein Lin28 and its target HMGA2 [193] |
| Pazopanib (GW786034) | c-KIT, FGFR, PDGFR, and VEGFR | Induced DNA damage and cellular senescence [206] | ↓ ABCB1 and ABCG2 functional activity [198] | Inhibited EMT (↓ VE-cadherin) [207] | ND |
| PD173074 | FGFR1 and VEGFR | ND | ↓ ABCB1 and ABCC10 functional activity [208,209] | Induced MET (↑ E-cadherin, ↓ Snail1) [173] | ND |
| Axitinib | VEGFR1, VEGFR2, VEGFR3, PDGFRβ, and c-Kit | Induced senescence and/or mitotic catastrophe [293,294,295] Activated DDR (↑ γ-H2AX, Chk1, p21) and G2/M arrest [293,295] | ↓ ABCG2 functional activity [235] | Inhibited EMT (↓ Vimentin) [238] | Targeted CSC-like cells [235] Decrease CSC proliferation [239] |
| Regorafenib | VEGFR1-2, TIE-2, PDGFRβ, FGFR1, RET, c-KIT, BRAF, and c-RAF/Raf-1 | Inhibited radiation-induced DDR [248] Induced DNA damage and decreased repair ability [249] | ↓ ABCB1 functional activity [243] ↓ ABCG2 functional activity [245] | Inhibited EMT (↑ E-cadherin, ↓ SNAI2 and Vimentin) [252] | Decreased the stemness phenotypes [254] |
| Cabozantinib (XL184) | VEGFR2 and MET | ND | ↓ ABCB1 functional activity [260] ↓ ABCG2 functional activity [257,258,259] | Inhibited EMT [262] | Downregulated CD133 (↓ SOX2) [261] |
| Sunitinib | PDGFR, VEGFR, Kit, and FLT-3 | Attenuated HR-mediated DNA repair [265] Induced non-repaired DNA DSBs and G1/S cell cycle arrest [266] Induced chromosome instability, DNA damage, and p53-dependent apoptosis [267] Enhanced radiation-induced DNA DSBs and induced G2/M arrest [268] | ↓ ABCB1 functional activity [264] ↓ ABCG2 functional activity [263,264] | ND | Downregulated ALDHhigh CSC-like cells [270] Decreased CSC proliferation [239] Disrupted CSC s differentiation into endothelial cells in vitro and vasculo-genesis induced by CSCs in vivo [273] |
| Motesanib (AMG-706) | VEGFR1, VEGFR2, VEGFR3, Kit, PDGFR, and Ret | ND | ↓ ABCB1 and ABCG2 functional activity [278] | ND | ND |
| Telatinib (BAY 57-9352) | VEGFR2/3, c-Kit, and PDGFRα | ND | ↓ ABCG2 functional activity [279] | ND | Targeted FSL3, overexpressed in colorectal CSCs [296] |
| SKLB-610 | VEGFR2, FGFR2, and PDGFR | ND | ↓ ABCG2 functional activity [280] | ND | ND |
| Crizotinib | c-Met, ALK, and ROS1 | Enhanced radiation-induced DNA DSBs [284] | ↓ ABCB1 and ABCG2 functional activity [258,281] | Inhibited EMT (↑ E-cadherin; ↓ Vimentin) [282] | Targeted CSCs [283] |
| Imatinib | v-Abl, c-Kit, and PDGFR | Induced DNA alkali-labile sites [285] Inhibited the S–G2–M transition after Adriamycin exposure and inactivated ATM/ATR signaling pathway [286] | ↓ ABCB1 and ABBC10 functional activity [287,288] | Inhibited EMT (↑ E-cadherin; ↓ Fibronectin, SNAI2) by modulating the Notch signaling pathway [289] | Decreased proliferation of human MSCs [290] Inhibited differentiation of CSCs [291] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boichuk, S.; Gessel, T. Repurposing the Tyrosine Kinase Inhibitors Targeting FGFR and VEGFR Pathways for Cancer Therapy: A Comprehensive Review. Cancers 2025, 17, 3354. https://doi.org/10.3390/cancers17203354
Boichuk S, Gessel T. Repurposing the Tyrosine Kinase Inhibitors Targeting FGFR and VEGFR Pathways for Cancer Therapy: A Comprehensive Review. Cancers. 2025; 17(20):3354. https://doi.org/10.3390/cancers17203354
Chicago/Turabian StyleBoichuk, Sergei, and Tatyana Gessel. 2025. "Repurposing the Tyrosine Kinase Inhibitors Targeting FGFR and VEGFR Pathways for Cancer Therapy: A Comprehensive Review" Cancers 17, no. 20: 3354. https://doi.org/10.3390/cancers17203354
APA StyleBoichuk, S., & Gessel, T. (2025). Repurposing the Tyrosine Kinase Inhibitors Targeting FGFR and VEGFR Pathways for Cancer Therapy: A Comprehensive Review. Cancers, 17(20), 3354. https://doi.org/10.3390/cancers17203354






