Vascular Access Devices for Stem Cell Transplantation: A Review of Catheter Types—A Crucial Step Towards the Enhancement of Patient Care
Simple Summary
Abstract
1. Introduction
2. Review Methodology
3. Classification and Types of Vascular Access Devices
- •
- Duration of access:
- ∘
- Short-term (few days or few weeks);
- ∘
- Medium-term (up to 3–4 months);
- ∘
- Long term (>3–4 months).
- •
- Site of insertion:
- ∘
- PICCs (Peripherally inserted central catheters) are inserted into deep veins of the arm (basilic vein, brachial veins, brachial tract of the axillary vein) or the cephalic vein at the arm.
- ∘
- CICCs (Centrally inserted central catheters)—inserted into deep veins of the supra-clavicular area (internal jugular vein, brachiocephalic vein, subclavian vein, deep tract of the external jugular vein) or of the infra-clavicular area (thoracic tract of the cephalic vein, thoracic tract of the axillary vein).
- ∘
- FICCs (Femorally inserted central catheters)—inserted into veins of the lower limb (common femoral vein, superficial femoral vein, saphenous vein).
- ∘
- TIVAD Totally implantable venous access device (or port).
- ▪
- Chest ports, also known as CICC ports.
- ▪
- Femoral ports, also known as FICC port.
- ▪
- Brachial ports, also known as PICC ports, also known as arm ports.
- •
- Number of lumens (single, double, or multi-lumen).
- •
- Characteristic of tip (open tip or valve tip).
- •
- Materials to reduce complications (e.g., silicone or polyurethane catheters impregnated with heparin, antibiotics, or silver).
- PVAD—peripheral venous access devices:
- SPC—Short peripheral catheter;
- LPC—Long peripheral catheter (also known as mini-midline or short midline);
- MC—Midline catheter (also known as midclavicular catheter).
- 2.
- CVAD—central venous access devices:
- PICC—Peripherally inserted central catheters;
- CICC—Centrally inserted central catheter;
- FICC—Femorally inserted central catheter;
- TIVAD—Totally implantable venous access device (or port).
- i.
- Chest ports, also known as CICC ports;
- ii.
- Femoral ports, also known as FICC ports;
- iii.
- Brachial ports, also known as PICC ports, also known as arm ports.
- 3.
- Tunneled CVAD:
- Tc—Tunneled, and cuffed: Tc-CICC, Tc-PICC, Tc-FICC;
- Tnc—Tunneled, but non-cuffed: Tnc-CICC, Tnc-PICC, Tnc-FICC.
4. Catheter Lifespan
4.1. Short-Term and Medium-Term Devices
4.2. Long-Term Devices
5. Material Considerations
- −
- In ports (TIVADs), a large series report higher infection and thrombosis with PU vs. SI, though SI shows more mechanical failures [24].
- −
- In PICCs, older reviews suggest similar or slightly lower infection risk with PU, but outcomes depend more on lumen size, catheter-to-vein ratio, and maintenance bundles than on material alone [25].
6. Types of CVADs
6.1. Centrally Inserted Central Catheters (CICCs)
6.2. Peripherally Inserted Central Catheters (PICCs)
6.3. Tunneled CVADs
6.4. TIVAD—Totally Implantable Venous Access Devices
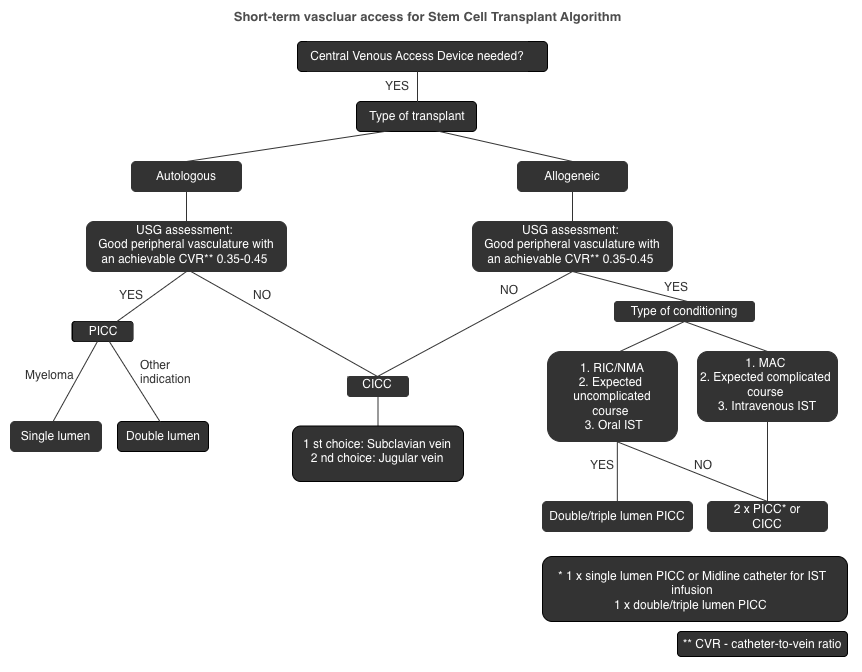
7. General Principles and Indications
Role of Totally Implantable Venous Access Devices (Ports) in HSCT
| Clinical Scenario | Preferred Device | Evidence Type/Key References |
|---|---|---|
| Allo-HSCT, MAC, inpatient aplasia (high multi-infusion, frequent sampling) | Tunneled multi-lumen CVC (SCV > IJV when feasible) | HSCT-specific [11,34] |
| Allo-HSCT, RIC/NMA, ambulatory (lower concurrent infusion demand) | Port or PICC (per center logistics) | Indirect [6,8,9] |
| Auto-HSCT (outpatient programs), limited concurrent infusions | Port or PICC | Indirect [8,9,49] |
| Long-term PN after engraftment (ambulatory) | Dedicated port (TIVAD) | Indirect [5,6,7] |
8. Complications and Important Clinical Aspects
8.1. Insertion-Related Mechanical Complications
8.2. Catheter-Related Infections
8.3. Catheter-Related Thrombosis (CRT)
8.4. Upper-Extremity CRT with PICCs in HSCT: Evidence, Thresholds, and Risk Mitigation
- •
- French size vs. CRT: Symptomatic CRT risk rises steeply with size: 3 Fr~0.9%, 4 Fr~3.3%, 5 Fr~5.5%, 6 Fr~10.7% (oncology data) [64].
- •
- CVR: Maintain ≤ 45% (measured by ultrasound without compression) as recommended by Infusion Therapy Standards [6].
- •
- Lumen number: Single-lumen is preferred; dual-lumen only when clinically essential (e.g., allo-MAC with high infusion demand) [28].
8.5. Comparative Summary of Vascular Access Devices in HSCT
| Parameter | PICC | CICC | Tunneled CVAD | References |
|---|---|---|---|---|
| CLABSI rate (per 1000 catheter-days) | 2.12 | 4.09 | 0.1–1.0 | [9,28,29] |
| CRT incidence (%) | 2–8% (↑ with >4 Fr, multi-lumen) | 5–18% | <5% | [59,64,65] |
| Mechanical complications | Rare | 5–19% (↓ with US guidance) | Moderate | [20,38,53] |
| Multi-infusion support | Limited by lumen size (4–5 Fr) | Good (multi-lumen, high flow) | Good (multi-lumen) | [69,70] |
| Cryopreserved graft infusion | Feasible with dual PICCs (≥5 Fr) | Preferred | Feasible | [6,44] |
| Catheter-to-vein ratio | ≤45% recommended | Not applicable | Not applicable | [6,45] |
| Cumulative thrombosis risk | ↑ with multiple PICCs | Lower | Lower | [64,66] |
| Patient comfort/QoL | High | Lower | Highest | [70,71] |
| Removal feasibility | Easy | Easy | Surgical, delayed | [16,20] |
8.6. Monitoring of Immunosuppressive Drugs
- •
- Stop infusion time: pause drug infusion for at least 2–5 min before sampling.
- •
- Flush volume: ≥10–20 mL 0.9% NaCl using pulsatile technique.
- •
- Discard volume: at least 2–3 times the catheter dead space (typically 5 mL).
- •
- Dedicated lumen: use a lumen not used for drug infusion whenever possible.
- •
- Documentation: record stop time, flush, and discard volumes in the patient chart.
- •
- If these conditions cannot be met, venipuncture should be prioritized. Operationally, venipuncture ensures accuracy but may increase patient discomfort and staff workload; therefore, a dedicated lumen with strict adherence to protocol can be an acceptable alternative in selected cases. The full recommended protocol and a comparison of venipuncture versus PICC sampling are provided in Supplementary Materials S1 and S2.
8.7. Patient Comfort
8.8. Insertion Site Considerations in HSCT
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiffer, C.A.; Mangu, P.B.; Wade, J.C.; Camp-Sorrell, D.; Cope, D.G.; El-Rayes, B.F.; Gorman, M.; Ligibel, J.; Mansfield, P.; Levine, M. Central Venous Catheter Care for the Patient With Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2013, 31, 1357–1370. [Google Scholar] [CrossRef]
- Sousa, B.; Furlanetto, J.; Hutka, M.; Gouveia, P.; Wuerstlein, R.; Mariz, J.M.; Pinto, D.; Cardoso, F. Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann. Oncol. 2015, 26, v152–v168. [Google Scholar] [CrossRef]
- Rens, M.R.v.; Lee, R.v.d.; Spencer, T.R.; Boxtel, T.v.; Barone, G.; Crocoli, A.; Pinelli, F.; Pittiruti, M. The NAVIGATE project: A GloVANet–WoCoVA position statement on the nomenclature for vascular access devices. J. Vasc. Access 2025, 26, 1439–1446. [Google Scholar] [CrossRef]
- Jahanzeb, M.; Wu, C.-Y.; Lim, H.; Muro, K.; Xu, L.; Somashekhar, M.; Somashekhar, S.P.S.P.; Zhang, X.; Qiu, X.; Fu, Y.; et al. International experts consensus on optimal central vascular access device selection and management for patients with cancer. J. Vasc. Access 2024, 26, 1447–1458. [Google Scholar] [CrossRef]
- Okamura, N.; Yamato, T.; Yamaoka, I.; Doi, K.; Koyama, Y. How to perform appropriate flushing after lipid emulsion administration using totally implantable venous access devices in long-term total parenteral nutrition and home parenteral nutrition. Clin. Nutr. ESPEN 2021, 41, 287–292. [Google Scholar] [CrossRef]
- Gorski, L.A.M.; Hadaway, L.M.; Hagle, M.E.P.; Broadhurst, D.M.; Clare, S.M.; Kleidon, T.M.P.; Meyer, B.M.P.; Nickel, B.A.-C.; Rowley, S.M.; Sharpe, E.D.; et al. Infusion Therapy Standards of Practice, 8th Edition. J. Infus. Nurs. 2021, 44, S1–S224. [Google Scholar] [CrossRef]
- Cabrero, E.L.; Robledo, R.T.; Cuñado, A.C.; Sardelli, D.G.; López, C.H.; Formatger, D.G.; Perez, L.L.; López, C.E.; Moreno, A.T. Risk factors of catheter- associated bloodstream infection: Systematic review and meta-analysis. PLoS ONE 2023, 18, e0282290. [Google Scholar] [CrossRef]
- Wu, O.; McCartney, E.; Heggie, R.; Germeni, E.; Paul, J.; Soulis, E.; Dillon, S.; Ryan, C.; Sim, M.; Dixon-Hughes, J.; et al. Venous access devices for the delivery of long-term chemotherapy: The CAVA three-arm RCT. Health Technol. Assess. 2021, 25, 1–126. [Google Scholar] [CrossRef]
- Walser, E.M. Venous Access Ports: Indications, Implantation Technique, Follow-Up, and Complications. Cardiovasc. Interv. Radiol. 2012, 35, 751–764. [Google Scholar] [CrossRef]
- Zingg, W.; Pittet, D. Peripheral venous catheters: An under-evaluated problem. Int. J. Antimicrob. Agents 2009, 34, S38–S42. [Google Scholar] [CrossRef] [PubMed]
- Blennow, O.; Ljungman, P.; Sparrelid, E.; Mattsson, J.; Remberger, M. Incidence, risk factors, and outcome of bloodstream infections during the pre-engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl. Infect. Dis. 2014, 16, 106–114. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Jones, M.; Okano, S.; Looke, D.; Kennedy, G.; Pavilion, G.; Clouston, J.; Van Kuilenburg, R.; Geary, A.; Joubert, W.; Eastgate, M.; et al. Catheter-associated bloodstream infection in patients with cancer: Comparison of left- and right-sided insertions. J. Hosp. Infect. 2021, 118, 70–76. [Google Scholar] [CrossRef]
- Celebi, S.; Sezgin, M.E.; Cakır, D.; Baytan, B.; Demirkaya, M.; Sevinir, B.; Bozdemir, S.E.; Gunes, A.M.; Hacimustafaoglu, M. Catheter-associated Bloodstream Infections in Pediatric Hematology-Oncology Patients. Pediatr. Hematol. Oncol. 2013, 30, 187–194. [Google Scholar] [CrossRef]
- Machat, S.; Eisenhuber, E.; Pfarl, G.; Stübler, J.; Koelblinger, C.; Zacherl, J.; Schima, W. Complications of central venous port systems: A pictorial review. Insights Imaging 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Kakkos, A.; Bresson, L.; Hudry, D.; Cousin, S.; Lervat, C.; Bogart, E.; Meurant, J.; El Bedoui, S.; Decanter, G.; Hannebicque, K.; et al. Complication-related removal of totally implantable venous access port systems: Does the interval between placement and first use and the neutropenia-inducing potential of chemotherapy regimens influence their incidence? A four-year prospective study of 4045 patients. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 689–695. [Google Scholar] [CrossRef]
- Akers, A.S.; Chelluri, L. Peripherally inserted central catheter use in the hospitalized patient: Is there a role for the hospitalist? J. Hosp. Med. 2009, 4, E1–E4. [Google Scholar] [CrossRef]
- Kang, J.; Chen, W.; Sun, W.; Ge, R.; Li, H.; Ma, E.; Su, Q.; Cheng, F.; Hong, J.; Zhang, Y.; et al. Health-Related Quality of Life of Cancer Patients with Peripherally Inserted Central Catheter: A Pilot Study. J. Vasc. Access 2017, 18, 396–401. [Google Scholar] [CrossRef]
- Yeow, M.; Soh, S.; Yap, R.; Tay, D.; Low, Y.F.; Goh, S.S.N.; Yeo, C.S.; Lo, Z.J. A systematic review and network meta-analysis of randomized controlled trials on choice of central venous access device for delivery of chemotherapy. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 1184–1191.e8. [Google Scholar] [CrossRef]
- Chatani, S.; Tsukii, R.; Nagasawa, K.; Hasegawa, T.; Murata, S.; Kato, M.; Yamaura, H.; Onaya, H.; Matsuo, K.; Watanabe, Y.; et al. Difficult removal of totally implantable venous access devices in adult patients: Incidence, risk factors, and management. J. Vasc. Access 2023, 24, 1150–1157. [Google Scholar] [CrossRef]
- Lin, D.M.; Wu, Y. Implantable vascular access devices – past, present, and future. Transfusion 2018, 58, 545–548. [Google Scholar] [CrossRef]
- Frasca, D.; Dahyot-Fizelier, C.; Mimoz, O. Prevention of central venous catheter-related infection in the intensive care unit. Crit. Care 2010, 14, 212–218. [Google Scholar] [CrossRef]
- Borretta, L.; MacDonald, T.; Digout, C.; Smith, N.; Fernandez, C.V.; Kulkarni, K. Peripherally Inserted Central Catheters in Pediatric Oncology Patients: A 15-Year Population-based Review From Maritimes, Canada. J. Pediatr. Hematol. 2018, 40, e55–e60. [Google Scholar] [CrossRef]
- Sakai, T.; Kohda, K.; Konuma, Y.; Hiraoka, Y.; Ichikawa, Y.; Ono, K.; Horiguchi, H.; Tatekoshi, A.; Takada, K.; Iyama, S.; et al. A role for peripherally inserted central venous catheters in the prevention of catheter-related blood stream infections in patients with hematological malignancies. Int. J. Hematol. 2014, 100, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tong, H.; Liu, H.; Wang, Y.; Wang, R.; Gao, H.; Yu, P.; Lv, Y.; Chen, S.; Wang, G.; et al. Effectiveness of antimicrobial-coated central venous catheters for preventing catheter-related blood-stream infections with the implementation of bundles: A systematic review and network meta-analysis. Ann. Intensiv. Care 2018, 8, 71. [Google Scholar] [CrossRef]
- Dünser, M.W.; Mayr, A.J.; Hinterberger, G.; Flörl, C.L.; Ulmer, H.; Schmid, S.; Friesenecker, B.; Lorenz, I.; Hasibeder, W.R. Central Venous Catheter Colonization in Critically Ill Patients: A Prospective, Randomized, Controlled Study Comparing Standard with Two Antiseptic-Impregnated Catheters. Anesth. Analg. 2005, 101, 1778–1784. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Hahn, U.; Salwender, H.-J.; Haas, R.; Jansen, B.; Wolbring, P.; Rinck, M.; Hunstein, W. Prevention of Catheter-related Infections by Silver Coated Central Venous Catheters in Oncological Patients. Zentralblatt Bakteriol. 1995, 283, 215–223. [Google Scholar] [CrossRef]
- Schears, G.J.; Ferko, N.; Syed, I.; Arpino, J.-M.; Alsbrooks, K. Peripherally inserted central catheters inserted with current best practices have low deep vein thrombosis and central line–associated bloodstream infection risk compared with centrally inserted central catheters: A contemporary meta-analysis. J. Vasc. Access 2020, 22, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Imasaki, M.; Shirano, M.; Shimizu, K.; Yagi, N.; Tsutsumi, M.; Yoshida, M.; Yoshimura, T.; Hayashi, Y.; Nakao, T.; et al. Peripherally inserted central venous catheters decrease central line-associated bloodstream infections and change microbiological epidemiology in adult hematology unit: A propensity score-adjusted analysis. Ann. Hematol. 2022, 101, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Wildgruber, M.; Lueg, C.; Borgmeyer, S.; Karimov, I.; Braun, U.; Kiechle, M.; Meier, R.; Koehler, M.; Ettl, J.; Berger, H. Polyurethane versus silicone catheters for central venous port devices implanted at the forearm. Eur. J. Cancer 2016, 59, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Seo, T.-S.; Song, M.G.; Cha, I.-H.; Kim, J.S.; Choi, C.W.; Seo, J.H.; Oh, S.C. Incidence and risk factors of infectious complications related to implantable venous-access ports. Korean J. Radiol. 2014, 15(4), 494–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kozlowski, K.M.; Jalaeian, H.; Travis, L.M.; Zikria, J.F. A comparative analysis of infection and complication rates between single- and double-lumen ports. Infect. Control Hosp. Epidemiol. 2024, 45, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, D.; Hansen, E.; Kreil, S.; Nolte, F.; Jawhar, M.; Hecht, A.; Hofmann, W.; Klein, S.A. The insertion site is the main risk factor for central venous catheter-related complications in patients with hematologic malignancies. Am. J. Hematol. 2022, 97, 303–310. [Google Scholar] [CrossRef]
- Snarski, E.; Stringer, J.; Mikulska, M.; Gil, L.; Tridello, G.; Bosman, P.; Lippinkhof, A.; Hoek, J.; Karas, M.; Zver, S.; et al. Risk of infectious complications in adult patients after allogeneic hematopoietic stem cell transplantation depending on the site of central venous catheter insertion—Multicenter prospective observational study, from the IDWP EBMT and Nurses Group of EBMT. Bone Marrow Transplant. 2021, 56, 2929–2933. [Google Scholar] [CrossRef]
- Hentrich, M.; Böll, B.; Teschner, D.; Panse, J.; Schmitt, T.; Naendrup, J.H.; Schmidt-Hieber, M.; Neitz, J.; Fiegle, E.; Schalk, E. Impact of the insertion site of central venous catheters on central venous catheter-related bloodstream infections in patients with cancer: Results from a large prospective registry. Infection 2023, 51, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/infection-control/media/pdfs/Strive-CLABSI103-508.pdf (accessed on 25 July 2025).
- Zochios, V.; Umar, I.; Simpson, N.; Jones, N. Peripherally Inserted Central Catheter (PICC)-Related Thrombosis in Critically Ill Patients. J. Vasc. Access 2014, 15, 329–337. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.C.; Gould, M.K. Preventing Complications of Central Venous Catheterization. N. Engl. J. Med. 2003, 348, 1123–1133. [Google Scholar] [CrossRef]
- Sapp, K.; Mahmoudjafari, Z.; Lutfi, F.; Leiker, B.; Cole, M. Challenging the Practice of Utilizing Central Venous Catheter Samples to Guide Tacrolimus Therapeutic Drug Monitoring Results. Biol. Blood Marrow Transplant. 2024, 30, S127–S128. [Google Scholar] [CrossRef]
- Miller, L.M.; Clark, E.; Dipchand, C.; Hiremath, S.; Kappel, J.; Kiaii, M.; Lok, C.; Luscombe, R.; Moist, L.; Oliver, M.; et al. Hemodialysis Tunneled Catheter-Related Infections. Can. J. Kidney Health Dis. 2016, 3. [Google Scholar] [CrossRef]
- Randolph, A.G.; Cook, D.J.; Gonzales, C.A.; Brun-Buisson, C. Tunneling short-term central venous catheters to prevent catheter-related infection. Crit. Care Med. 1998, 26, 1452–1457. [Google Scholar] [CrossRef]
- Saber, W.; Moua, T.; Williams, E.C.; Verso, M.; Agnelli, G.; Couban, S.; Young, A.; DE Cicco, M.; Biffi, R.; van Rooden, C.J.; et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: A patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J. Thromb. Haemost. 2011, 9, 312–319. [Google Scholar] [CrossRef]
- Flick, A.I.; Winters, R. Vascular Tunneled Central Catheter Access; StatPearls Publishing: Petersburg, FL, USA, 2024. [Google Scholar] [PubMed]
- Milczarek, S.; Kulig, P.; Piotrowska, O.; Zuchmanska, A.; Bielikowicz, A.; Machalinski, B. Catheter-related thrombosis in stem cell recipients: Comparison of different types of catheter. Haematologica 2024, 109, 1285–1288. [Google Scholar] [CrossRef]
- Baskin, J.L.; Pui, C.-H.; Reiss, U.; A Wilimas, J.; Metzger, M.L.; Ribeiro, R.C.; Howard, S.C. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169. [Google Scholar] [CrossRef]
- Murray, J.; Precious, E.; Alikhan, R. Catheter-related thrombosis in cancer patients. Br. J. Haematol. 2013, 162, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Levine, M.N.; Butler, G.; Webb, C.; Costantini, L.; Gu, C.; Julian, J.A. Incidence, Risk Factors, and Outcomes of Catheter-Related Thrombosis in Adult Patients With Cancer. J. Clin. Oncol. 2006, 24, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The Risk of Bloodstream Infection in Adults With Different Intravascular Devices: A Systematic Review of 200 Published Prospective Studies. Mayo Clin. Proc. 2006, 81, 1159–1171. [Google Scholar] [CrossRef]
- Bellesi, S.; Chiusolo, P.; De Pascale, G.; Pittiruti, M.; Scoppettuolo, G.; Metafuni, E.; Giammarco, S.; Sorà, F.; Laurenti, L.; Leone, G.; et al. Peripherally inserted central catheters (PICCs) in the management of oncohematological patients submitted to autologous stem cell transplantation. Support. Care Cancer 2013, 21, 531–535. [Google Scholar] [CrossRef]
- Baier, C.; Linke, L.; Eder, M.; Schwab, F.; Chaberny, I.F.; Vonberg, R.-P.; Ebadi, E. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLoS ONE 2020, 15, e0227772. [Google Scholar] [CrossRef]
- Boubekri, A. Reducing Central Line-Associated Bloodstream Infections in the Blood and Marrow Transplantation Population: A Review of the Literature. Clin. J. Oncol. Nurs. 2013, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ruesch, S.; Walder, B.; Tramèr, M.R. Complications of central venous catheters: Internal jugular versus subclavian access—A systematic review. Crit. Care Med. 2002, 30, 454–460. [Google Scholar] [CrossRef]
- Pittiruti, M.; Hamilton, H.; Biffi, R.; MacFie, J.; Pertkiewicz, M. Espen ESPEN Guidelines on Parenteral Nutrition: Central Venous Catheters (access, care, diagnosis and therapy of complications). Clin. Nutr. 2009, 28, 365–377. [Google Scholar] [CrossRef]
- Jeon, M.H.; Kim, C.S.; Han, K.D.; Kim, M.J. Efficacy and Safety of Midline Catheters with Integrated Wire Accelerated Seldinger Technique. Vasc. Spéc. Int. 2022, 38, 2. [Google Scholar] [CrossRef]
- Bell, T.; O’Grady, N.P. Prevention of Central Line–Associated Bloodstream Infections. Infect. Dis. Clin. N. Am. 2017, 31, 551–559. [Google Scholar] [CrossRef]
- Böll, B.; Schalk, E.; Buchheidt, D.; Hasenkamp, J.; Kiehl, M.; Kiderlen, T.R.; Kochanek, M.; Koldehoff, M.; Kostrewa, P.; Claßen, A.Y.; et al. Central venous catheter–related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 239–259. [Google Scholar] [CrossRef]
- Milczarek, S.; Kulig, P.; Piotrowska, O.; Zuchmańska, A.; Wilk-Milczarek, E.; Machaliński, B. Incidence of Catheter-Associated Bloodstream Infections in Stem Cell Recipients—Should We Be “PICCy”? Cancers 2024, 16, 1239. [Google Scholar] [CrossRef]
- Morano, S.G.; Latagliata, R.; Girmenia, C.; Massaro, F.; Berneschi, P.; Guerriero, A.; Giampaoletti, M.; Sammarco, A.; Annechini, G.; Fama, A.; et al. Catheter-associated bloodstream infections and thrombotic risk in hematologic patients with peripherally inserted central catheters (PICC). Support. Care Cancer 2015, 23, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Mariggiò, E.; Iori, A.P.; Micozzi, A.; Chistolini, A.; Latagliata, R.; Berneschi, P.; Giampaoletti, M.; La Rocca, U.; Bruzzese, A.; Barberi, W.; et al. Peripherally inserted central catheters in allogeneic hematopoietic stem cell transplant recipients. Support. Care Cancer 2020, 28, 4193–4199. [Google Scholar] [CrossRef]
- Dix, C.H.K.; Yeung, D.T.O.; Rule, M.L.; Ma, D.D.F. Essential, but at what risk? A prospective study on central venous access in patients with haematological malignancies. Intern. Med. J. 2012, 42, 901–906. [Google Scholar] [CrossRef]
- Kamphuisen, A.Y.L. Catheter-related thrombosis: Lifeline or a pain in the neck? Am. Soc. Hematol. 2012, 2012, 638–644. [Google Scholar] [CrossRef]
- Lee, A.Y.; Levine, M.N. Venous Thromboembolism and Cancer: Risks and Outcomes. Circulation 2003, 107, I-17–I-21. [Google Scholar] [CrossRef]
- Chopra, V.; O’HOro, J.C.; Rogers, M.A.M.; Maki, D.G.; Safdar, N. The Risk of Bloodstream Infection Associated with Peripherally Inserted Central Catheters Compared with Central Venous Catheters in Adults: A Systematic Review and Meta-Analysis. Infect. Control. Hosp. Epidemiol. 2013, 34, 908–918. [Google Scholar] [CrossRef]
- Fracchiolla, N.S.; Todisco, E.; Bilancia, A.; Gandolfi, S.; Mancini, V.; Marbello, L.; Bernardi, M.; Assanelli, A.; Orofino, N.; Cassin, R.; et al. Peripherally Inserted Central Catheters (PICCs) Implantation in the Clinical Management of Oncohematologic Patients: Results of a Large Multicenter, Retrospective Study of the REL Group (Rete Ematologica Lombarda - Lombardy Hematologic Network, Italy). Blood 2015, 126, 5611. [Google Scholar] [CrossRef]
- Adrian, M.; Borgquist, O.; Kröger, T.; Linné, E.; Bentzer, P.; Spångfors, M.; Åkeson, J.; Holmström, A.; Linnér, R.; Kander, T. Mechanical complications after central venous catheterisation in the ultrasound-guided era: A prospective multicentre cohort study. Br. J. Anaesth. 2022, 129, 843–850. [Google Scholar] [CrossRef]
- Lv, S.; Liu, Y.; Wei, G.; Shi, X.; Chen, S.; Zhang, X. The anticoagulants rivaroxaban and low molecular weight heparin prevent PICC-related upper extremity venous thrombosis in cancer patients. Medicine 2019, 98, e17894. [Google Scholar] [CrossRef]
- Lee, A.; Badgley, C.; Lo, M.; Banez, M.T.; Graff, L.; Damon, L.; Martin, T.; Dzundza, J.; Wong, M.; Olin, R. Evaluation of venous thromboembolism prophylaxis protocol in hematopoietic cell transplant patients. Bone Marrow Transplant. 2023, 58, 1247–1253. [Google Scholar] [CrossRef]
- Shih, Y.; Teng, C.J.; Chen, T.; Chang, K.; Chen, M. Dual-lumen power injectable peripherally inserted central catheters in allogeneic hematopoietic stem cell transplantation: A prospective observational study. J. Clin. Nurs. 2022, 31, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yang, J.; Song, L.; Jiang, Y.; Liu, Y. Comparison of three types of central venous catheters in patients with malignant tumor receiving chemotherapy. Patient Prefer. Adherence 2017, ume 11, 1197–1204. [Google Scholar] [CrossRef]
- Chen, Y.B.; Bao, H.S.; Hu, T.T.; He, Z.; Wen, B.; Liu, F.T.; Su, F.X.; Deng, H.R.; Wu, J.N. Comparison of comfort and complications of Implantable Venous Access Port (IVAP) with ultrasound guided Internal Jugular Vein (IJV) and Axillary Vein/Subclavian Vein (AxV/SCV) puncture in breast cancer patients: A randomized controlled study. BMC Cancer 2022, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.W.; A Crowther, M.; Jamula, E.; El-Sharkawy, R.; Brown, M.; Paterson, G.; Lui, M.; Don-Wauchope, A.C. Assessment of the Measurement Error in Cyclosporine Levels Drawn Between Peripheral and Central Sources. Am. J. Clin. Pathol. 2017, 149, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.G.; Babu, M.C.S.; Lokanatha, D.; Bhat, G.R. Outcomes, cost comparison, and patient satisfaction during long-term central venous access in cancer patients: Experience from a Tertiary Care Cancer Institute in South India. Indian J. Med Paediatr. Oncol. 2016, 37, 232–238. [Google Scholar] [CrossRef]
| Catheter Type | Peripherally Inserted Central Catheters (PICCs) | Tunneled Central Venous Access Devices | Totally Implantable Venous Access Device | Centrally Inserted Central Catheters (CICCs) |
|---|---|---|---|---|
| Description | Inserted into a peripheral vein and advanced to the central venous system. | Surgically implanted with a portion tunneled subcutaneously before entering a central vein. | A port implanted under the skin connected to a catheter placed in a central vein. | Inserted directly into a central vein (e.g., subclavian or jugular). |
| Advantages | 1. Minimally invasive 2. No significant adverse events upon insertion 3. Suitable for long-term use 4. Facilitates multidrug treatment and TPN administration | 1. Lower infection rates compared to non-tunneled catheters 2. Suitable for long-term use 3. Facilitates multidrug treatment and TPN administration | 1. Lower maintenance 2. Reduced infection rates 3. Suitable for long-term use | 1. Rapid access 2. Suitable for short-term use 3. Facilitates multidrug treatment and TPN administration 4. Accurate immunosuppression levels |
| Disadvantages | 1. Increased risk of thrombosis and occlusion 2. Potential for catheter dislodgement 3. Falsely elevated immunosuppression levels | 1. Requires surgical placement and removal 2. Can be uncomfortable for patients 3. Difficult to remove in case of infection | 1. Requires surgical insertion and removal 2. Potential for needle dislodgement 3. Difficult to remove in case of infection 4. PN/TPN feasible with a dedicated lumen and standardized maintenance; occlusion risk may increase if flushing/locking is suboptimal [5,6,7] | 1. Highest risk of serious mechanical complications upon implantation 2. Higher risk of infection 3. Less comfortable for patients |
| Clinical Determinant | Comparator/Setting | Outcome (Unit) | Effect Size/Absolute Rate | Population/Evidence Tag | Key Sources |
|---|---|---|---|---|---|
| PICC vs. CICC | Contemporary practice (meta-analysis) | CLABSI (per 1000 catheter-days/IRR) | IRR 0.52 (95% CI 0.30–0.92)—PICC vs. CICC ↓ infection | Indirect (mixed hospital/oncology) | Schears et al. 2020 [28] |
| PICC vs. CICC | Adult hematology ward (propensity-adjusted) | CLABSI (per 1000 catheter-days) | PICC 3.29 vs. CICC 5.11; HR 0.48 (95% CI 0.31–0.75) | Indirect (hematology) | Nakaya 2022 [29] |
| PICC vs. CICC | Contemporary practice (meta-analysis) | Venous thrombosis (CRT/DVT) | RR 2.08 (95% CI 1.47–2.94)—↑ with PICC; attenuation with single-lumen & ≤4–5 Fr | Indirect (mixed) | Schears et al. 2020[28] |
| Ports (TIVAD)—material | PU vs. SI (forearm ports, large cohort) | Infection/thrombosis; mechanical failure | Complications 1.8 (PU) vs. 0.3 (SI)/1000 cath-days; explantation 10.6% PU vs. 4.6% SI; SI ↑ mechanical failures | Indirect (oncology) | Wildgruber et al. 2016 [30] |
| Ports (TIVAD)—benchmark | Long-term use | CLABSI (per 1000 catheter-days) | Typowo 0.06–0.30 | Indirect (oncology) | Walser 2012 [9] ; Shim 2014 [31] |
| Ports—number of lumens | Double- vs. single-lumen | Bloodstream infection and dysfunction | HR 2.98 (95% CI 1.12–7.94) for bacteremia (double vs. single); ↑ malfunction/fibrin sheath with double | Indirect (oncology) | Kozlowski et al. 2024 [32] |
| CICC—insertion site | SCV vs. IJV in hematologic malignancies | CRBSI/CLABSI | CRBSI 1.2 (SCV) vs. 5.7 (IJV)/1000 cath-days; CLABSI 8% vs. 26%; IJV risk ↑ (HR 5.4 for CRBSI) | Indirect (hematology) | Heidenreich et al. 2022 [33] |
| CICC—insertion site (HSCT) | IJV vs. SCV in allo-HSCT | Infectious complications | OR 2.03 (95% CI 1.01–4.06)—IJV vs. SCV | HSCT-specific | Snarski et al. 2021 [34] |
| CICC—femoral site | Femoral vs. IJV/SCV (matched analysis) | CRBSI | 5.7 vs. 14.2/1000 cath-days; no significant difference short-term | Indirect (oncology) | Hentrich et al. 2023 [35] |
| Antimicrobial strategies | CHG-alcohol vs. povidone-iodine; antimicrobial/silver-coated CVCs | CLABSI/CRBSI | RR 0.51 (95% CI 0.27–0.97) for CHG vs. PI; antimicrobial coatings reduce CLABSI in networks/RCTs | Indirect (ICU/oncology) | CDC/CLEAN trial [36]; Wang 2018 et al. [25] |
| Catheter-to-vein ratio | ≤45% vs. >45% (upper-arm veins) | CRT | Guideline threshold ≤45% to mitigate CRT; quantitative effect varies by cohort/design | Indirect (standards/ICU) | Gorski et al. 2021 [6]; Zochios et al. 2014 [37] |
| Determinant | Key Point | Population | Evidence Tag | Sources |
|---|---|---|---|---|
| PICC vs. CICC—CLABSI | IRR~0.52 (PICC ↓ infection) | Oncology | Indirect | [28] |
| PICC vs. CICC—CRT | RR~2.0; attenuates with ≤4 Fr, single-lumen | Oncology | Indirect | [28,64] |
| PICC CRT in HSCT | ~9% (allo-HSCT) | HSCT | HSCT-specific | [59] |
| CVR | ≤45% threshold reduces CRT | Practice | Indirect | [6] |
| Bundle (USG + CVR) | CRT ≈ CICC after implementation | HSCT | HSCT-specific | [44] |
| 2024 Consensus | Smallest size, minimal lumens; no HSCT-specific CVR | Oncology | Indirect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milczarek, S.; Kulig, P.; Piotrowska, O.; Zuchmańska, A.; Brzosko, M.; Machaliński, B. Vascular Access Devices for Stem Cell Transplantation: A Review of Catheter Types—A Crucial Step Towards the Enhancement of Patient Care. Cancers 2025, 17, 3325. https://doi.org/10.3390/cancers17203325
Milczarek S, Kulig P, Piotrowska O, Zuchmańska A, Brzosko M, Machaliński B. Vascular Access Devices for Stem Cell Transplantation: A Review of Catheter Types—A Crucial Step Towards the Enhancement of Patient Care. Cancers. 2025; 17(20):3325. https://doi.org/10.3390/cancers17203325
Chicago/Turabian StyleMilczarek, Sławomir, Piotr Kulig, Oliwia Piotrowska, Alina Zuchmańska, Martyna Brzosko, and Bogusław Machaliński. 2025. "Vascular Access Devices for Stem Cell Transplantation: A Review of Catheter Types—A Crucial Step Towards the Enhancement of Patient Care" Cancers 17, no. 20: 3325. https://doi.org/10.3390/cancers17203325
APA StyleMilczarek, S., Kulig, P., Piotrowska, O., Zuchmańska, A., Brzosko, M., & Machaliński, B. (2025). Vascular Access Devices for Stem Cell Transplantation: A Review of Catheter Types—A Crucial Step Towards the Enhancement of Patient Care. Cancers, 17(20), 3325. https://doi.org/10.3390/cancers17203325






