TCN1 Drives Malignant Progression of Pancreatic Cancer Through STAT4-Mediated Transcriptional Activation of the DUOX2/ROS Signaling Axis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Patient Cohort and Specimen Collection
3. Results
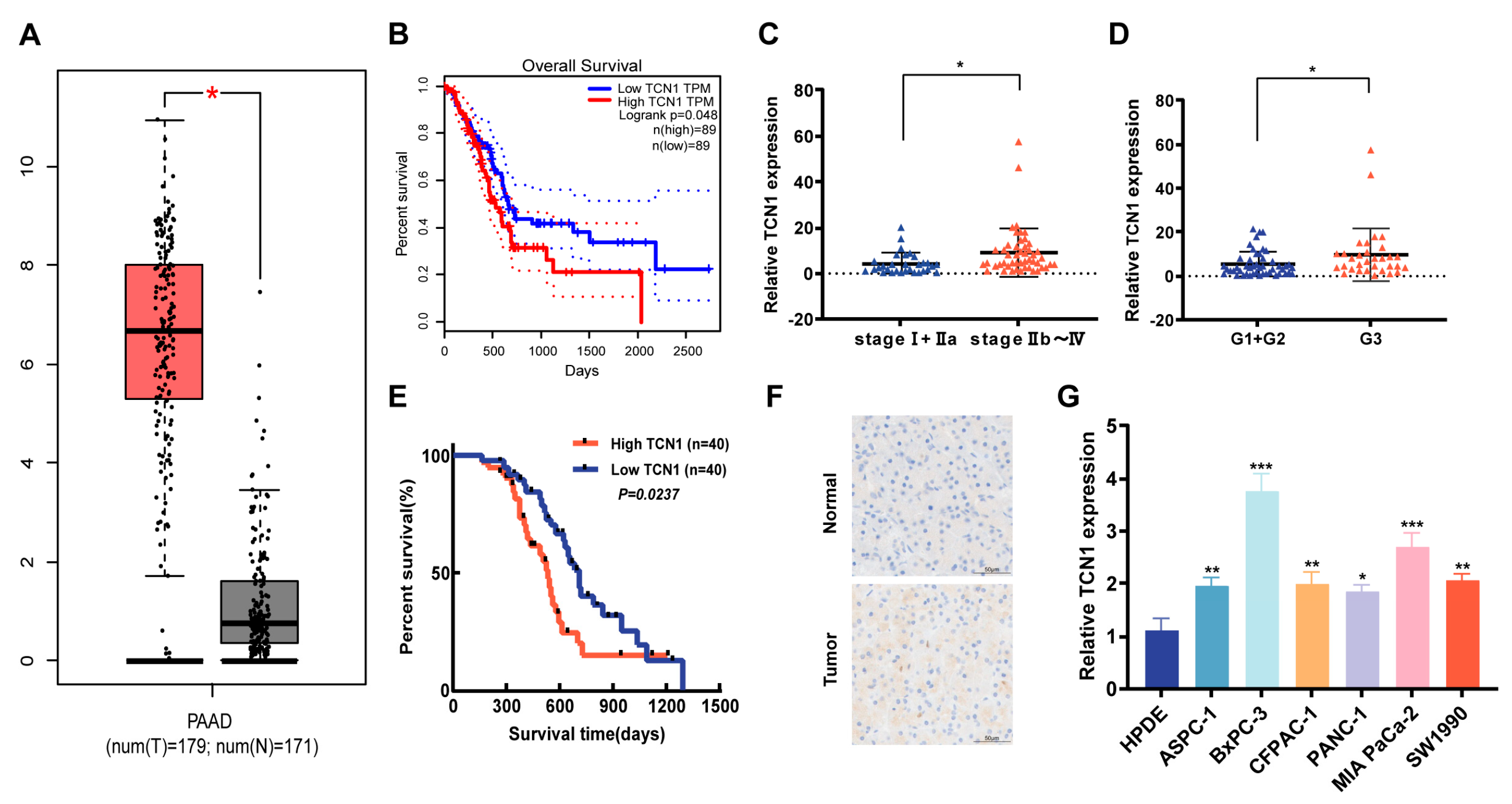
3.1. Expression Characteristics and Clinical Significance of TCN1 in Pancreatic Cancer
3.2. Regulatory Role of TCN1 in Pancreatic Cancer Cell Proliferation
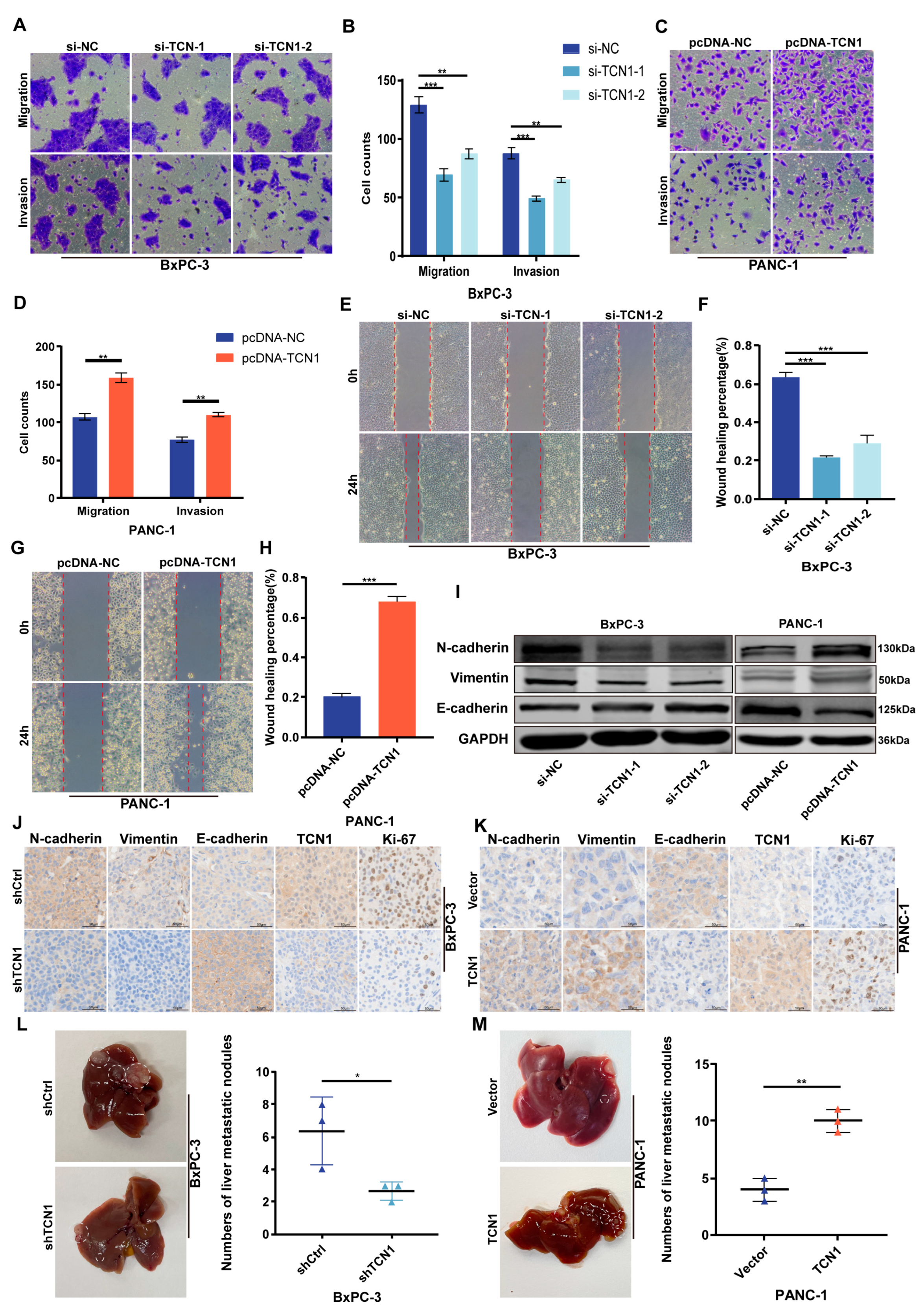
3.3. TCN1 Promotes Migration, Invasion, and EMT Progression in Pancreatic Cancer
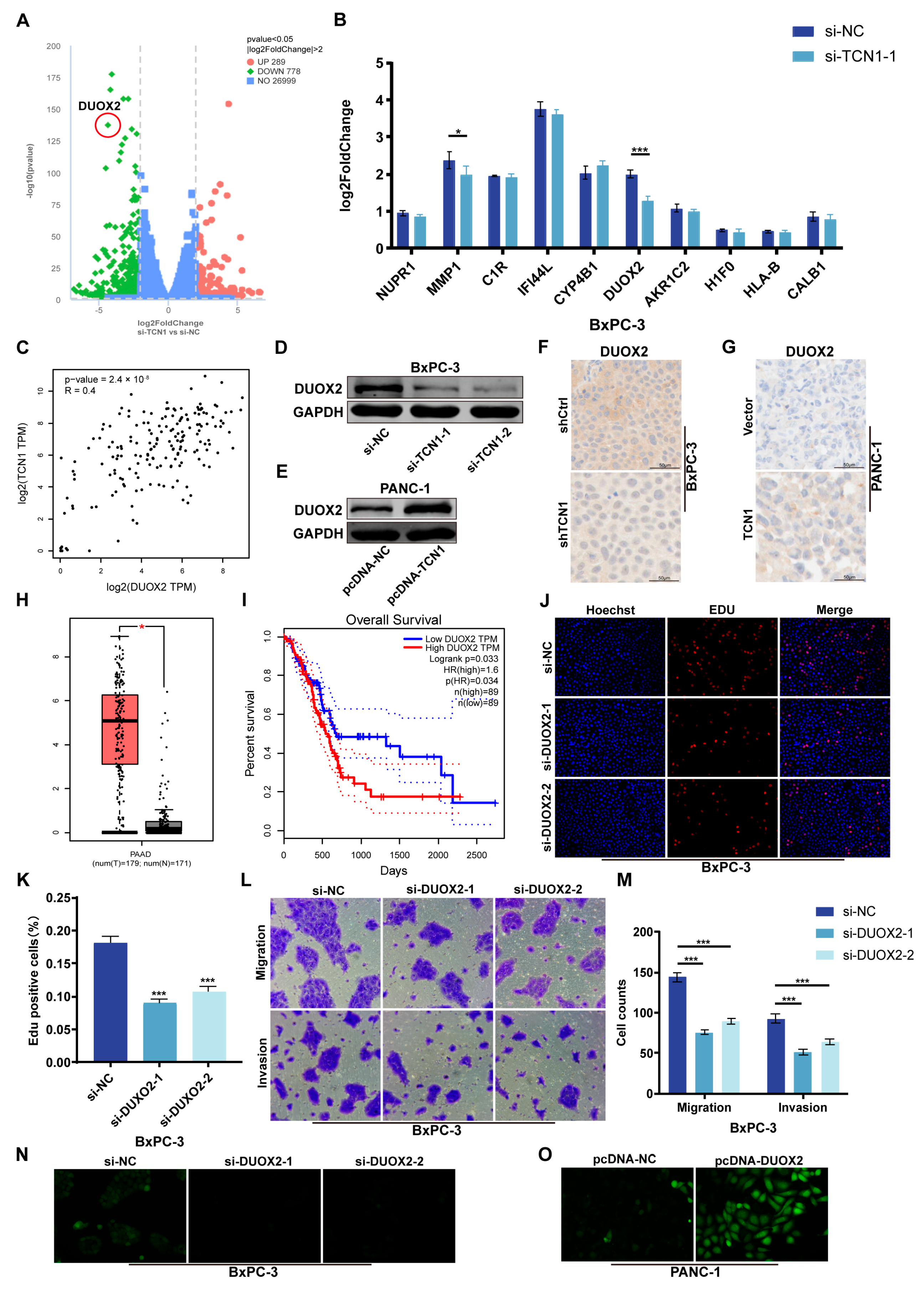
3.4. DUOX2 Is Transcriptionally Regulated by TCN1 and Drives Malignant Phenotypes in Pancreatic Cancer
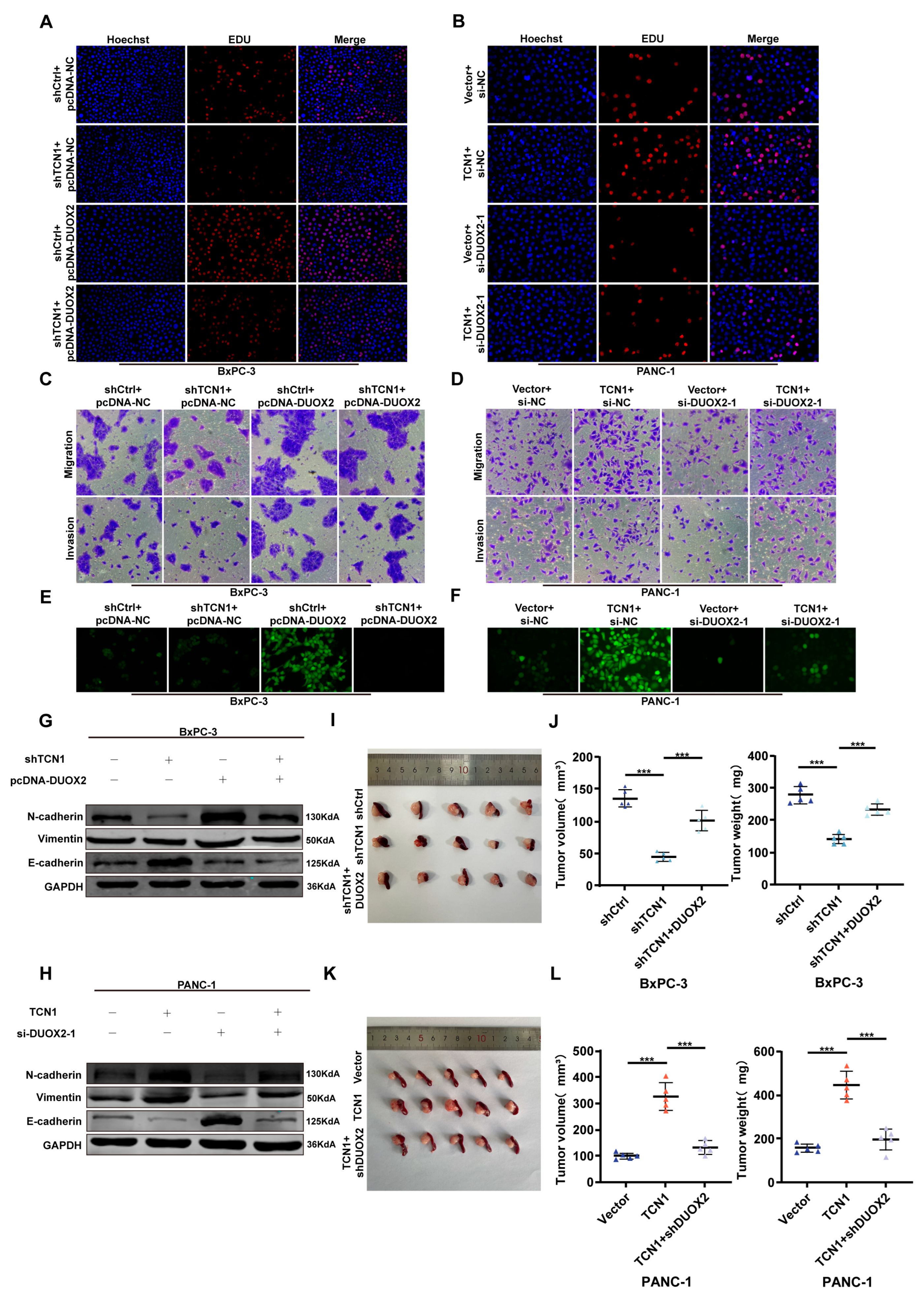
3.5. TCN1 Promotes Pancreatic Cancer Malignancy by Activating DUOX2
3.6. TCN1 Binds and Regulates Transcription Factor STAT4 to Promote DUOX2 Transcription
3.7. TCN1/STAT4/DUOX2 Axis Drives Pancreatic Cancer Progression Through ROS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, H.; Wu, L.; Lai, Z.; Geng, D.; Yang, W.; Zhang, J.; Fan, Z.; Qin, W.; Wang, Y.; et al. O-GlcNAcylation promotes pancreatic tumor growth by regulating malate dehydrogenase 1. Nat. Chem. Biol. 2022, 18, 1087–1095. [Google Scholar] [CrossRef]
- Zhu, X.G.; Chudnovskiy, A.; Baudrier, L.; Prizer, B.; Liu, Y.; Ostendorf, B.N.; Yamaguchi, N.; Arab, A.; Tavora, B.; Timson, R.; et al. Functional genomics in vivo reveal metabolic dependencies of pancreatic cancer cells. Cell Metab. 2021, 33, 211–221.e6. [Google Scholar] [CrossRef]
- Sherman, M.H.; Beatty, G.L. Tumor microenvironment in pancreatic cancer pathogenesis and therapeutic resistance. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 123–148. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer-Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Niu, N.; Shen, X.; Wang, Z.; Chen, Y.; Weng, Y.; Yu, F.; Tang, Y.; Lu, P.; Liu, M.; Wang, L.; et al. Tumor cell-intrinsic epigenetic dysregulation shapes can-cer-associated fibroblasts heterogeneity to metabolically support pancreatic cancer. Cancer Cell 2024, 42, 869–884.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immu-notherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Lao, M.; Sun, K.; He, L.; Xu, J.; Duan, Y.; Chen, Y.; Ying, H.; Li, M.; et al. Targeting ubiquitin-specific protease 8 sensitizes anti-programmed death-ligand 1 immunotherapy of pancreatic cancer. Cell Death Differ. 2023, 30, 560–575. [Google Scholar] [CrossRef]
- Ge, W.; Yue, M.; Lin, R.; Zhou, T.; Xu, H.; Wang, Y.; Mao, T.; Li, S.; Wu, X.; Zhang, X.; et al. PLA2G2A+ cancer-associated fibroblasts mediate pancreatic cancer immune escape via impeding antitumor immune response of CD8+ cytotoxic T cells. Cancer Lett. 2023, 558, 216095. [Google Scholar] [CrossRef] [PubMed]
- Rather, G.M.; Pramono, A.A.; Szekely, Z.; Bertino, J.R.; Tedeschi, P.M. In cancer, all roads lead to NADPH. Pharmacol. Ther. 2021, 226, 107864. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.-H.; Chen, Z.-S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Update. 2018, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Park, M.T.; Kim, M.J.; Suh, Y.; Kim, R.K.; Kim, H.; Lim, E.J.; Yoo, K.-C.; Lee, G.-H.; Kim, Y.-H.; Hwang, S.-G.; et al. Novel signaling axis for ROS generation during K-Ras-induced cellular transformation. Cell Death Differ. 2014, 21, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wu, Z.; Huang, Z.; Hao, X.; Wang, S.; Deng, J.; Yin, Y.; Tan, C. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 2021, 45, 102051. [Google Scholar] [CrossRef]
- Shinohara, M.; Adachi, Y.; Mitsushita, J.; Kuwabara, M.; Nagasawa, A.; Harada, S.; Furuta, S.; Zhang, Y.; Seheli, K.; Miyazaki, H.; et al. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J. Biol. Chem. 2010, 285, 4481–4488. [Google Scholar] [CrossRef]
- Mori, K.; Uchida, T.; Yoshie, T.; Mizote, Y.; Ishikawa, F.; Katsuyama, M.; Shibanuma, M. A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS J. 2019, 286, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Nieborowska-Skorska, M.; Kopinski, P.K.; Ray, R.; Hoser, G.; Ngaba, D.; Flis, S.; Cramer, K.; Reddy, M.M.; Koptyra, M.; Penserga, T.; et al. Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors. Blood 2012, 119, 4253–4263. [Google Scholar] [CrossRef]
- Radisky, D.C.; Levy, D.D.; Littlepage, L.E.; Liu, H.; Nelson, C.M.; Fata, J.E.; Leake, D.; Godden, E.L.; Albertson, D.G.; Nieto, M.A.; et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005, 436, 123–127. [Google Scholar] [CrossRef]
- Chandra, V.; Li, L.; Le Roux, O.; Zhang, Y.; Howell, R.M.; Rupani, D.N.; Baydogan, S.; Miller, H.D.; Riquelme, E.; Petrosino, J.; et al. Gut epithelial interleukin-17 receptor A signaling can modulate distant tumors growth through microbial regulation. Cancer Cell 2024, 42, 85–100.e6. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Fritsch, J.; González, E.E.; Landau, K.S.; Santander, A.M.; Fernández, I.; Hazime, H.; Davies, J.M.; Santaolalla, R.; Phillips, M.C.; et al. Epithelial TLR4 signaling activates DUOX2 to induce microbiota-driven tumorigenesis. Gastroenterology 2021, 60, 797–808.e6. [Google Scholar] [CrossRef]
- Kang, K.A.; Ryu, Y.S.; Piao, M.J.; Shilnikova, K.; Kang, H.K.; Yi, J.M.; Boulanger, M.; Paolillo, R.; Bossis, G.; Yoon, S.Y.; et al. DUOX2-mediated production of reactive oxygen species induces epithelial mesenchymal transition in 5-fluorouracil resistant human colon cancer cells. Redox Biol. 2018, 17, 224–235. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Feng, L.; Zhi, L.; Jiang, D.; Yu, B.; Zhang, Z.; Gao, B.; Zhang, C.; Li, M.; et al. DUOX2 promotes the progression of colorectal cancer cells by regulating the AKT pathway and interacting with RPL3. Carcinogenesis 2021, 42, 105–117. [Google Scholar] [CrossRef]
- Parekh, P.R.; Solano-Gonzalez, E.; Martins, M.B.; Ma, X.; Tighe, K.; Casildo, A.; Zodda, A.; Johnstone, C.; Poirier, Y.; Mahmood, J.; et al. DUOX2, a new biomarker for disseminated gastric Cancer’s response to low dose radiation in mice. Cancers 2021, 13, 4186. [Google Scholar] [CrossRef]
- Lacombe, V.; Lenaers, G.; Urbanski, G. Diagnostic and therapeutic perspectives associated to cobalamin-dependent me-tabolism and transcobalamins’ synthesis in solid cancers. Nutrients 2022, 14, 2058. [Google Scholar] [CrossRef]
- Joslin, A.C.; Green, R.; German, J.B.; Lange, M.C. Concept mapping One-Carbon Metabolism to model future ontologies for nutrient-gene-phenotype interactions. Genes Nutr. 2014, 9, 419. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Zhang, Q.; Huang, H.; Shi, X.; Hou, D.; Xing, C. TCN1 deficiency inhibits the malignancy of colorectal cancer cells by regulating the ITGB4 pathway. Gut Liver 2023, 17, 412–429. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Guéant, J.L.; Caillerez-Fofou, M.; Battaglia-Hsu, S.; Alberto, J.M.; Freund, J.N.; Dulluc, I.; Adjalla, C.; Maury, F.; Merle, C.; Nicolas, J.-P.; et al. Molecular and cellular effects of vitamin B12 in brain, myocardium and liver through its role as co-factor of methionine synthase. Biochimie 2013, 95, 1033–1040. [Google Scholar] [CrossRef]
- Kovatcheva, M.; Melendez, E.; Chondronasiou, D.; Pietrocola, F.; Bernad, R.; Caballe, A.; Junza, A.; Capellades, J.; Holguín-Horcajo, A.; Prats, N.; et al. Vitamin B12 is a limiting factor for induced cellular plasticity and tissue repair. Nat. Metab. 2013, 5, 1911–1930. [Google Scholar] [CrossRef]
- Deng, M.; Ran, P.; Chen, L.; Wang, Y.; Yu, Z.; Cai, K.; Feng, J.; Qin, Z.; Yin, Y.; Tan, S.; et al. Proteogenomic characterization of cholangiocarcinoma. Hepatology 2023, 77, 411–429. [Google Scholar] [CrossRef]
- Liu, G.J.; Wang, Y.J.; Yue, M.; Zhao, L.M.; Guo, Y.D.; Liu, Y.P.; Yang, H.-C.; Liu, F.; Zhang, X.; Zhi, L.-H.; et al. High expression of TCN1 is a negative prognostic biomarker and can predict neoadjuvant chemosensitivity of colon cancer. Sci. Rep. 2020, 10, 11951. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial mesenchymal transition in tumor metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, F.; Wang, L.; Peng, C.; Huang, R.; Peng, F. STAT4 facilitates PD-L1 level via IL-12R/JAK2/STAT3 axis and predicts immunotherapy response in breast cancer. MedComm 2023, 4, e464. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Moriarty, A.K.; De Melo, P.; Keeter, W.C.; Alakhras, N.S.; Nelson, A.S.; Hoover, M.; Barrios, M.S.; Nadler, J.L.; Serezani, C.H.; et al. STAT4 is expressed in neutrophils and promotes antimicrobial immunity. JCI Insight 2021, 6, e141326. [Google Scholar] [CrossRef]
- Pileckaite, E.; Vilkeviciute, A.; Kriauciuniene, L.; Liutkevicius, V.; Liutkeviciene, R. Investigating the link between STAT4 genetic variants, STAT4 protein concentrations, and laryngeal squamous cell carcinoma: A comprehensive analysis of clinical manifestations. Int. J. Mol. Sci. 2024, 25, 10180. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Tuveson, D.A. ROS in cancer: The burning question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Niu, Z.S.; Wang, W.H.; Niu, X.J. Recent progress in molecular mechanisms of postoperative recurrence and metastasis of hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 6433–6477. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-cadherin and N-cadherin switch in epi-thelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.N. Thermodynamic aspects and reprogramming cellular energy metabolism during the fibrosis process. Int. J. Mol. Sci. 2017, 18, 2537. [Google Scholar] [CrossRef]



| Clinical Characteristic | Total | TCN1 Expression | p Value | |
|---|---|---|---|---|
| Low (40) | High (40) | |||
| Age (years) | ||||
| <60 | 32 | 14 | 18 | 0.361 |
| ≥60 | 48 | 26 | 22 | |
| Gender | ||||
| Male | 44 | 23 | 21 | 0.653 |
| Female | 36 | 17 | 19 | |
| TNM stage | ||||
| Ⅰ+Ⅱa | 31 | 21 | 10 | 0.012 * |
| Ⅱb+Ⅲ | 49 | 19 | 30 | |
| Nodal metastasis | ||||
| Yes | 45 | 20 | 25 | 0.259 |
| No | 35 | 20 | 15 | |
| pathologic stage | ||||
| G1+G2 | 48 | 29 | 19 | 0.022 * |
| G3 | 32 | 11 | 21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ju, D.; Yu, Z.; Zhang, B.; Xue, D.; Wang, Y. TCN1 Drives Malignant Progression of Pancreatic Cancer Through STAT4-Mediated Transcriptional Activation of the DUOX2/ROS Signaling Axis. Cancers 2025, 17, 3300. https://doi.org/10.3390/cancers17203300
Liu Z, Ju D, Yu Z, Zhang B, Xue D, Wang Y. TCN1 Drives Malignant Progression of Pancreatic Cancer Through STAT4-Mediated Transcriptional Activation of the DUOX2/ROS Signaling Axis. Cancers. 2025; 17(20):3300. https://doi.org/10.3390/cancers17203300
Chicago/Turabian StyleLiu, Zonglin, Dongxue Ju, Ze Yu, Binru Zhang, Dongbo Xue, and Yongwei Wang. 2025. "TCN1 Drives Malignant Progression of Pancreatic Cancer Through STAT4-Mediated Transcriptional Activation of the DUOX2/ROS Signaling Axis" Cancers 17, no. 20: 3300. https://doi.org/10.3390/cancers17203300
APA StyleLiu, Z., Ju, D., Yu, Z., Zhang, B., Xue, D., & Wang, Y. (2025). TCN1 Drives Malignant Progression of Pancreatic Cancer Through STAT4-Mediated Transcriptional Activation of the DUOX2/ROS Signaling Axis. Cancers, 17(20), 3300. https://doi.org/10.3390/cancers17203300







