Neoadjuvant 177Lutetium-PSMA-617 Radioligand Therapy for High-Risk Localized Prostate Cancer: Rationale, Early Clinical Evidence, and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
2. Defining the High-Risk Patient and the Limits of Standard of Care
2.1. A Heterogeneous Definition: Comparing International Guidelines
2.2. The Sobering Reality of Recurrence and Mortality
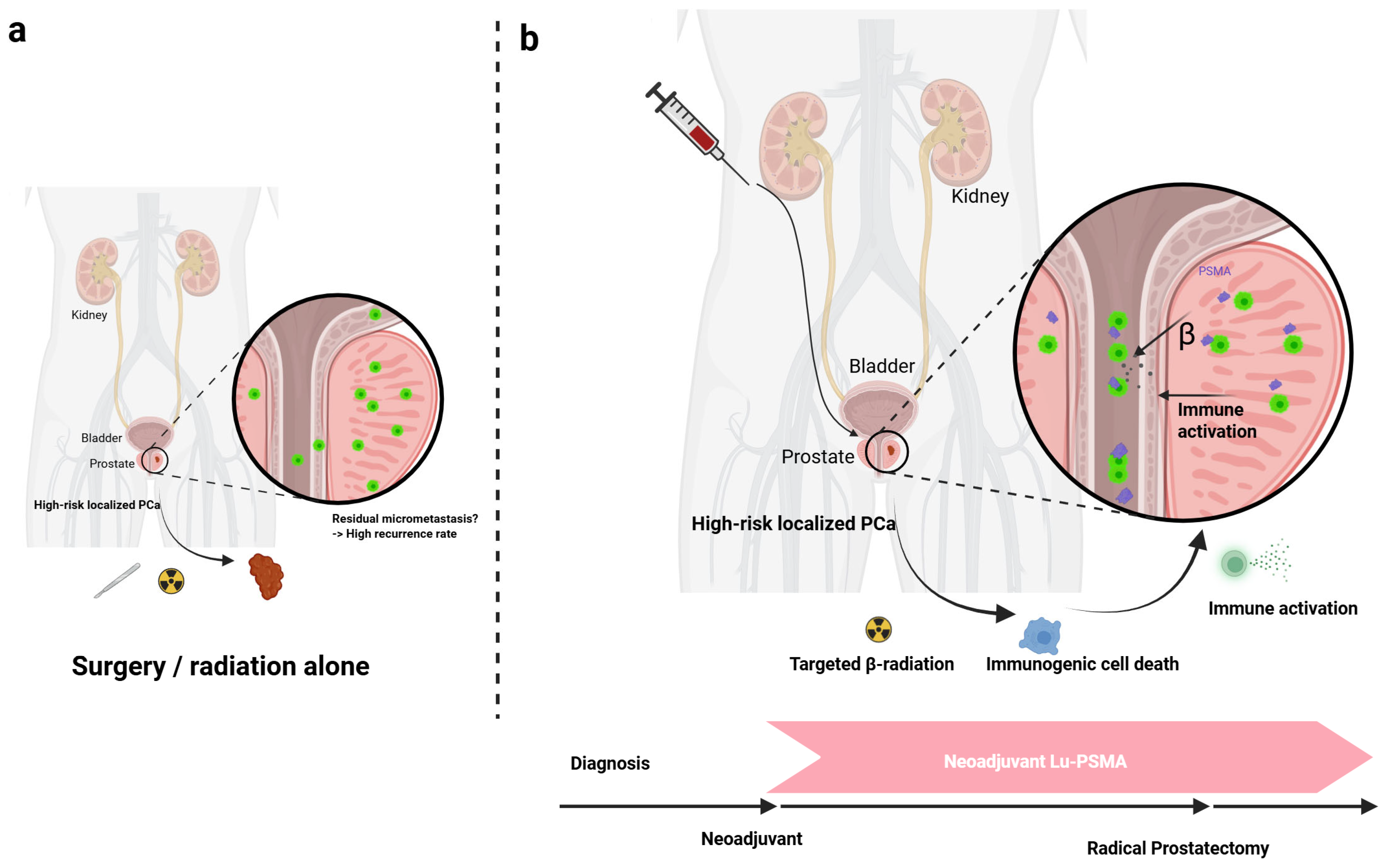
2.3. The Rationale for a Neoadjuvant Approach
3. The Theranostic Foundation: PSMA-Targeted RLT
3.1. Mechanism of Action of 177Lu-PSMA-617
3.2. Pivotal Evidence from the Metastatic Setting
3.3. The Radiobiological Imperative: β- Versus Alpha-Emitters
| Radionuclide | Particle Emitted | Half-Life (days) | Max Energy (MeV) | Max Range in Tissue | Typical LET (keV/µm) |
|---|---|---|---|---|---|
| 177Lu [52,53] | β | 6.7 | 0.497 | ~2 mm | ~0.2 |
| 90Y [54] | β | 2.7 | 2.3 | ~11 mm | ~0.2 |
| 223Ra [55] | α | 11.4 | 5.0–7.5 | 40–100 µm | ~80 |
| 225Ac [56,57] | α | 9.9 | 5.0–8.4 | 40–100 µm | ~100 |
4. Emerging Evidence for Neoadjuvant 177Lu-PSMA RLT
4.1. Preclinical Rationale and Early Human Experience
4.2. Initial Clinical Trial Data: The LuTectomy and Golan Studies
4.3. The Next Frontier in Trial Design: An Overview of Ongoing Studies
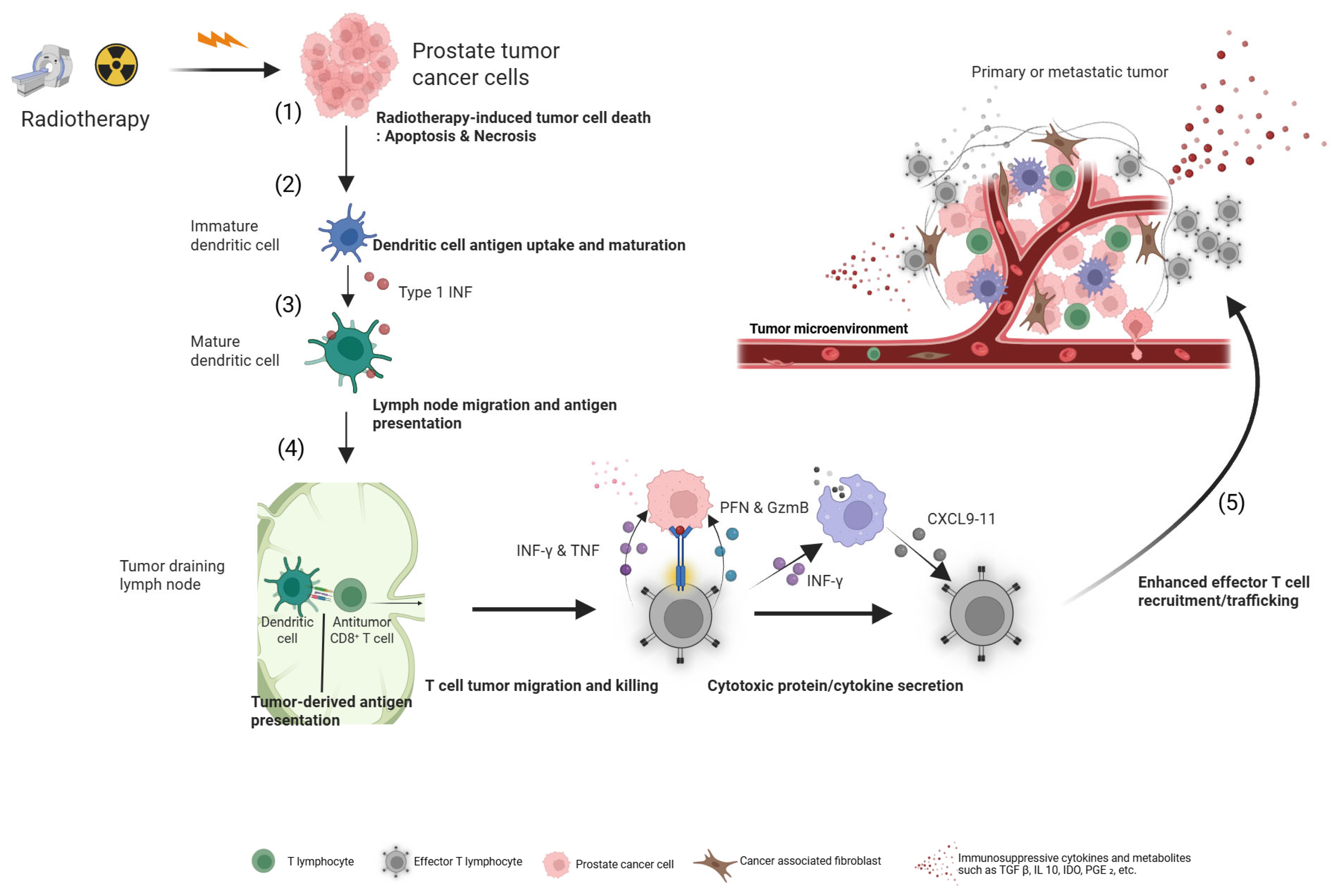
5. The Immunomodulatory Potential of Neoadjuvant RLT
5.1. Inducing an In Situ Vaccine Effect
5.2. A Hypothesis-Generating Biomarker: The PD-L2 Signature
6. Optimizing Efficacy: Combination Strategies and Sequencing
6.1. Synergy with Immunotherapy
6.2. Interplay with PARP Inhibitors: Evidence of Cross-Resistance
6.3. Modulating the Target: The “PSMA Flare” Phenomenon
7. Navigating the Path to Clinical Implementation
7.1. Validating Endpoints for Accelerated Approval: The Role of MFS
7.2. Identifying MRD by Detecting Circulating Tumor DNA
7.3. Key Hurdles in Clinical Adoption: From Efficacy to Global Implementation
8. Conclusion and Future Directions
8.1. Summary of the Potential for Neoadjuvant RLT to Reshape the Treatment Paradigm
8.2. Key Unanswered Questions and Roadmap for Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Bitting, R.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; Desai, N.; Dorff, T.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 3.2024: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2024, 22, 140–150. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer-Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Gómez Rivas, J.; Ortega Polledo, L.E.; De La Parra Sánchez, I.; Gutiérrez Hidalgo, B.; Martín Monterrubio, J.; Marugán Álvarez, M.J.; Somani, B.K.; Enikeev, D.; Puente Vázquez, J.; Sanmamed Salgado, N. Current Status of Neoadjuvant Treatment Before Surgery in High-Risk Localized Prostate Cancer. Cancers 2024, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, Y.; Smith, J.J.; Fokas, E.; Watanabe, J.; Cercek, A.; Greten, F.R.; Bando, H.; Shi, Q.; Garcia-Aguilar, J.; Romesser, P.B. Future direction of total neoadjuvant therapy for locally advanced rectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.; Nguyen, N.T.; Sainsbury, F.; Kimizuka, N.; Muyldermans, S.; Benešová-Schäfer, M. PSMA-targeted radiotheranostics in modern nuclear medicine: Then, now, and what of the future? Theranostics 2024, 14, 3043–3079. [Google Scholar] [CrossRef] [PubMed]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate cancer review: Genetics, diagnosis, treatment options, and alternative approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Prostate Cancer Version 2.2025. Available online: https://www.nccn.org (accessed on 27 July 2025).
- European Association of Urology. EAU Guidelines on Prostate Cancer; EAU Guidelines Office: Arnhem, The Netherlands, 2025; Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 26 July 2025).
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E. Clinically localized prostate cancer: AUA/ASTRO guideline, part I: Introduction, risk assessment, staging, and risk-based management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Crocetto, F.; Musone, M.; Chianese, S.; Conforti, P.; Digitale Selvaggio, G.; Caputo, V.F.; Falabella, R.; Del Giudice, F.; Giulioni, C.; Cafarelli, A.; et al. Blood and urine-based biomarkers in prostate cancer: Current advances, clinical applications, and future directions. J. Liq. Biopsy 2025, 9, 100305. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Combes, A.D.; Palma, C.A.; Calopedos, R.; Wen, L.; Woo, H.; Fulham, M.; Leslie, S. PSMA PET-CT in the Diagnosis and Staging of Prostate Cancer. Diagnostics 2022, 12, 2594. [Google Scholar] [CrossRef]
- Karpinski, M.J.; Rahbar, K.; Bögemann, M.; Nikoukar, L.R.; Schäfers, M.; Hoberück, S.; Miederer, M.; Hölscher, T.; Rasul, S.; Miszczyk, M.; et al. Updated Prostate Cancer Risk Groups by Prostate-specific Membrane Antigen Positron Emission Tomography Prostate Cancer Molecular Imaging Standardized Evaluation (PPP2): Results from an International Multicentre Registry Study. Eur. Urol. 2025, 88, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Ravizzini, G.; Chapin, B.F.; Kundra, V. Imaging Biochemical Recurrence After Prostatectomy: Where Are We Headed? Am. J. Roentgenol. 2020, 214, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Lewinshtein, D.; Teng, B.; Valencia, A.; Gibbons, R.; Porter, C.R. The long-term outcomes after radical prostatectomy of patients with pathologic Gleason 8–10 disease. Adv. Urol. 2012, 2012, 428098. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef]
- Leichman, L. Neoadjuvant chemotherapy for disseminated colorectal cancer: Changing the paradigm. J. Clin. Oncol. 2006, 24, 3817–3818. [Google Scholar] [CrossRef]
- Hirmas, N.; Holtschmidt, J.; Loibl, S. Shifting the paradigm: The transformative role of neoadjuvant therapy in early breast cancer. Cancers 2024, 16, 3236. [Google Scholar] [CrossRef]
- Häberle, L.; Erber, R.; Gass, P.; Hein, A.; Niklos, M.; Volz, B.; Hack, C.C.; Schulz-Wendtland, R.; Huebner, H.; Goossens, C. Prediction of pathological complete response after neoadjuvant chemotherapy for HER2-negative breast cancer patients with routine immunohistochemical markers. Breast Cancer Res. 2025, 27, 13. [Google Scholar] [CrossRef]
- Howard, F.M.; He, G.; Peterson, J.R.; Pfeiffer, J.; Earnest, T.; Pearson, A.T.; Abe, H.; Cole, J.A.; Nanda, R. Highly accurate response prediction in high-risk early breast cancer patients using a biophysical simulation platform. Breast Cancer Res. Treat. 2022, 196, 57–66. [Google Scholar] [CrossRef]
- van Der Slot, M.A.; Remmers, S.; Kweldam, C.F.; den Bakker, M.A.; Nieboer, D.; Busstra, M.B.; Gan, M.; Klaver, S.; Rietbergen, J.B.; van Leenders, G.J. Biopsy prostate cancer perineural invasion and tumour load are associated with positive posterolateral margins at radical prostatectomy: Implications for planning of nerve-sparing surgery. Histopathology 2023, 83, 348–356. [Google Scholar] [CrossRef]
- Aredo, J.V.; Jamali, A.; Zhu, J.; Heater, N.; Wakelee, H.A.; Vaklavas, C.; Anagnostou, V.; Lu, J. Liquid Biopsy Approaches for Cancer Characterization, Residual Disease Detection, and Therapy Monitoring. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e481114. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Skelton, W.P. Prostate Cancer. [Updated 4 October 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470550/ (accessed on 31 August 2025).
- Perera, M.; Beech, B.B.; De Jesus Escano, M.; Gmelich, C.; Yip, W.; Boorjian, S.A.; Eastham, J.A. Neoadjuvant Systemic Therapy Prior to Radical Prostatectomy for Clinically Localized High-Risk Prostate Cancer. Front. Urol. 2022, 2, 864646. [Google Scholar] [CrossRef]
- Sartor, O.; Bono, J.d.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. New Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Eapen, R.S.; Buteau, J.P.; Jackson, P.; Mitchell, C.; Oon, S.F.; Alghazo, O.; McIntosh, L.; Dhiantravan, N.; Scalzo, M.J.; O’Brien, J.; et al. Administering [177Lu]Lu-PSMA-617 Prior to Radical Prostatectomy in Men with High-risk Localised Prostate Cancer (LuTectomy): A Single-centre, Single-arm, Phase 1/2 Study. Eur. Urol. 2024, 85, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Golan, S.; Frumer, M.; Zohar, Y.; Rosenbaum, E.; Yakimov, M.; Kedar, D.; Margel, D.; Baniel, J.; Steinmetz, A.P.; Groshar, D.; et al. Neoadjuvant 177Lu-PSMA-I&T Radionuclide Treatment in Patients with High-risk Prostate Cancer Before Radical Prostatectomy: A Single-arm Phase 1 Trial. Eur. Urol. Oncol. 2023, 6, 151–159. [Google Scholar] [CrossRef]
- Krafft, U.; Grünwald, V.; Fendler, W.P.; Herrmann, K.; Reis, H.; Roghmann, F.; Rahbar, K.; Heidenreich, A.; Giesel, F.; Niegisch, G.; et al. A randomized phase I/II study of neoadjuvant treatment with 177-Lutetium-PSMA-617 with or without ipilimumab in patients with very high-risk prostate cancer who are candidates for radical prostatectomy (NEPI trial). J. Clin. Oncol. 2024, 42, TPS352. [Google Scholar] [CrossRef]
- Le, T.K.; Duong, Q.H.; Baylot, V.; Fargette, C.; Baboudjian, M.; Colleaux, L.; Taïeb, D.; Rocchi, P. Castration-Resistant Prostate Cancer: From Uncovered Resistance Mechanisms to Current Treatments. Cancers 2023, 15, 5047. [Google Scholar] [CrossRef]
- Corpetti, M.; Müller, C.; Beltran, H.; de Bono, J.; Theurillat, J.P. Prostate-Specific Membrane Antigen-Targeted Therapies for Prostate Cancer: Towards Improving Therapeutic Outcomes. Eur. Urol. 2024, 85, 193–204. [Google Scholar] [CrossRef]
- Jiang, Z.; Kadeerhan, G.; Zhang, J.; Guo, W.; Guo, H.; Wang, D. Advances in prostate-specific membrane antigen-targeted theranostics: From radionuclides to near-infrared fluorescence technology. Front. Immunol. 2025, 15, 1533532. [Google Scholar] [CrossRef]
- Bakht, M.K.; Beltran, H. Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat. Rev. Urol. 2025, 22, 26–45. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [177Lu] Lu-PSMA-617 (PluvictoTM): The first FDA-approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Cross, W.; Wong, P.; Freedman, N. Dose distributions for electrons and beta rays incident normally on water. Radiat. Prot. Dosim. 1991, 35, 77–91. [Google Scholar] [CrossRef]
- Delbart, W.; Karabet, J.; Marin, G.; Penninckx, S.; Derrien, J.; Ghanem, G.E.; Flamen, P.; Wimana, Z. Understanding the radiobiological mechanisms induced by 177Lu-DOTATATE in comparison to external beam radiation therapy. Int. J. Mol. Sci. 2022, 23, 12369. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef]
- Kuo, P.; Hesterman, J.; Rahbar, K.; Kendi, A.T.; Wei, X.X.; Fang, B.; Adra, N.; Armstrong, A.J.; Garje, R.; Michalski, J.M. [68Ga] Ga-PSMA-11 PET baseline imaging as a prognostic tool for clinical outcomes to [177Lu] Lu-PSMA-617 in patients with mCRPC: A VISION substudy. J. Clin. Oncol. 2022, 40, 16. [Google Scholar] [CrossRef]
- Ling, S.W.; de Lussanet de la Sablonière, Q.; Ananta, M.; de Blois, E.; Koolen, S.L.W.; Drexhage, R.C.; Hofland, J.; Robbrecht, D.G.J.; van der Veldt, A.A.M.; Verburg, F.A.; et al. First real-world clinical experience with [177Lu]Lu-PSMA-I&T in patients with metastatic castration-resistant prostate cancer beyond VISION and TheraP criteria. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2034–2040. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Buteau, J.P.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D. Overall survival with [177Lu] Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2024, 25, 99–107. [Google Scholar]
- Almuradova, E.; Seyyar, M.; Arak, H.; Tamer, F.; Kefeli, U.; Koca, S.; Sen, E.; Telli, T.A.; Karatas, F.; Gokmen, I.; et al. The real-world outcomes of Lutetium-177 PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer: Turkish Oncology Group multicenter study. Int. J. Cancer 2024, 154, 692–700. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Russ, E.; Davis, C.M.; Slaven, J.E.; Bradfield, D.T.; Selwyn, R.G.; Day, R.M. Comparison of the medical uses and cellular effects of high and low linear energy transfer radiation. Toxics 2022, 10, 628. [Google Scholar] [CrossRef]
- Bertinetti, A.; Palmer, B.; Bradshaw, T.; Culberson, W. Investigation of a measurement-based dosimetry approach to beta particle-emitting radiopharmaceutical therapy nuclides across tissue interfaces. Phys. Med. Biol. 2024, 69, 125008. [Google Scholar]
- Shi, M.; Jakobsson, V.; Greifenstein, L.; Khong, P.-L.; Chen, X.; Baum, R.P.; Zhang, J. Alpha-peptide receptor radionuclide therapy using actinium-225 labeled somatostatin receptor agonists and antagonists. Front. Med. 2022, 9, 1034315. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, I.V.; Nikitaki, Z.; Kalospyros, S.A.; Georgakilas, A.G. Ionizing radiation and complex DNA damage: From prediction to detection challenges and biological significance. Cancers 2019, 11, 1789. [Google Scholar] [CrossRef]
- King, A.P.; Lin, F.I.; Escorcia, F.E. Why bother with alpha particles? Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 7–17. [Google Scholar]
- Ballisat, L.; De Sio, C.; Beck, L.; Guatelli, S.; Sakata, D.; Shi, Y.; Duan, J.; Velthuis, J.; Rosenfeld, A. Dose and DNA damage modelling of diffusing alpha-emitters radiation therapy using Geant4. Phys. Medica 2024, 121, 103367. [Google Scholar] [CrossRef]
- Lepareur, N.; Ramée, B.; Mougin-Degraef, M.; Bourgeois, M. Clinical Advances and Perspectives in Targeted Radionuclide Therapy. Pharmaceutics 2023, 15, 1733. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Fung, E.; Niaz, M.O.; Bissassar, M.; Singh, S.; Patel, A.; Tan, A.; Zuloaga, J.M.; Castellanos, S.H.; Nauseef, J.T. Abstract CT143: Results of combined targeting of prostate-specific membrane antigen (PSMA) with alpha-radiolabeled antibody 225Ac-J591 and beta-radiolabeled ligand 177Lu-PSMA I&T: Preclinical and initial phase 1 clinical data in patients with metastatic castration-resistant prostate cancer (mCRPC). Cancer Res. 2022, 82, CT143. [Google Scholar]
- Bruchertseifer, F.; Kellerbauer, A.; Malmbeck, R.; Morgenstern, A. Targeted alpha therapy with bismuth-213 and actinium-225: Meeting future demand. J. Label. Compd. Radiopharm. 2019, 62, 794–802. [Google Scholar]
- Dash, A.; Pillai, M.R.; Knapp, F.F., Jr. Production of 177Lu for Targeted Radionuclide Therapy: Available Options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Kouri, M.A.; Georgopoulos, A.; Manios, G.E.; Maratou, E.; Spathis, A.; Chatziioannou, S.; Platoni, K.; Efstathopoulos, E.P. Preliminary Study on Lutetium-177 and Gold Nanoparticles: Apoptosis and Radiation Enhancement in Hepatic Cancer Cell Line. Curr. Issues Mol. Biol. 2024, 46, 12244–12259. [Google Scholar] [CrossRef]
- Brans, B.; Mottaghy, F.M.; Kessels, A. 90Y/177Lu-DOTATATE therapy: Survival of the fittest? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1785–1787. [Google Scholar] [CrossRef]
- Henriksen, G.; Breistøl, K.; Bruland, Ø.S.; Fodstad, Ø.; Larsen, R.H. Significant antitumor effect from bone-seeking, α-particle-emitting 223Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002, 62, 3120–3125. [Google Scholar]
- Miederer, M.; Scheinberg, D.A.; McDevitt, M.R. Realizing the potential of the Actinium-225 radionuclide generator in targeted alpha particle therapy applications. Adv. Drug Deliv. Rev. 2008, 60, 1371–1382. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef]
- Parent, E.E.; Savir-Baruch, B.; Gayed, I.W.; Almaguel, F.; Chin, B.B.; Pantel, A.R.; Armstrong, E.; Morley, A.; Ippisch, R.C.; Flavell, R.R. 177Lu-PSMA therapy. J. Nucl. Med. Technol. 2022, 50, 205–212. [Google Scholar] [CrossRef]
- Foxton, C.; Waldron, B.; Grønlund, R.V.; Simón, J.J.; Cornelissen, B.; O’Neill, E.; Stevens, D. Preclinical Evaluation of 177Lu-rhPSMA-10.1, a Radiopharmaceutical for Prostate Cancer: Biodistribution and Therapeutic Efficacy. J. Nucl. Med. 2025, 66, 599–604. [Google Scholar] [CrossRef]
- Alghazo, O.; Eapen, R.; Dhiantravan, N.; Violet, J.A.; Jackson, P.; Scalzo, M.; Keam, S.P.; Mitchell, C.; Neeson, P.J.; Sandhu, S.K.; et al. Study of the dosimetry, safety, and potential benefit of 177Lu-PSMA-617 radionuclide therapy prior to radical prostatectomy in men with high-risk localized prostate cancer (LuTectomy study). J. Clin. Oncol. 2021, 39, TPS264. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Soydal, C.; Virgolini, I.; Tuncel, M.; Kairemo, K.; Kapp, D.S.; von Eyben, F.E. Management Based on Pretreatment PSMA PET of Patients with Localized High-Risk Prostate Cancer Part 2: Prediction of Recurrence—A Systematic Review and Meta-Analysis. Cancers 2025, 17, 841. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef]
- Handke, A.; Kesch, C.; Fendler, W.P.; Telli, T.; Liu, Y.; Hakansson, A.; Davicioni, E.; Hughes, J.; Song, H.; Lueckerath, K.; et al. Analysing the tumor transcriptome of prostate cancer to predict efficacy of Lu-PSMA therapy. J. Immunother. Cancer 2023, 11, e007354. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Barjij, I.; Meliani, M. Immunogenic Cell Death as a Target for Combination Therapies in Solid Tumors: A Systematic Review Toward a New Paradigm in Immuno-Oncology. Cureus 2025, 17, e85776. [Google Scholar] [CrossRef]
- Sandhu, S.; Subramaniam, S.; Hofman, M.S.; Stockler, M.R.; Martin, A.J.; Pokorski, I.; Goh, J.C.; Pattison, D.A.; Dhiantravan, N.; Gedye, C. Evolution: Phase II study of radionuclide 177Lu-PSMA-617 therapy versus 177Lu-PSMA-617 in combination with ipilimumab and nivolumab for men with metastatic castration-resistant prostate cancer (mCRPC; ANZUP 2001). J. Clin. Oncol. 2023, 41, 6. [Google Scholar] [CrossRef]
- Strati, A.; Adamopoulos, C.; Kotsantis, I.; Psyrri, A.; Lianidou, E.; Papavassiliou, A.G. Targeting the PD-1/PD-L1 signaling pathway for cancer therapy: Focus on biomarkers. Int. J. Mol. Sci. 2025, 26, 1235. [Google Scholar] [CrossRef]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012, 2012, 656340. [Google Scholar] [CrossRef]
- Zhu, L.; Qu, Y.; Yang, J.; Shao, T.; Kuang, J.; Liu, C.; Qi, Y.; Li, M.; Li, Y.; Zhang, S.; et al. PD-L2 act as an independent immune checkpoint in colorectal cancer beyond PD-L1. Front. Immunol. 2024, 15, 1486888. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Gu, R.; Chen, J.; Chen, Q.; Lin, T.; Wu, J.; Hu, Y.; Yuan, A. In Situ Vaccination with Mitochondria-Targeting Immunogenic Death Inducer Elicits CD8(+) T Cell-Dependent Antitumor Immunity to Boost Tumor Immunotherapy. Adv. Sci. 2023, 10, e2300286. [Google Scholar] [CrossRef]
- Bono, J.d.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Sun, C.; Chu, A.; Song, R.; Liu, S.; Chai, T.; Wang, X.; Liu, Z. PARP inhibitors combined with radiotherapy: Are we ready? Front. Pharmacol. 2023, 14, 1234973. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Tuchayi, A.M.; Low, S.K.; Arafa, A.T.; Graham, L.S.; Gulati, R.; Pritchard, C.C.; Montgomery, R.B.; Haffner, M.C.; Nelson, P.S.; et al. Association of Prior PARP Inhibitor Exposure with Clinical Outcomes after 177Lu-PSMA-617 in Men with Castration-resistant Prostate Cancer and Mutations in DNA Homologous Recombination Repair Genes. Eur. Urol. Oncol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Berchuck, J.E.; Zhang, Z.; Silver, R.; Kwak, L.; Xie, W.; Lee, G.-S.M.; Freedman, M.L.; Kibel, A.S.; Van Allen, E.M.; McKay, R.R.; et al. Impact of Pathogenic Germline DNA Damage Repair alterations on Response to Intense Neoadjuvant Androgen Deprivation Therapy in High-risk Localized Prostate Cancer. Eur. Urol. 2021, 80, 295–303. [Google Scholar] [CrossRef]
- Sengupta, S.; Kasireddy, S.R.; Mukkamala, R.; Srinivasarao, M.; Low, P.S. Abstract 590: Prostate specific membrane antigen targeted PRMT5 inhibition re-sensitizes cancer cells to radiation therapies. Cancer Res. 2025, 85, 590. [Google Scholar] [CrossRef]
- van der Gaag, S.; Vis, A.N.; Bartelink, I.H.; Koppes, J.C.C.; Hodolic, M.; Hendrikse, H.; Oprea-Lager, D.E. Exploring the Flare Phenomenon in Patients with Castration-Resistant Prostate Cancer: Enzalutamide-Induced PSMA Upregulation Observed on PSMA PET. J. Nucl. Med. 2025, 66, 373–376. [Google Scholar] [CrossRef]
- Mei, R.; Bracarda, S.; Emmett, L.; Farolfi, A.; Lambertini, A.; Fanti, S.; Castellucci, P. Androgen deprivation therapy and its modulation of PSMA expression in prostate cancer: Mini review and case series of patients studied with sequential [68Ga]-Ga-PSMA-11 PET/CT. Clin. Transl. Imaging 2021, 9, 215–220. [Google Scholar] [CrossRef]
- Zacho, H.D.; Petersen, L.J. Bone flare to androgen deprivation therapy in metastatic, hormone-sensitive prostate cancer on 68Ga-prostate-specific membrane antigen PET/CT. Clin. Nucl. Med. 2018, 43, e404–e406. [Google Scholar] [CrossRef]
- Emmett, L.; Subramaniam, S.; Crumbaker, M.; Nguyen, A.; Joshua, A.M.; Weickhardt, A.; Lee, S.T.; Ng, S.; Francis, R.J.; Goh, J.C.; et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 563–571. [Google Scholar] [CrossRef]
- Emmett, L.; Subramaniam, S.; Crumbaker, M.; Joshua, A.M.; Sandhu, S.; Nguyen, A.; Weickhardt, A.; Lee, S.-T.; Ng, S.; Francis, R.J.; et al. Overall survival and quality of life with [177Lu]Lu-PSMA-617 plus enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer (ENZA-p): Secondary outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2025, 26, 291–299. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Kattan, M.W.; Eastham, J.A.; Bianco, F.J., Jr.; Yossepowitch, O.; Vickers, A.J.; Klein, E.A.; Wood, D.P.; Scardino, P.T. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J. Clin. Oncol. 2009, 27, 4300–4305. [Google Scholar] [CrossRef]
- Xie, W.; Regan, M.M.; Buyse, M.; Halabi, S.; Kantoff, P.W.; Sartor, O.; Soule, H.; Clarke, N.W.; Collette, L.; Dignam, J.J.; et al. Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J. Clin. Oncol. 2017, 35, 3097–3104. [Google Scholar] [CrossRef]
- Xie, W.; Ravi, P.; Buyse, M.; Halabi, S.; Kantoff, P.; Sartor, O.; Soule, H.; Clarke, N.; Dignam, J.; James, N.; et al. Validation of metastasis-free survival as a surrogate endpoint for overall survival in localized prostate cancer in the era of docetaxel for castration-resistant prostate cancer. Ann. Oncol. 2024, 35, 285–292. [Google Scholar] [CrossRef]
- Peng, Y.; Mei, W.; Ma, K.; Zeng, C. Circulating Tumor DNA and Minimal Residual Disease (MRD) in Solid Tumors: Current Horizons and Future Perspectives. Front. Oncol. 2021, 11, 763790. [Google Scholar] [CrossRef]
- Zang, P.D.; Lama, D.; Huang, Z.; Jaime-Casas, S.; Contente-Cuomo, T.; Stampar, M.; Marshall, A.; Dinwiddie, D.; Berens, M.E.; Pond, S.; et al. Feasibility of enriched amplicon circulating tumor DNA sequencing to detect minimal residual disease (MRD) after prostatectomy in localized prostate cancer. J. Clin. Oncol. 2025, 43, 402. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Kairemo, K.; Kapp, D.S. Prostate-Specific Antigen as an Ultrasensitive Biomarker for Patients with Early Recurrent Prostate Cancer: How Low Shall We Go? A Systematic Review. Biomedicines 2024, 12, 822. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Izumi, K.; Matsumoto, R.; Ito, Y.; Hoshi, S.; Matsubara, N.; Yamasaki, T.; Mizowaki, T.; Komaru, A.; Nomura, S.; Hattori, T.; et al. [177Lu]Lu-PSMA-617 in Patients with Progressive PSMA+ mCRPC Treated With or Without Prior Taxane-Based Chemotherapy: A Phase 2, Open-Label, Single-Arm Trial in Japan. Cancers 2025, 17, 2351. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Expands Pluvicto’s Metastatic Castration-Resistant Prostate Cancer Indication. 28 March 2025. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-pluvictos-metastatic-castration-resistant-prostate-cancer-indication?utm (accessed on 31 August 2025).
- European Medicines Agency. Pluvicto; European Medicines Agency: Amsterdam, The Netherlands, 2025 27 March 2025; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/pluvicto?utm (accessed on 31 August 2025).
- Health Canada. Details for: PLUVICTO; Government of Canada: Ottawa, ON, Canada, 2025 30 August 2025; Available online: https://dhpp.hpfb-dgpsa.ca/dhpp/resource/101939?utm (accessed on 31 August 2025).
- Giammarile, F.; Paez, D.; Zimmermann, R.; Cutler, C.S.; Jalilian, A.; Korde, A.; Knoll, P.; Ayati, N.; Lewis, J.S.; Lapi, S.E.; et al. Production and regulatory issues for theranostics. Lancet Oncol. 2024, 25, e260–e269. [Google Scholar] [CrossRef]
- Zagni, F.; Vetrone, L.; Farolfi, A.; Vadalà, M.; Rizzini, E.L.; Golemi, A.; Strigari, L.; Fanti, S. Feasibility of 177Lu-PSMA Administration as Outpatient Procedure for Prostate Cancer. J. Nucl. Med. 2024, 65, 1848–1849. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Radiation Protection of Patients in Therapeutic Nuclear Medicine; International Atomic Energy Agency: Vienna, Austria, 2025; Available online: https://www.iaea.org/resources/rpop/health-professionals/nuclear-medicine/therapeutic-nuclear-medicine/patients?utm (accessed on 31 August 2025).
- Herrmann, K.; Giovanella, L.; Santos, A.; Gear, J.; Kiratli, P.O.; Kurth, J.; Denis-Bacelar, A.M.; Hustinx, R.; Patt, M.; Wahl, R.L.; et al. Joint EANM, SNMMI and IAEA enabling guide: How to set up a theranostics centre. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2300–2309. [Google Scholar] [CrossRef]
- Hooijman, E.L.; Radchenko, V.; Ling, S.W.; Konijnenberg, M.; Brabander, T.; Koolen, S.L.W.; de Blois, E. Implementing Ac-225 labelled radiopharmaceuticals: Practical considerations and (pre-)clinical perspectives. EJNMMI Radiopharm. Chem. 2024, 9, 9. [Google Scholar] [CrossRef]
- Pound, C.R.; Partin, A.W.; Eisenberger, M.A.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M.; et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [177Lu]Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Berg, S.A.; Sayan, M. The Clinical Impact of the Decipher Genomic Classifier in Prostate Cancer. Eurasian J. Med. 2025, 57, e25828. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, N.M.; Maurice-Dror, C.; Herberts, C.; Tu, W.; Fan, W.; Murtha, A.J.; Kollmannsberger, C.; Kwan, E.M.; Parekh, K.; Schönlau, E.; et al. Prediction of plasma ctDNA fraction and prognostic implications of liquid biopsy in advanced prostate cancer. Nat. Commun. 2024, 15, 1828. [Google Scholar] [CrossRef]
- Cherny, N.I.; Oosting, S.F.; Dafni, U.; Latino, N.J.; Galotti, M.; Zygoura, P.; Dimopoulou, G.; Amaral, T.; Barriuso, J.; Calles, A.; et al. ESMO-Magnitude of Clinical Benefit Scale version 2.0 (ESMO-MCBS v2.0). Ann. Oncol. 2025, 36, 866–908. [Google Scholar] [CrossRef] [PubMed]
- Hofland, J.; Brabander, T.; Verburg, F.A.; Feelders, R.A.; de Herder, W.W. Peptide Receptor Radionuclide Therapy. J. Clin. Endocrinol. Metab. 2022, 107, 3199–3208. [Google Scholar] [CrossRef]
- Eifer, M.; Sutherland, D.E.K.; Goncalves, I.; Buteau, J.P.; Au, L.; Azad, A.A.; Emmett, L.; Kong, G.; Kostos, L.; Ravi Kumar, A.S.; et al. Therapy-Related Myeloid Neoplasms After [177Lu]Lu-PSMA Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer: A Case Series. J. Nucl. Med. 2025, 66, 579–584. [Google Scholar] [CrossRef]
- Mehrens, D.; Kramer, K.K.M.; Unterrainer, L.M.; Beyer, L.; Bartenstein, P.; Froelich, M.F.; Tollens, F.; Ricke, J.; Rübenthaler, J.; Schmidt-Hegemann, N.S.; et al. Cost-Effectiveness Analysis of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. J. Natl. Compr. Canc Netw. 2023, 21, 43–50.e42. [Google Scholar] [CrossRef]
| Guideline | High-Risk Criteria | Very High-Risk Criteria | Key Features and Differences |
|---|---|---|---|
| NCCN (2025) [7] | One or more of:
| Two or more of:
|
|
| EAU (2025) [8] | Two or more of:
|
|
|
| AUA/ASTRO/SUO (2022) [9] | One or more of:
| No separate category. Instead, classified as “Locally Advanced Disease”:
|
|
| Trial Name (Identifier) | Phase | Patient Population | Intervention(s) | Primary Endpoint(s) | Key Secondary Endpoints |
|---|---|---|---|---|---|
| LuTectomy (NCT04430192) [60] | I/II | High-risk localized/locoregional PCa (n = 20) | Single cycle of 177Lu-PSMA-617 prior to RP | Absorbed radiation dose | Safety, surgical feasibility, PSA response, imaging response, pathological response |
| NEPI (EudraCT 2021-004894-30) [28] | I/II | Very high-risk localized PCa (n = 46) | ADT + 2 cycles of 177Lu-PSMA-617 +/- 4 cycles of ipilimumab prior to RP | Feasibility of RP, pCR | Safety, DFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, W.-A.; Joung, J.Y. Neoadjuvant 177Lutetium-PSMA-617 Radioligand Therapy for High-Risk Localized Prostate Cancer: Rationale, Early Clinical Evidence, and Future Directions. Cancers 2025, 17, 3330. https://doi.org/10.3390/cancers17203330
Kwon W-A, Joung JY. Neoadjuvant 177Lutetium-PSMA-617 Radioligand Therapy for High-Risk Localized Prostate Cancer: Rationale, Early Clinical Evidence, and Future Directions. Cancers. 2025; 17(20):3330. https://doi.org/10.3390/cancers17203330
Chicago/Turabian StyleKwon, Whi-An, and Jae Young Joung. 2025. "Neoadjuvant 177Lutetium-PSMA-617 Radioligand Therapy for High-Risk Localized Prostate Cancer: Rationale, Early Clinical Evidence, and Future Directions" Cancers 17, no. 20: 3330. https://doi.org/10.3390/cancers17203330
APA StyleKwon, W.-A., & Joung, J. Y. (2025). Neoadjuvant 177Lutetium-PSMA-617 Radioligand Therapy for High-Risk Localized Prostate Cancer: Rationale, Early Clinical Evidence, and Future Directions. Cancers, 17(20), 3330. https://doi.org/10.3390/cancers17203330






