Fusobacteriumnucleatum: Pathophysiological and Clinical Involvement in Inflammatory Bowel Diseases, Colorectal Cancer and Cardiovascular Diseases
Simple Summary
Abstract
1. Introduction
2. Methods
- ◦
- Published in English with an available abstract;
- ◦
- Reported original data from clinical studies, cohort investigations, randomized controlled trials, or quantitative meta-analyses of such studies;
- ◦
- Addressed at least one of the following topics: Fusobacterium nucleatum and inflammatory bowel disease (IBD), F. nucleatum and colorectal cancer (CRC), F. nucleatum and cardiovascular diseases (CVD), microbial biomarkers, immune modulation, or microbiota–host interactions [16].
- ◦
- Narrative or systematic reviews without pooled analysis, case reports, and editorials;
- ◦
- Studies lacking a direct investigation of Fusobacterium nucleatum;
- ◦
- Experimental works unrelated to IBD, CRC, or CVD.
- ◦
- “Fusobacterium nucleatum AND colorectal cancer” → n = 182 articles;
- ◦
- “Fusobacterium nucleatum AND inflammatory bowel disease” → n = 64 articles;
- ◦
- “Fusobacterium nucleatum AND cardiovascular disease” → n = 71 articles;
- ◦
- “Fusobacterium nucleatum AND immune modulation/microbiota” → n = 98 articles.
3. Fusobacterium nucleatum: An Overview of Clinical Functions and Pathogenesis
4. Fusobacterium nucleatum and Inflammatory Bowel Diseases: Pathogenesis and Clinical Evidence
5. Fusobacterium nucleatum and Colorectal Cancer Risk: Cancer Pathways and Clinical Evidence
Fusobacterium nucleatum and Immune Response in CRC
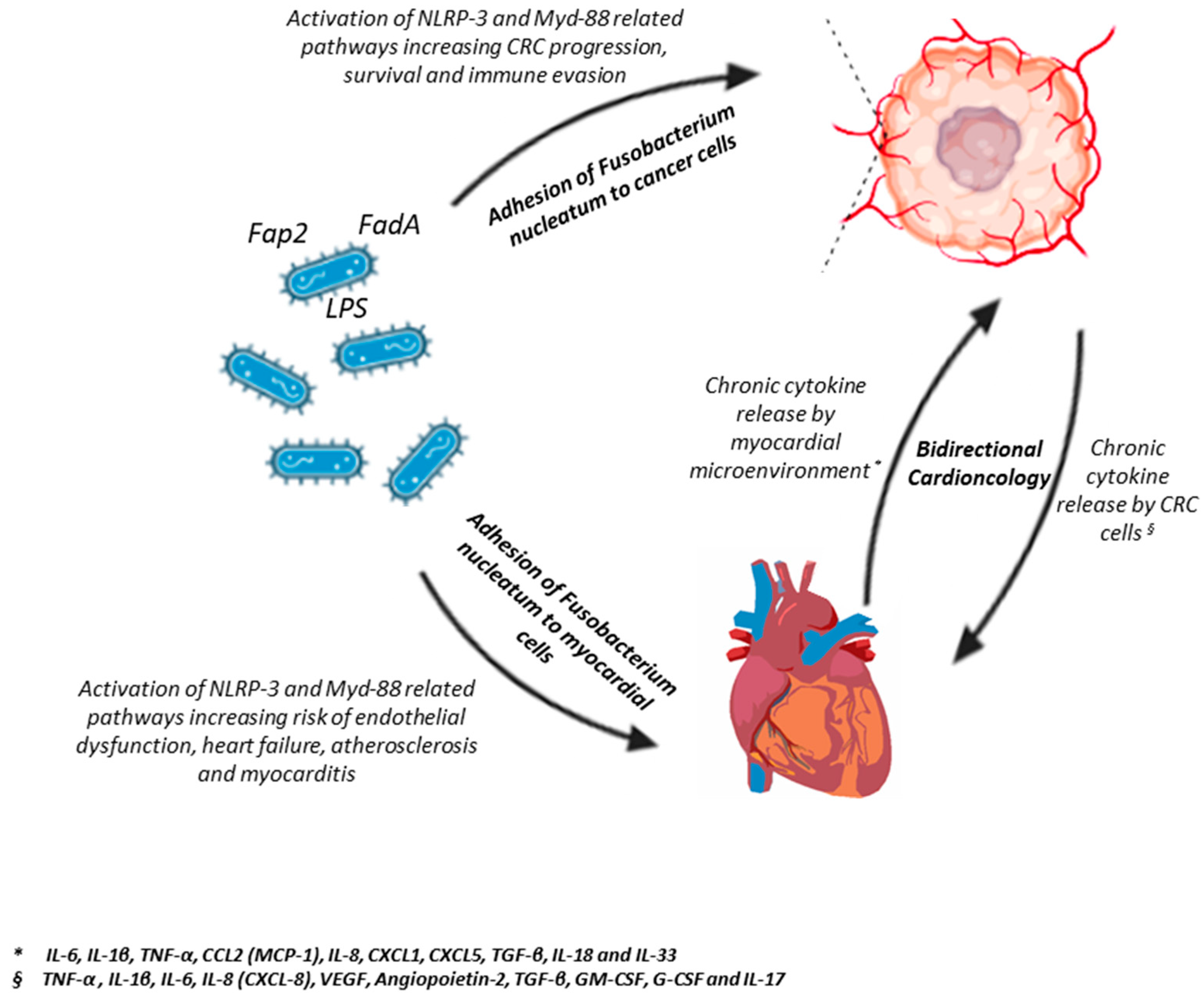
6. Fusobacterium nucleatum and Cardiovascular Diseases: A Putative Deep Interaction
6.1. Molecular Mechanisms of Vascular Colonization and Endothelial Dysfunction
6.2. Clinical Evidence of Systemic Dissemination
6.3. Preclinical Data on Immunological Crosstalk and Chronic Systemic Inflammation
7. Fusobacterium nucleatum Strains and Disease Associations
8. Pharmacological and Nutritional Approaches to Influence Fusobacterium nucleatum Levels
8.1. Nutritional Strategies and Dietary Modulation
8.2. Integrative and Translational Perspectives
9. Discussion
10. Limitations and Controversies
Outstanding Research Questions
- Strain specificity and virulence heterogeneity. Current data suggest that pathogenic potential varies markedly across Fusobacterium nucleatum subspecies and clades, yet strain-level determinants of virulence, such as adhesin repertoires (FadA, Fap2 variants), mobile genetic elements, and metabolic traits, remain poorly characterized. Large-scale, strain-resolved genomics and phenotypic profiling are essential to delineate pathogenic versus commensal lineages and their host tropisms.
- Host–microbe and microbe–microbe interactions. The mechanisms by which Fusobacterium nucleatum interfaces with host immunity, barrier integrity, and the broader microbial community require deeper exploration. Multi-omics integration (metatranscriptomics, proteomics, and metabolomics) could unravel context-dependent interactions that modulate inflammation, tumorigenesis, and endothelial dysfunction. Defining host genetic or epigenetic susceptibilities that facilitate colonization will be pivotal for risk stratification.
- Diagnostic and quantitative standardization. Heterogeneity in sampling matrices (saliva, stool, tissue), extraction protocols, and quantitative thresholds hampers reproducibility across studies. Standardized diagnostic pipelines, combining molecular quantification (qPCR, digital PCR, metagenomics) with host biomarkers of inflammation and immune activation, are needed to translate Fusobacterium nucleatum detection into clinically actionable information.
- Therapeutic targeting and intervention studies. Despite encouraging preclinical evidence, the translational impact of modulating Fusobacterium nucleatum remains largely theoretical. Future trials should evaluate whether reducing bacterial burden through antimicrobial, probiotic, or dietary interventions translates into improved outcomes in inflammatory bowel disease, colorectal cancer, or cardiovascular cohorts. Mechanism-based strategies, such as blocking FadA–cadherin or Fap2–TIGIT interactions, should be explored in controlled, ethically sound frameworks.
- Longitudinal and interventional research designs. The field urgently requires longitudinal cohort studies that track oral–intestinal–vascular colonization dynamics and intervention trials that test causality rather than association. Such designs will be crucial to move from correlative evidence to mechanistic and therapeutic validation.
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stokowa-Sołtys, K.; Wojtkowiak, K.; Jagiełło, K. Fusobacterium nucleatum—Friend or foe? J. Inorg. Biochem. 2021, 224, 111586. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontol 2000 2022, 89, 166–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgård, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate from the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Queen, J.; Domingue, J.C.; White, J.R.; Stevens, C.; Udayasuryan, B.; Nguyen, T.T.D.; Wu, S.; Ding, H.; Fan, H.; McMann, M.; et al. Comparative Analysis of Colon Cancer-Derived Fusobacterium nucleatum Subspecies: Inflammation and Colon Tumorigenesis in Murine Models. mBio 2021, 13, e0299121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, W.; Han, J.; Peng, X.; Zhou, X.; Gong, T.; Zheng, X. The role of Fusobacterium nucleatum in cancer and its implications for clinical applications. Mol. Oral Microbiol. 2024, 39, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in tumors: From tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 2024, 25, 2306676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, L.; Chen, H.; He, C.; Li, J. Effects of gut microbiota in breast cancer. Front. Oncol. 2025, 15, 1617410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gholizadeh, P.; Eslami, H.; Kafil, H.S. Carcinogenesis mechanisms of Fusobacterium nucleatum. Biomed. Pharmacother. 2017, 89, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Li, J.; Zhou, Z.; Huang, X.; Gopinath, D.; Luo, P.; Wang, Q.; Shan, D. Fusobacterium in the microbiome: From health to disease across the oral-gut axis and beyond. NPJ Biofilms Microbiomes 2025, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Miskeen, A.Y.; Bhat, A.; Fazili, K.M.; Ganai, B.A. Fusobacterium nucleatum: An emerging bug in colorectal tumorigenesis. Eur. J. Cancer Prev. 2015, 24, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Allen-Vercoe, E.; Strauss, J.; Chadee, K. Fusobacterium nucleatum: An emerging gut pathogen? Gut Microbes. 2011, 2, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Rivera, M.A.; Dewhirst, F.E.; Bullman, S.; Johnston, C.D. Addressing controversy in Fusobacterium nomenclature: What exactly does "F. nucleatum" refer to? Gut Microbes 2025, 17, 2514797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajendiran, E.; Ramadass, B.; Ramprasath, V. Understanding connections and roles of gut microbiome in cardiovascular diseases. Can. J. Microbiol. 2021, 67, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Battson, M.L.; Lee, D.M.; Weir, T.L.; Gentile, C.L. The gut microbiota as a novel regulator of cardiovascular function and disease. J. Nutr. Biochem. 2018, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.; Wang, H.; Tao, Y.; Luo, K.; Ye, J.; Ran, S.; Guan, Z.; Wang, Y.; Hu, H.; Huang, R. Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front. Cell Infect. Microbiol. 2022, 12, 1020583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strauss, J.; Kaplan, G.G.; Beck, P.L.; Rioux, K.; Panaccione, R.; Devinney, R.; Lynch, T.; Allen-Vercoe, E. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm. Bowel Dis. 2011, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Storm, J.C.; Ford, B.A.; Streit, J.A. Myocardial infection due to Fusobacterium nucleatum. Diagn. Microbiol. Infect. Dis. 2013, 77, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Sukawa, Y.; Adachi, Y.; Ito, M.; Mitsuhashi, K.; Kurihara, H.; Kanno, S.; Yamamoto, I.; Ishigami, K.; Igarashi, H.; et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 2016, 22, 557–566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Antonio, D.L.; Zenoniani, A.; Umme, S.; Piattelli, A.; Curia, M.C. Intratumoral Fusobacterium nucleatum in Pancreatic Cancer: Current and Future Perspectives. Pathogens. 2024, 14, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadeghloo, Z.; Ebrahimi, S.; Hakemi-Vala, M.; Totonchi, M.; Sadeghi, A.; Fatemi, N. Fusobacterium nucleatum and non-coding RNAs: Orchestrating oncogenic pathways in colorectal cancer. Gut Pathog. 2025, 17, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Leng, X.X.; Qi, J.; Wang, N.; Han, J.X.; Tao, Z.H.; Zhuang, Z.Y.; Ren, Y.; Xie, Y.L.; Jiang, S.S.; et al. The adhesin RadD enhances Fusobacterium nucleatum tumour colonization and colorectal carcinogenesis. Nat. Microbiol. 2024, 9, 2292–2307. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vello, P.; Di Lorenzo, F.; Lamprinaki, D.; Notaro, A.; Speciale, I.; Molinaro, A.; Juge, N.; De Castro, C. Structure of the O-Antigen and the Lipid A from the Lipopolysaccharide of Fusobacterium nucleatum ATCC 51191. Chembiochem 2021, 22, 1252–1260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groeger, S.; Zhou, Y.; Ruf, S.; Meyle, J. Pathogenic Mechanisms of Fusobacterium nucleatum on Oral Epithelial Cells. Front. Oral Health. 2022, 3, 831607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbari, E.; Epstein, J.B.; Samim, F. Unveiling the Hidden Links: Periodontal Disease, Fusobacterium nucleatum, and Cancers. Curr. Oncol. Rep. 2024, 26, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Redline, R.W.; Han, Y.W. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J. Immunol. 2007, 214, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Jeepipalli, S.; Aravindraja, C.; Duncan, W.; Krishna, V.M.; Sahay, B.; Chan, E.K.L.; Kesavalu, L. Exploring the complex interplay between oral Fusobacterium nucleatum infection, periodontitis, and robust microRNA induction, including multiple known oncogenic miRNAs. mSystems 2025, 10, e0173224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tefiku, U.; Popovska, M.; Cana, A.; Zendeli-Bedxeti, L.; Recica, B.; Spasovska-Gjorgovska, A.; Spasovski, S. Determination of the Role of Fusobacterium nucleatum in the Pathogenesis in and Out the Mouth. Prilozi 2020, 41, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Tattoli, I.; Mascellino, M.T. The Impact of Fusobacterium nucleatum and the Genotypic Biomarker KRAS on Colorectal Cancer Pathogenesis. Int. J. Mol. Sci. 2025, 26, 6958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schöpf, F.; Marongiu, G.L.; Milaj, K.; Sprink, T.; Kikhney, J.; Moter, A.; Roderer, D. Structural basis of Fusobacterium nucleatum adhesin Fap2 interaction with receptors on cancer and immune cells. Nat. Commun. 2025, 16, 8104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, X.; Liu, J.; Jiang, K.; Lian, S.; Shi, Y.; Fu, S.; Zhao, P.; Xiao, J.; Sun, D.; Guo, D. The outer membrane protein of Fusobacterium necrophorum, 43K OMP, stimulates inflammatory cytokine production through nuclear factor kappa B activation. Anaerobe. 2023, 82, 102768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Udayasuryan, B.; Ahmad, R.N.; Nguyen, T.T.D.; Umaña, A.; Monét Roberts, L.; Sobol, P.; Jones, S.D.; Munson, J.M.; Slade, D.J.; Verbridge, S.S. Fusobacterium nucleatum induces proliferation and migration in pancreatic cancer cells through host autocrine and paracrine signaling. Sci Signal. 2022, 15, eabn4948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slade, D.J. New Roles for Fusobacterium nucleatum in Cancer: Target the Bacteria, Host, or Both? Trends Cancer 2021, 7, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Oska, N.; Peterson, D.; Brothers, K.; Maramraju, R.; Ayyad, A.; Hohmann, B. Isolated Facial Vein Thrombophlebitis Caused by Fusobacterium nucleatum: A Lemierre-Variant Case. Case Rep. Infect. Dis. 2025, 2025, 9938125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, Y.W.; Houcken, W.; Loos, B.G.; Schenkein, H.A.; Tezal, M. Periodontal disease, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Adv. Dent. Res. 2014, 26, 47–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mendes, R.T.; Nguyen, D.; Stephens, D.; Pamuk, F.; Fernandes, D.; Van Dyke, T.E.; Kantarci, A. Endothelial Cell Response to Fusobacterium nucleatum. Infect. Immun. 2016, 84, 2141–2148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, X.; Song, L.J.; Li, L.X.; Liu, T.; Zhang, M.M.; Li, Z.; Wang, P.; Li, M.; Zuo, X.L. Fusobacterium nucleatum Causes Microbial Dysbiosis and Exacerbates Visceral Hypersensitivity in a Colonization-Independent Manner. Front Microbiol. 2020, 11, 1281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, P.; Chen, Y.; Guo, X.; Chen, Y.; Su, W.; Zhan, N.; Dong, W. Fusobacterium nucleatum Activates Endoplasmic Reticulum Stress to Promote Crohn’s Disease Development via the Upregulation of CARD3 Expression. Front. Pharmacol. 2020, 11, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Golob, J.; Rao, K.; Berinstein, J.A.; Chey, W.D.; Owyang, C.; Kamada, N.; Higgins, P.D.R.; Young, V.; Bishu, S.; Lee, A.A. The Microbiome in Quiescent Crohn’s Disease with Persistent Symptoms Show Disruptions in Microbial Sulfur and Tryptophan Pathways. Gastro Hep. Adv. 2023, 3, 167–177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Zhang, D.; Liu, C.; Tang, B.; Cui, Y.; Guo, D.; Duan, M.; Tu, Y.; Zheng, H.; Ning, X.; et al. Outer Membrane Vesicles Derived from Fusobacterium nucleatum Trigger Periodontitis Through Host Overimmunity. Adv. Sci. 2024, 11, e2400882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Zhang, Z.; Wu, R.; Mao, M.; Ji, Y.; Wang, X.; Dou, S.; Yan, M.; Chen, W. Fusobacterium nucleatum-Derived Outer Membrane Vesicles Promote Immunotherapy Resistance via Changes in Tryptophan Metabolism in Tumour-Associated Macrophages. J. Extracell. Vesicles 2025, 14, e70070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, G.; Gao, C.; Jiang, S.; Cai, Q.; Li, R.; Sun, Q.; Xiao, C.; Xu, Y.; Wu, B.; Zhou, H. Fusobacterium nucleatum outer membrane vesicles activate autophagy to promote oral cancer metastasis. J. Adv. Res. 2024, 56, 167–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, G.; Sun, Q.; Cai, Q.; Zhou, H. Outer Membrane Vesicles from Fusobacterium nucleatum Switch M0-Like Macrophages Toward the M1 Phenotype to Destroy Periodontal Tissues in Mice. Front. Microbiol. 2022, 13, 815638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Wang, Y.; Fan, R.; Zhang, L.; Li, Z.; Zhang, Y.; Zheng, W.; Wang, L.; Liu, B.; Quan, C. Quantitative Proteomic Analysis of Outer Membrane Vesicles from Fusobacterium nucleatum Cultivated in the Mimic Cancer Environment. Microbiol. Spectr. 2023, 11, e0039423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Li, B.; Bao, L.; Chen, Y.; Yang, J.; Xu, F.; Shi, S.; Chen, W.; Wang, B.; Liu, Y. Fusobacterium nucleatum Aggravates Intestinal Barrier Impairment and Colitis Through IL-8 Induced Neutrophil Chemotaxis by Activating Epithelial Cells. J. Inflamm. Res. 2024, 17, 8407–8420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, S.S.; Chen, Y.X.; Fang, J.Y. Fusobacterium nucleatum: Ecology, pathogenesis and clinical implications. Nat. Rev. Microbiol. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, X.; Wang, X.; Deng, B.; He, H.; Xu, M.; Wu, X.; Tan, C.; Liu, Y.; Yu, B.; et al. Fusobacterium nucleatum exacerbates colitis via STAT3 activation induced by Acetyl-CoA accumulation. Gut Microbes 2025, 17, 2489070. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera-Kweh, M.F.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Fusobacterium nucleatum Alters Atherosclerosis Risk Factors and Enhances Inflammatory Markers with an Atheroprotective Immune Response in ApoE(null) Mice. PLoS ONE 2015, 10, e0129795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, S.K.; Xu, Y.; Dai, S.T.; Wang, Y.Z.; Zhang, J.; Hou, D.Z.; Li, R.G.; Gong, K.M. Gut microbiota diversity and composition in patients with atherosclerosis analyzed using full-length 16S rRNA gene sequencing. Minerva Cardiol. Angiol. 2025, 73, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Valadbeigi, H.; Khoshnood, S.; Negahdari, B.; Maleki, A.; Mohammadinejat, M.; Haddadi, M.H. Mixed oral biofilms are controlled by the interspecies interactions of Fusobacterium nucleatum. Oral Dis. 2024, 30, 3582–3590. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.; Chattopadhyay, D.; Saha Mondal, P.; Das, S.; Mondal, A.; Das, A.; Samanta, S.; Saha, T. Fusobacterium nucleatum modulates the Wnt/β-catenin pathway in colorectal cancer development. Int. J. Biol. Macromol. 2025, 299, 140196. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Kiritkumar Makwana, R.; Shetty, V.; Mukherjee, S.; Narayan, P. Cardiovascular diseases and the heart-gut cross talk. Indian. Heart J. 2024, 76, 94–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaplan, C.W.; Lux, R.; Haake, S.K.; Shi, W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol. Microbiol. 2009, 71, 35–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tahara, T.; Yamamoto, E.; Suzuki, H.; Maruyama, R.; Chung, W.; Garriga, J.; Jelinek, J.; Yamano, H.O.; Sugai, T.; An, B.; et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curia, M.C.; Pignatelli, P.; D’Antonio, D.L.; D’Ardes, D.; Olmastroni, E.; Scorpiglione, L.; Cipollone, F.; Catapano, A.L.; Piattelli, A.; Bucci, M.; et al. Oral Porphyromonas gingivalis and Fusobacterium nucleatum Abundance in Subjects in Primary and Secondary Cardiovascular Prevention, with or without Heterozygous Familial Hypercholesterolemia. Biomedicines 2022, 10, 2144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Advani, S.M.; Advani, P.; DeSantis, S.M.; Brown, D.; VonVille, H.M.; Lam, M.; Loree, J.M.; Mehrvarz Sarshekeh, A.; Bressler, J.; Lopez, D.S.; et al. Clinical, Pathological, and Molecular Characteristics of CpG Island Methylator Phenotype in Colorectal Cancer: A Systematic Review and Meta-analysis. Transl Oncol. 2018, 11, 1188–1201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe. 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bashir, A.; Miskeen, A.Y.; Hazari, Y.M.; Asrafuzzaman, S.; Fazili, K.M. Fusobacterium nucleatum, inflammation, and immunity: The fire within human gut. Tumour Biol. 2016, 37, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kaplan, R.C.; Burk, R.D.; Qi, Q. The Oral Microbiota, Microbial Metabolites, and Immuno-Inflammatory Mechanisms in Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 12337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Zhao, R.; Kang, Z.; Cao, Z.; Liu, N.; Shen, J.; Wang, C.; Pan, F.; Zhou, X.; Liu, Z.; et al. Delivery of short chain fatty acid butyrate to overcome Fusobacterium nucleatum-induced chemoresistance. J. Control Release 2023, 363, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrand Rudin, A.; Khamzeh, A.; Venkatakrishnan, V.; Basic, A.; Christenson, K.; Bylund, J. Short chain fatty acids released by Fusobacterium nucleatum are neutrophil chemoattractants acting via free fatty acid receptor 2 (FFAR2). Cell Microbiol. 2021, 23, e13348. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Steinberg, T.; Scholz, K.J.; Kruse, A.; Rezasoltani, S.; Conrads, G.; Al-Ahmad, A.; Cieplik, F. The rise and evolving role of Fusobacterium nucleatum subspecies. Curr. Res. Microb. Sci. 2025, 9, 100414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Socio, G.V.; Mencacci, A.; Bini, P.; Pasticci, M.B. Fusobacterium nucleatum endocarditis mimicking polymyalgia rheumatica. South. Med. J. 2009, 102, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Tavana, A.M. Heart failure and oral bacteria: How could be prevented? J. Cardiovasc. Dis. Res. 2010, 1, 161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- King, M.; Hurley, H.; Davidson, K.R.; Dempsey, E.C.; Barron, M.A.; Chan, E.D.; Frey, A. The Link between Fusobacteria and Colon Cancer: A Fulminant Example and Review of the Evidence. Immune Netw. 2020, 20, e30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shigematsu, Y.; Saito, R.; Amori, G.; Kanda, H.; Takahashi, Y.; Takeuchi, K.; Takahashi, S.; Inamura, K. Fusobacterium nucleatum, immune responses, and metastatic organ diversity in colorectal cancer liver metastasis. Cancer Sci. 2024, 115, 3248–3255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamaguchi, M.; Nishimura, F.; Naruishi, H.; Soga, Y.; Kokeguchi, S.; Takashiba, S. Thiazolidinedione (pioglitazone) blocks P. gingivalis- and F. nucleatum, but not E. coli, lipopolysaccharide (LPS)-induced interleukin-6 (IL-6) production in adipocytes. J. Dent. Res. 2005, 84, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S51–S69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grigore, I.; Ciobotaru, O.R.; Hînganu, D.; Gurau, G.; Tutunaru, D.; Hînganu, M.V. A Systemic Perspective of the Link Between Microbiota and Cardiac Health: A Literature Review. Life 2025, 15, 1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.D.; Zhang, X.; Zhang, Y.P.; Yue, C.B.; Wang, Y.L.; Pan, H.W.; Zhang, Y.L.; Liu, H.; Zhang, Y. Fusobacterium nucleatum Is a Risk Factor for Metastatic Colorectal Cancer. Curr. Med. Sci. 2022, 42, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Gethings-Behncke, C.; Coleman, H.G.; Jordao, H.W.T.; Longley, D.B.; Crawford, N.; Murray, L.J.; Kunzmann, A.T. Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomark. Prev. 2020, 29, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and its associated systemic diseases: Epidemiologic studies and possible mechanisms. J. Oral Microbiol. 2022, 15, 2145729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janati, A.I.; Karp, I.; Laprise, C.; Sabri, H.; Emami, E. Detection of Fusobaterium nucleatum in feces and colorectal mucosa as a risk factor for colorectal cancer: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, B.; Lu, X.; Tong, Y.; Feng, Y.; Mao, Y.; Dun, G.; Li, J.; Xu, Q.; Tang, J.; Zhang, T.; et al. MicroRNA-31 induced by Fusobacterium nucleatum infection promotes colorectal cancer tumorigenesis. iScience. 2023, 26, 106770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hullar, M.A.J.; Kahsai, O.J.; Hill, C.; Levy, L.; Malen, R.C.; Curtis, K.R.; Ammar, H.; Sillah, A.; Reedy, A.M.; Lampe, J.W.; et al. Highly Sensitive DNA Testing of Fusobacterium nucleatum in Colorectal Tumors. Cancer Epidemiol Biomarkers Prev. 2025, 34, 1377–1385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Wang, Y.; Yu, L.; Zhang, Y.; Wang, Y.; Shang, Z.; Xin, Y.; Li, X.; Ning, N.; Zhang, Y.; et al. Fusobacterium nucleatum promotes colorectal cancer metastasis by excretion of miR-122-5p from cells via exosomes. iScience 2023, 26, 107686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bu, X.; Wang, L. Iron metabolism and the tumor microenvironment: A new perspective on cancer intervention and therapy (Review). Int. J. Mol. Med. 2025, 55, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamane, T.; Kanamori, Y.; Sawayama, H.; Yano, H.; Nita, A.; Ohta, Y.; Hinokuma, H.; Maeda, A.; Iwai, A.; Matsumoto, T.; et al. Iron accelerates Fusobacterium nucleatum-induced CCL8 expression in macrophages and is associated with colorectal cancer progression. JCI Insight 2022, 7, e156802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamura, K.; Izumi, D.; Kandimalla, R.; Sonohara, F.; Baba, Y.; Yoshida, N.; Kodera, Y.; Baba, H.; Goel, A. Intratumoral Fusobacterium nucleatum Levels Predict Therapeutic Response to Neoadjuvant Chemotherapy in Esophageal Squamous Cell Carcinoma. Clin. Cancer Res. 2019, 25, 6170–6179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Lin, S.; Ji, Y.; Puqiong, C.; Gao, J.; Li, S. Prognostic impact of Fusobacterium nucleatum on survival in colorectal cancer: A systematic review and meta-analysis. J. Cancer Res. Ther. 2025, 21, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Casasanta, M.A.; Yoo, C.C.; Udayasuryan, B.; Sanders, B.E.; Umaña, A.; Zhang, Y.; Peng, H.; Duncan, A.J.; Wang, Y.; Li, L.; et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci Signal. 2020, 13, eaba9157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karta, J.; Meyers, M.; Rodriguez, F.; Koncina, E.; Gilson, C.; Klein, E.; Gabola, M.; Benzarti, M.; Pérez Escriva, P.; Molina Tijeras, J.A.; et al. Fusobacterium nucleatum interacts with cancer-associated fibroblasts to promote colorectal cancer. EMBO J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Queen, J.; Cing, Z.; Minsky, H.; Nandi, A.; Southward, T.; Ferri, J.; McMann, M.; Iyadorai, T.; Vadivelu, J.; Roslani, A.; et al. Fusobacterium nucleatum is enriched in invasive biofilms in colorectal cancer. NPJ Biofilms Microbiom. 2025, 11, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borowsky, J.; Haruki, K.; Lau, M.C.; Dias Costa, A.; Väyrynen, J.P.; Ugai, T.; Arima, K.; da Silva, A.; Felt, K.D.; Zhao, M.; et al. Association of Fusobacterium nucleatum with Specific T-cell Subsets in the Colorectal Carcinoma Microenvironment. Clin. Cancer Res. 2021, 27, 2816–2826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shenker, B.J.; Datar, S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect. Immun. 1995, 63, 4830–4836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ismail, F.; Mansoor, U.; Mehmood Qadri, H.; Zaman, M.; Ali, A.; Asif, M.A.; Kaleem, M.; Khan, H.; Bashir, R.A.; Irshad, S.; et al. Fusobacterium nucleatum as an Emerging Culprit in Brain Abscesses: A Narrative Synthesis of 25 Years of Clinical and Diagnostic Data. Cureus 2025, 17, e89421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, B.Y.; Zhu, H.; Li, Y.L.; Zhang, J.; Xu, S.; Zhou, L.J.; Du, L.J.; Liu, T.; Sun, X.N.; Tian, G.C.; et al. Oral Pathobionts Aggravate Myocardial Infarction Through Mobilization of B2 Cells. Circulation 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, L.; Wu, P.; Zhao, L.; Wu, Y. Fusobacterium nucleatum Accelerates Atherosclerosis via Macrophage-Driven Aberrant Proinflammatory Response and Lipid Metabolism. Front. Microbiol. 2022, 13, 798685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michel, J.; Joly, L.M.; Lvovschi, V.E. A discrepant presentation of bacteremia in the emergency department linked to a Fusobacterium nucleatum infection: A case report. J. Med. Case Rep. 2022, 16, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Connolly, J.P.; Kelly, L. The physical biogeography of Fusobacterium nucleatum in health and disease. mBio 2025, 16, e0298924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zanzoni, A.; Spinelli, L.; Braham, S.; Brun, C. Perturbed human sub-networks by Fusobacterium nucleatum candidate virulence proteins. Microbiome 2017, 5, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, A.; Jaaback, K.; Boulton, A.; Wong-Brown, M.; Raymond, S.; Dutta, P.; Bowden, N.A.; Ghosh, A. Fusobacterium nucleatum: An Overview of Evidence, Demi-Decadal Trends, and Its Role in Adverse Pregnancy Outcomes and Various Gynecological Diseases, including Cancers. Cells 2024, 13, 717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ellett, F.; Kacamak, N.I.; Alvarez, C.R.; Oliveira, E.H.S.; Hasturk, H.; Paster, B.J.; Kantarci, A.; Irimia, D. Fusobacterium nucleatum dissemination by neutrophils. J. Oral Microbiol. 2023, 15, 2217067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narii, N.; Zha, L.; Sobue, T.; Kitamura, T.; Shiba, S.; Mizutani, S.; Yamada, T.; Yachida, S. Association Between Diet and Fusobacterium nucleatum in the Feces of Healthy Adults: A Hospital-based Cross-sectional Study. Cancer Prev. Res. 2023, 16, OF1–OF8. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-So, J.; Zhang, X.; Yang, X.; Rubinstein, M.R.; Mao, Y.; Kitajewski, J.; Liu, K.; Han, Y.W. Omega-3 fatty acids suppress Fusobacterium nucleatum-induced placental inflammation originating from maternal endothelial cells. JCI Insight 2019, 4, e125436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, M.; Fang, H.; Niu, Y.; Lu, J.; Ma, Y.; Zhang, B.; Zhu, H.; Chen, P. Interspecies interactions mediated by arginine metabolism enhance the stress tolerance of Fusobacterium nucleatum against Bifidobacterium animalis. Microbiol. Spectr. 2025, 13, e0223524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, R.S.; Nishihara, R.; Cao, Y.; Song, M.; Mima, K.; Qian, Z.R.; Nowak, J.A.; Kosumi, K.; Hamada, T.; Masugi, Y.; et al. Association of Dietary Patterns with Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017, 3, 921–927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alam, J.; Baek, K.J.; Choi, Y.S.; Kim, Y.C.; Choi, Y. N-acetylcysteine and the human serum components that inhibit bacterial invasion of gingival epithelial cells prevent experimental periodontitis in mice. J. Periodontal Implant. Sci. 2014, 44, 266–273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muchova, M.; Kuehne, S.A.; Grant, M.M.; Smith, P.P.; Nagi, M.; Chapple, I.L.C.; Hirschfeld, J. Fusobacterium nucleatum elicits subspecies-specific responses in human neutrophils. Front. Cell Infect. Microbiol. 2024, 14, 1449539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Luo, W.; Xiao, L.; Xu, X.; Peng, X.; Cheng, L.; Zhou, X.; Zheng, X. Microbial manipulators: Fusobacterium nucleatum modulates the tumor immune microenvironment in colorectal cancer. J. Oral Microbiol. 2025, 17, 2544169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, P.; Tian, Z.; Kong, X.; Yang, L.; Shan, X.; Dong, B.; Ding, X.; Jing, X.; Jiang, C.; Jiang, N. FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J. Exp. Clin. Cancer Res. 2020, 39, 202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omran, T.A.; Subirats Camacho, J.L.; Senthakumaran, T.; Gundersen, G.; Alte, A.K.; Randen, U.; Tunsjø, H.S.; Sæther, P.C.; Bemanian, V. Fusobacterium-associated molecular and immunological alterations in colorectal cancer: Insights from a Norwegian cohort. Front. Immunol. 2025, 16, 1601423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lau, H.C.; Yuan, X.; Huang, H.; Zhang, M.; Hsueh, C.Y.; Gong, H. Fusobacterium nucleatum facilitates proliferation and autophagy by activating miR-361-3p/NUDT1 axis through oxidative stress in hypopharyngeal squamous cell carcinoma. BMC Cancer 2023, 23, 990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, J.H.; Xie, Y.H.; Zou, T.H.; Qian, Y.; Kang, Z.R.; Zhou, C.B.; Pan, S.Y.; Xia, T.X.; Chen, Y.X.; Fang, J.Y. Fecal Fusobacterium nucleatum as a predictor for metachronous colorectal adenoma after endoscopic polypectomy. J. Gastroenterol. Hepatol. 2021, 36, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Graciano-España, M.C.; Barnhart, K.; Gonzalez-Monfort, M.; Arenas-Barrero, M.; Legro, R.S.; Thomas, T.R.; Rush, M.A.; Vilella, F.; Fernández-Sánchez, M.; Simon, C. Fusobacterium nucleatum is not significantly present in eutopic endometrium from patients with minimal-mild and moderate-severe endometriosis. Fertil. Steril. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Z.; Jin, W.; Guo, W.; Jin, Z.; Zuo, Y. Oral Fusobacterium nucleatum exacerbates ulcerative colitis via the oral-gut axis: Mechanisms and therapeutic implications. Front. Cell Infect. Microbiol. 2025, 15, 1564169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brennan, C.A.; Clay, S.L.; Lavoie, S.L.; Bae, S.; Lang, J.K.; Fonseca-Pereira, D.; Rosinski, K.G.; Ou, N.; Glickman, J.N.; Garrett, W.S. Fusobacterium nucleatum drives a pro-inflammatory intestinal microenvironment through metabolite receptor-dependent modulation of IL-17 expression. Gut Microbes 2021, 13, 1987780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Y.; Yu, Y.; Yin, Y.; Wang, L.; Yang, H.; Luo, S.; Zheng, Q.; Pan, Y.; Zhang, D. Potential role of epithelial-mesenchymal transition induced by periodontal pathogens in oral cancer. J. Cell Mol. Med. 2024, 28, e18064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hui, B.; Zhou, C.; Xu, Y.; Wang, R.; Dong, Y.; Zhou, Y.; Ding, J.; Zhang, X.; Xu, J.; Gu, Y. Exosomes secreted by Fusobacterium nucleatum-infected colon cancer cells transmit resistance to oxaliplatin and 5-FU by delivering hsa_circ_0004085. J. Nanobiotechnology 2024, 22, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fardini, Y.; Wang, X.; Témoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zheng, S.; Li, M.; Xu, C.; Jia, D.; Qi, Y.; Hou, T.; Wang, L.; Wang, B.; et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microbes 2022, 14, 2038852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prusa, J.; Gorelik, M.G.; Blake, K.S.; Dantas, G. State of omics-based microbial diagnostics of CRC. Gut Microbes 2025, 17, 2526132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Q.; Lu, X.; Li, J.; Feng, Y.; Tang, J.; Zhang, T.; Mao, Y.; Lan, Y.; Luo, H.; Zeng, L.; et al. Fusobacterium nucleatum induces excess methyltransferase-like 3-mediated microRNA-4717-3p maturation to promote colorectal cancer cell proliferation. Cancer Sci. 2022, 113, 3787–3800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A.; et al. Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jamieson, P.E.; Carbonero, F.; Stevens, J.F. Dietary (poly)phenols mitigate inflammatory bowel disease: Therapeutic targets, mechanisms of action, and clinical observations. Curr. Res. Food Sci. 2023, 6, 100521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Travassos, L.H.; Carneiro, L.A.; Ramjeet, M.; Hussey, S.; Kim, Y.G.; Magalhães, J.G.; Yuan, L.; Soares, F.; Chea, E.; Le Bourhis, L.; et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 2010, 11, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Chen, Y.; Cao, P.; Chen, Y.; Guo, Y.; Wang, S.; Dong, W. Fusobacterium nucleatum Promotes the Development of Ulcerative Colitis by Inducing the Autophagic Cell Death of Intestinal Epithelial. Front. Cell Infect. Microbiol. 2020, 10, 594806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quagliariello, V.; D’Aiuto, G.; Iaffaioli, R.V.; Berretta, M.; Buccolo, S.; Iovine, M.; Paccone, A.; Cerrone, F.; Bonanno, S.; Nunnari, G.; et al. Reasons why COVID-19 survivors should follow dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations: From hyper-inflammation to cardiac dysfunctions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- Daulagala, A.C.; Bridges, M.C.; Kourtidis, A. E-cadherin Beyond Structure: A Signaling Hub in Colon Homeostasis and Disease. Int. J. Mol. Sci. 2019, 20, 2756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P.; Liu, X.; Gu, Z.; Jiang, Z.; Zhao, S.; Song, Y.; Yu, J. Targeting TIGIT for cancer immunotherapy: Recent advances and future directions. Biomark. Res. 2024, 12, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Database | Search String |
|---|---|
| Medline | “Fusobacterium nucleatum AND colorectal cancer” OR “Fusobacterium nucleatum AND inflammatory bowel disease” OR “Fusobacterium nucleatum AND cardiovascular disease” OR “Fusobacterium nucleatum AND immune modulation” OR “oral microbiota AND systemic diseases” |
| EMBASE | “Fusobacterium nucleatum AND colorectal cancer” OR “Fusobacterium nucleatum AND IBD” OR “Fusobacterium nucleatum AND cardiovascular disease” OR “Fusobacterium nucleatum AND microbiota” |
| Mechanism | Molecular Pathway | Pathogenic Effects in IBD | Pathogenic Effects in CRC | Pathogenic Effects in CVD | Preclinical Evidences | Clinical Evidences |
|---|---|---|---|---|---|---|
| Mucosal colonization and persistence | Isolation from intestinal biopsies; subspecies tropism (Fna C2 clade in CRC) | Persistent presence in IBD mucosa, driving local inflammation and barrier disruption | Selective enrichment in right-sided CRC; enhanced tumor colonization and acid resistance | Bacteremia and vascular tissue seeding following oral dissemination | In vitro and in vivo models confirm mucosal adhesion and invasion [51,52,53,54] | Frequent detection in IBD biopsies and CRC tissues; F. nucleatum DNA in vascular plaques [51,52,55,56] |
| Adhesion and epithelial/endothelial invasion | Adhesins FadA, RadD binding to E-/VE-cadherin | Disrupts epithelial junctions, increases permeability | Activates β-catenin signaling → oncogene transcription and epithelial proliferation | Alters endothelial junctions and vascular permeability | Cellular invasion assays; β-catenin pathway activation [53,57,58,59] | Elevated FadA expression in CRC and CVD lesions [57,58,59] |
| Biofilm formation and interspecies aggregation | Outer membrane proteins RadD, Fap2 | Supports microbial persistence and mucosal colonization | Enhances biofilm stability in tumor niches, promoting immune evasion | Facilitates oral–vascular dissemination via stable multispecies biofilms | Demonstrated interbacterial co-aggregation in vitro [60] | Identified in oral biofilms from IBD and periodontitis patients [60] |
| Host signaling and inflammatory activation | FadA–E-cadherin → β-catenin; TLR2/4 → NF-κB, MAPK | Induces IL-6, TNF-α, IL-1β release, amplifying mucosal inflammation | Promotes oncogenic signaling, proliferation, and cytokine production | Upregulates VCAM-1, ICAM-1, and E-selectin → leukocyte adhesion, foam-cell formation | Functional models confirm cytokine cascades and NF-κB activation [61,62,63] | Elevated inflammatory mediators in affected tissues [62,63] |
| Immune evasion and immunomodulation | Fap2–Gal-GalNAc binding; TIGIT inhibition; macrophage polarization | Modulates local immune response and delays mucosal healing | Suppresses NK and T-cell cytotoxicity; promotes M2 macrophage phenotype | Induces Th17 polarization, IL-17 production, and NET formation | Immune-cell assays and murine CRC/CVD models [64,65,66] | Fap2 detected in tumor and vascular tissues; Th17 signature in patients [64,66] |
| Stemness and crypt colonization | LY6A receptor activation → RPS14 upregulation | Not reported | Induces cancer stem-like cell phenotype, sustaining proliferation | Not reported | Observed in colonic crypts of experimental models [65] | Association with aggressive CRC subtypes [62,64,65] |
| Endotoxin activity and oxidative stress | LPS → NADPH oxidase activation | Contributes to epithelial injury and oxidative stress | Sustains tumor microenvironmental inflammation | Generates ROS, oxidized LDL, and plaque destabilization | Pro-oxidant and pro atherogenic effects in vitro [67] | Strong correlation with plaque inflammation and instability [67,68] |
| Metabolic and epigenetic modulation | Local SCFAs (butyrate, acetate, propionate) | Alters immune-cell metabolism and histone acetylation in crypts | May modulate tumor cell signaling under dysbiotic conditions | Promotes vascular inflammation when co-occurring with LPS and barrier injury | SCFA exposure experiments in cell and animal models [69] | Observed link between SCFA imbalance and inflammatory burden [69] |
| Systemic dissemination and direct infection | Bacteremia, translocation from oral niches | Extraintestinal flares linked to oral inflammation | Hematogenous spread to extraintestinal tumors (breast, pancreas) | Infective endocarditis; bacterial DNA in heart valves and atheromas | Animal and in vitro infection models [55,56,70,71] | Detection of F. nucleatum DNA in endocarditis and vascular lesions [55,56,70,71] |
| Chronic inflammation and tissue remodeling | Cytokine cascade (IL-6, TNF-α, IL-8, CXCL1) | Drives recurrent flares and mucosal ulceration | Reinforces tumor growth and invasion | Promotes endothelial damage and myocardial fibrosis | Induced chronic inflammation in experimental models [65,72] | Correlation with disease severity and cardiac dysfunction [65,72] |
| Therapeutic implications | Targeted eradication; periodontal therapy; TLR4/NF-κB inhibition | Reduces local inflammation, improves barrier repair | Attenuates tumor growth; potential microbial “Trojan horse” vectors | Improves endothelial function and lowers CRP; experimental inhibitors in development | Preclinical antibiotic and microbiota-modulating trials [73,74,75,76] | Clinical periodontal interventions improve vascular outcomes [74,75,76,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, V.; Forte, P.; Ciappina, G.; Colarusso, L.; Giorgi, C.; Fiorica, F.; Bottari, A.; Di Mauro, G.; Maurea, N.; Berretta, M. Fusobacteriumnucleatum: Pathophysiological and Clinical Involvement in Inflammatory Bowel Diseases, Colorectal Cancer and Cardiovascular Diseases. Cancers 2025, 17, 3348. https://doi.org/10.3390/cancers17203348
Quagliariello V, Forte P, Ciappina G, Colarusso L, Giorgi C, Fiorica F, Bottari A, Di Mauro G, Maurea N, Berretta M. Fusobacteriumnucleatum: Pathophysiological and Clinical Involvement in Inflammatory Bowel Diseases, Colorectal Cancer and Cardiovascular Diseases. Cancers. 2025; 17(20):3348. https://doi.org/10.3390/cancers17203348
Chicago/Turabian StyleQuagliariello, Vincenzo, Pietro Forte, Giuliana Ciappina, Luigi Colarusso, Carlotta Giorgi, Francesco Fiorica, Antonio Bottari, Giordana Di Mauro, Nicola Maurea, and Massimiliano Berretta. 2025. "Fusobacteriumnucleatum: Pathophysiological and Clinical Involvement in Inflammatory Bowel Diseases, Colorectal Cancer and Cardiovascular Diseases" Cancers 17, no. 20: 3348. https://doi.org/10.3390/cancers17203348
APA StyleQuagliariello, V., Forte, P., Ciappina, G., Colarusso, L., Giorgi, C., Fiorica, F., Bottari, A., Di Mauro, G., Maurea, N., & Berretta, M. (2025). Fusobacteriumnucleatum: Pathophysiological and Clinical Involvement in Inflammatory Bowel Diseases, Colorectal Cancer and Cardiovascular Diseases. Cancers, 17(20), 3348. https://doi.org/10.3390/cancers17203348










