The Prognostic Role of Geriatric Nutritional Risk Index in Periampullary Cancer Patients Undergoing Pancreaticoduodenectomy: A Propensity Score-Matched Survival Study
Abstract
Simple Summary
Abstract
1. Introduction
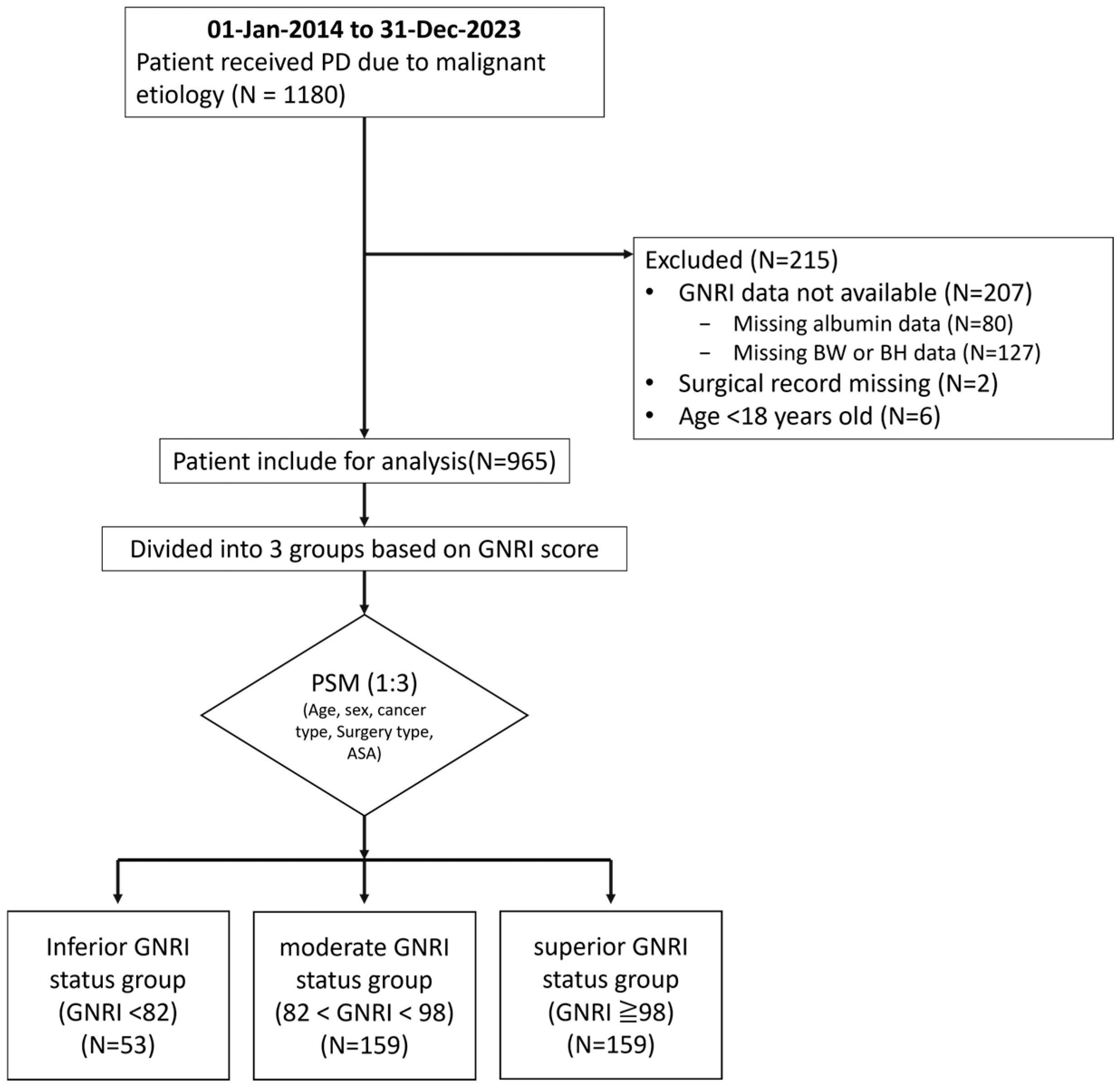
2. Materials and Methods
2.1. Patient
2.2. Nutritional Assessment Using GNRI
2.3. Outcome Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Association Between Different GNRI Groups and Short-Term Outcomes
3.3. Association Between Different GNRI Groups and Survival
3.3.1. Association Between GNRI and Survival
3.3.2. Subgroup Analysis: Pancreatic Cancer vs. Other Malignancies
3.4. Univariate and Multivariate Cox Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Pancreaticoduodenectomy |
| GNRI | Geriatric Nutritional Risk Index |
| OS | Overall Survival |
| RFS | Recurrence-Free Survival |
| HR | Hazard ratio |
| CI | Confidence Interval |
| MST | Median Survival Time |
| ACT | Adjuvant chemotherapy |
| NACT | Neoadjuvant chemotherapy |
| LN | Lymph node |
| ICU | Intensive Care Unit |
| TPN | Total Parenteral Nutrition |
| ASA | American Society of Anesthesiologists |
| AJCC | American Joint Committee on Cancer |
| PSM | Propensity Score Matching |
| CGRD | Chang Gung Research Database |
Appendix A
| Cohort (N = 1203) | Match (N = 371) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Inferior GNRI < 82 (n = 67) | Moderate 82 ≤ GNRI < 98 (n = 544) | Superior GNRI ≥ 98 (n = 592) | p-Value | Inferior GNRI < 82 (n = 53) | Moderate 82 ≤ GNRI < 98 (n = 159) | Superior GNRI ≥ 98 (n = 159) | p-Value |
| Tumor Type | ||||||||
| Pancreatic cancer, n (%) | 16 (23.88%) | 299 (42.1%) | 242 (40.88%) | 0.0158 | 16 (30.19%) | 48 (30.19%) | 48 (30.19%) | 1 |
| Distal CBD cancer, n (%) | 12 (17.91%) | 76 (13.97%) | 61 (10.3%) | 0.0637 | 12 (22.64%) | 37 (23.27%) | 34 (21.38%) | 0.9206 |
| Ampullary cancer, n (%) | 22 (32.84%) | 140 (25.74%) | 106 (17.91%) | 0.0007 | 22 (41.51%) | 63 (39.62%) | 63 (39.62%) | 0.9668 |
| Duodenal/gastric cancer, n (%) | 3 (4.48%) | 25 (4.6%) | 33 (5.57%) | 0.7349 | 3 (5.66%) | 11 (6.92%) | 14 (8.81%) | 0.6973 |
| Benign, n (%) | 14 (20.9%) | 74 (13.6%) | 150 (25.34%) | <0.0001 | ||||
| Age, year ± SD | 67.06 ± 13.62 | 66.08 ± 10.58 | 60.89 ± 11.69 | <0.0001 | 69.81 ± 10.26 | 68.03 ± 10.11 | 65.82 ± 10.29 | 0.9834 |
| Gender | 0.1015 | 0.904 | ||||||
| Male, n (%) | 39 (58.21%) | 330 (60.66%) | 322 (54.39%) | 28 (52.83%) | 82 (51.57%) | 86 (54.09%) | ||
| Female, n (%) | 28 (41.79%) | 214 (39.34%) | 270 (45.61%) | 25 (47.17%) | 77 (48.43%) | 73 (45.91%) | ||
| BMI, mean ± SD | 21.37 ± 2.29 | 22.21 ± 2.47 | 22.85 ± 2.72 | <0.0001 | 21.5 ± 2.48 | 22.33 ± 2.49 | 23.02 ± 2.63 | 0.0004 |
| Pre-OP biliary drainage, n (%) | 31 (46.27%) | 274 (50.37%) | 176 (29.73) | <0.0001 | 28 (52.83%) | 90 (56.6%) | 65 (40.88%) | 0.0169 |
| Underlying disease | ||||||||
| HTN, n (%) | 23 (34.33%) | 187 (34.38%) | 175 (29.56%) | 0.2023 | 19 (35.85%) | 68 (42.77%) | 55 (34.59%) | 0.3007 |
| DM, n (%) | 22 (32.84%) | 152 (27.94%) | 163 (27.53%) | 0.6565 | 20 (37.74%) | 51 (32.08%) | 44 (27.67%) | 0.3618 |
| Lab data, mean ± SD | ||||||||
| CA19-9 (ng/mL), mean ± SD | 1201.93 ± 4828.04 | 648.88 ± 2571.32 | 306.85 ± 1563.45 | <0.0001 | 1284.97 ± 5067.47 | 660.36 ± 2177.19 | 492.14 ± 2798.76 | <0.0001 |
| ALB (g/dL), mean ± SD | 2.5 ± 0.35 | 3.4 ± 0.3 | 4.24 ± 0.3 | <0.0001 | 2.53 ± 0.32 | 3.37 ± 0.3 | 4.17 ± 0.28 | <0.0001 |
| T-BIL (mg/dL), mean ± SD | 3.36 ± 3.64 | 3.4 ± 3.91 | 1.81 ±2.66 | <0.0001 | 3.44 ± 3.81 | 3.46 ± 3.92 | 2.21 ± 3.3 | <0.0001 |
| Surgery Type | 0.1979 | |||||||
| Open, n (%) | 525 (96.51%) | 599 (94.43%) | 52 (98.11%) | 155 (97.48%) | 155 (97.48%) | 0.8207 | ||
| MIS, n (%) | 19 (3.49%) | 33 (5.57%) | 1 (1.89%) | 4 (2.52%) | 4 (2.52%) | |||
| ASA physical status | 0.0017 | 0.9551 | ||||||
| I–II, n (%) | 12 (17.91%) | 106 (19.49%) | 166 (28.04%) | 10 (18.87%) | 32 (20.13%) | 30 (18.87%) | ||
| III–IV, n (%) | 55 (82.09%) | 438 (80.51%) | 426 (71.96%) | 43 (81.13%) | 127 (79.87%) | 129 (81.13%) | ||
| OP time (minutes), mean ± SD | 520.64 ± 147.07 | 525.75 ± 134.98 | 517.96 ± 145.16 | 0.2332 | 524.95 ± 146.68 | 515.29 ± 132.66 | 511.52 ± 155.08 | 0.5163 |
| OP blood loss (mL), mean ± SD | 791.42 ± 982.41 | 549.52 ± 836.49 | 420.24 ± 581.51 | <0.0001 | 604.34 ± 688.43 | 492.58 ± 861.86 | 473.02 ± 733.84 | 0.0577 |
| LN number, mean ± SD | 19.35 ± 11.48 | 19.87 ± 10.22 | 19.19 ± 10.39 | 0.5356 | 19.36 ± 11.65 | 20.01 ± 10.81 | 19.42 ± 10.54 | 0.7468 |
| positive LN number, mean ± SD | 1.96 ± 3.60 | 1.90 ± 2.49 | 1.85 ± 2.95 | 0.3376 | 1.9 ± 3.66 | 1.46 ± 2.23 | 1.33 ± 2.18 | 0.5032 |
| Tumor size (mm), mean ± SD | 33.10 ± 23.57 | 31.97 ± 18.26 | 30.04 ± 16.27 | 0.256 | 32.9 ± 23.92 | 32.97 ± 23.04 | 28.09 ± 14.66 | 0.1799 |
| Tumor margin | 0.1131 | 0.7125 | ||||||
| R0 resection, n (%) | 58 (86.57%) | 444 (81.62%) | 509 (85.98%) | 44 (83.02%) | 138 (86.79%) | 139 (87.42%) | ||
| Not R0 resection, n (%) | 9 (13.43%) | 100 (18.38%) | 83 (14.02%) | 9 (16.98%) | 21 (13.21%) | 20 (12.58%) | ||
| Pathological staging | 0.014 | 0.192 | ||||||
| Stage I | 10 (14.93%) | 87 (15.99%) | 108 (18.24%) | 10 (18.87%) | 29 (18.24%) | 40 (25.16%) | ||
| Stage II | 29 (43.28%) | 240 (44.12%) | 193 (32.60%) | 28 (52.83%) | 77 (48.43%) | 65 (40.88%) | ||
| Stage III | 7 (10.45%) | 109 (20.04%) | 79 (13.34%) | 6 (11.32%) | 31 (19.50%) | 27 (16.98%) | ||
| Stage IV | 4 (5.97%) | 11 (2.02%) | 19 (3.21%) | 4 (7.55%) | 3 (1.89%) | 6 (3.77%) | ||
| Perioperative chemotherapy in 3 months | ||||||||
| NACT | 2 (2.99%) | 11 (2.02%) | 30 (5.07%) | 0.021 | 1 (1.89%) | 2 (1.26%) | 7 (4.40%) | 0.207 |
| ACT | 16 (23.88%) | 212 (38.97%) | 214 (36.15) | 0.049 | 14 (26.42%) | 60 (37.74%) | 48 (30.19%) | 0.2 |
| Cohort (n = 1203) | Match (n = 371) | |||||||
|---|---|---|---|---|---|---|---|---|
| Prognostic Outcomes | GNRI < 82 (n = 67) | 82 ≤ GNRI < 98 (n = 544) | GNRI ≥ 98 (n = 592) | p-Value | GNRI < 82 (n = 53) | 82 ≤ GNRI < 98 (n = 159) | GNRI ≥ 98 (n = 159) | p-Value |
| Post-operative stays, day(s) ± SD | 24.28 ± 12.6 | 23.78 ± 10.4 | 24.14 ± 11.9 | 0.682 | 24.51 ± 11.43 | 23.57 ± 9.35 | 27.36 ± 12.7 | 0.0573 |
| Length of ICU stay, day(s) ± SD | 7.55 ± 8.53 | 4.74 ± 6.26 | 3.54 ± 3.66 | <0.0001 | 7.22 ± 8.79 | 4.73 ± 5.05 | 4.1 ± 4.59 | 0.0007 |
| Length of ventilator use, day(s) ± SD | 5.4 ± 7.32 | 3.18 ± 5.41 | 2.49 ± 2.61 | <0.0001 | 5.02 ± 7.44 | 2.9 ± 4.24 | 2.81 ± 3.19 | 0.0191 |
| Reintubation, n (%) | 9 (13.43%) | 18 (3.31%) | 17 (2.87%) | <0.0001 | 8 (15.09%) | 7 (4.4%) | 6 (3.77%) | 0.025 |
| Readmission to ICU, n (%) | 10 (13.43%) | 30 (5.51%) | 25 (4.22%) | 0.0012 | 9 (16.98%) | 11 (6.92%) | 9 (5.66%) | 0.3365 |
| TPN use, n (%) | 46 (68.66%) | 229 (42.1%) | 219 (36.99%) | <0.0001 | 37 (69.81%) | 74 (46.54%) | 66 (41.51%) | 0.0016 |
| ≥Grade 3 Complication, n (%) | 20 (29.85%) | 55 (10.11%) | 47(7.94%) | <0.0001 | 14 (26.42%) | 19 (11.95%) | 17 (10.69%) | 0.112 |
| Reoperation, n (%) | 14 (20.9%) | 30 (5.51%) | 25 (4.22%) | <0.0001 | 10 (18.87%) | 8 (5.03%) | 7 (4.4%) | 0.0007 |
| 30-day Mortality, n (%) | 10 (14.93%) | 12 (2.21%) | 9 (1.52%) | <0.0001 | 6 (11.32%) | 7 (4.4%) | 3 (1.89%) | 0.0137 |
| 90-day Mortality, n (%) | 18 (26.87%) | 30 (5.51%) | 18 (3.04%) | <0.0001 | 13 (24.53%) | 11 (6.92%) | 8 (5.03%) | <0.0001 |
| 1-Year Mortality, n (%) | 28 (41.79%) | 138 (25.37%) | 84 (14.19%) | <0.0001 | 23 (43.4%) | 41 (25.79%) | 23 (14.47%) | <0.0001 |
| GNRI Status | Stage II or Higher (N) | ACT Within 3 Months, n (%) | No ACT Within 3 Months, n (%) |
|---|---|---|---|
| Inferior GNRI status (GNRI < 82) | 38 | 14 (36.8%) * | 24 (63.2%) * |
| moderate GNRI status (82 ≤ GNRI < 98) | 111 | 60 (54.1%) * | 51 (45.9%) * |
| superior GNRI status (GNRI ≥ 98) | 98 | 48 (49%) * | 50 (51.0%) * |
| total | 247 | 122 (49.4%) * | 125 (50.6%) * |
References
- De Luca, R.; Gianotti, L.; Pedrazzoli, P.; Brunetti, O.; Rizzo, A.; Sandini, M.; Paiella, S.; Pecorelli, N.; Pugliese, L.; Pietrabissa, A.; et al. Immunonutrition and prehabilitation in pancreatic cancer surgery: A new concept in the era of ERAS® and neoadjuvant treatment. Eur. J. Surg. Oncol. 2023, 49, 542–549. [Google Scholar] [CrossRef]
- Kim, E.; Lee, D.H.; Jang, J.Y. Effects of Preoperative Malnutrition on Postoperative Surgical Outcomes and Quality of Life of Elderly Patients with Periampullary Neoplasms: A Single-Center Prospective Cohort Study. Gut Liver 2019, 13, 690–697. [Google Scholar] [CrossRef]
- du, Y.; Li, L.; Liu, Y.; Wang, S. Prevalence of Malnutrition and the Value of Predicting Pancreatic Fistula in Patients with Laparoscopic Pancreatoduodenectomy. J. Laparoendosc. Adv. Surg. Tech. A 2023, 33, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.N.; Gianotti, L.; Adiamah, A.; Barazzoni, R.; Deutz, N.E.P.; Dhatariya, K.; Greenhaff, P.L.; Hiesmayr, M.; Hjort Jakobsen, D.; Klek, S.; et al. Perioperative nutrition: Recommendations from the ESPEN expert group. Clin. Nutr. 2020, 39, 3211–3227. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Meguid, M.M.; Debonis, D.; Meguid, V.; Hill, L.R.; Terz, J.J. Complications of abdominal operations for malignant disease. Am. J. Surg. 1988, 156, 341–345. [Google Scholar] [CrossRef]
- Karim, S.A.M.; Abdulla, K.S.; Abdulkarim, Q.H.; Rahim, F.H. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int. J. Surg. 2018, 52, 383–387. [Google Scholar] [CrossRef]
- Lee, B.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Lee, J.S. Impact of preoperative malnutrition, based on albumin level and body mass index, on operative outcomes in patients with pancreatic head cancer. J. Hepatobiliary Pancreat. Sci. 2021, 28, 1069–1075. [Google Scholar] [CrossRef]
- Funamizu, N.; Nakabayashi, Y.; Iida, T.; Kurihara, K. Geriatric nutritional risk index predicts surgical site infection after pancreaticoduodenectomy. Mol. Clin. Oncol. 2018, 9, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Afaneh, C.; Gerszberg, D.; Slattery, E.; Seres, D.S.; Chabot, J.A.; Kluger, M.D. Pancreatic cancer surgery and nutrition management: A review of the current literature. Hepatobiliary Surg. Nutr. 2015, 4, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Cong, K.; Chunwei, G. Exploration of three different nutritional scores in predicting postoperative complications after pancreaticoduodenectomy. Nutr. Hosp. 2022, 39, 101–110. [Google Scholar] [CrossRef]
- Cheung, H.H.T.; Joynt, G.M.; Lee, A. Diagnostic test accuracy of preoperative nutritional screening tools in adults for malnutrition: A systematic review and network meta-analysis. Int. J. Surg. 2024, 110, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; Lin, Y.H.; Liu, Y.W.; Liu, Y.Y.; Li, W.F.; Kuo, M.C.; Huang, S.W.; Yeh, C.H.; Lin, Y.C.; Yin, S.M. The impact of preoperative nutritional status on postoperative outcomes: An insight from Geriatric Nutritional Risk Index in elderly pancreaticoduodenectomy patients. BMC Surg. 2024, 24, 100. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Pancreatic Adenocarcinoma; Version 3.2025; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Cui, J.; Jiao, F.; Li, Q.; Wang, Z.; Fu, D.; Liang, J.; Liang, H.; Xia, T.; Zhang, T.; Zhang, Y.; et al. Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of pancreatic cancer. J. Natl. Cancer Cent. 2022, 2, 205–215. [Google Scholar] [CrossRef]
- Martin-Perez, E.; Domínguez-Muñoz, J.E.; Botella-Romero, F.; Cerezo, L.; Matute Teresa, F.; Serrano, T.; Vera, R. Multidisciplinary consensus statement on the clinical management of patients with pancreatic cancer. Clin. Transl. Oncol. 2020, 22, 1963–1975. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Ampullary Adenocarcinoma. Version 2.2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ampullary.pdf (accessed on 3 February 2025).
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Wang, M.H.; Chen, C.Y.; Lin, Y.H.; Liu, Y.W.; Liu, Y.Y.; Li, W.F.; Lin, C.T.; Huang, S.W.; Yeh, C.H.; Yin, S.M. High Geriatric Nutritional Risk Index Risk as a Predictor of Postoperative Complications and Early Mortality in Older Adult Patients Undergoing Pancreatoduodenectomy for Periampullary Malignancies. J. Clin. Med. 2025, 14, 655. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kong, D.; Peng, J.; Wang, Z.; Chen, Y. Association of malnutrition with all-cause mortality in the elderly population: A 6-year cohort study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 52–59. [Google Scholar] [CrossRef]
- Nakayama, M.; Gosho, M.; Adachi, M.; Ii, R.; Matsumoto, S.; Miyamoto, H.; Hirose, Y.; Nishimura, B.; Tanaka, S.; Wada, T.; et al. The Geriatric Nutritional Risk Index as a Prognostic Factor in Patients with Advanced Head and Neck Cancer. Laryngoscope 2021, 131, E151–E156. [Google Scholar] [CrossRef]
- Thormann, M.; Hinnerichs, M.; Barajas Ordonez, F.; Saalfeld, S.; Perrakis, A.; Croner, R.; Omari, J.; Pech, M.; Zamsheva, M.; Meyer, H.J.; et al. Sarcopenia is an Independent Prognostic Factor in Patients With Pancreatic Cancer—A Meta-analysis. Acad. Radiol. 2023, 30, 1552–1561. [Google Scholar] [CrossRef]
- Miyamoto, H.; Toyokawa, T.; Ishidate, T.; Kuroda, K.; Miki, Y.; Yoshii, M.; Tamura, T.; Lee, S.; Maeda, K. Significance of the geriatric nutritional risk index and neutrophil-to-lymphocyte ratio as prognostic indicators in older patients with gastric cancer: A retrospective cohort study. BMC Cancer 2024, 24, 1396. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, L.; Tang, P.; Guo, R. Geriatric Nutritional Risk Index and Survival of Patients With Colorectal Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 906711. [Google Scholar] [CrossRef]
- Qiu, J.; Yu, Y.; Wang, Z.; Hong, L.; Shao, L.; Wu, J. Comprehensive analysis of the prognostic value of pre-treatment nutritional indicators in elderly rectal cancer patients. Sci. Rep. 2024, 14, 22078. [Google Scholar] [CrossRef]
- Kato, A.; Aoyama, T.; Maezawa, Y.; Hashimoto, I.; Hara, K.; Kazama, K.; Numata, M.; Sawazaki, S.; Tamagawa, A.; Cho, H.; et al. Geriatric Nutritional Risk Index Is an Independent Prognostic Factor for Patients With Esophageal Cancer Who Receive Curative Treatment. Anticancer. Res. 2024, 44, 331–337. [Google Scholar] [CrossRef]
- Yang, C.K.; Huang, K.T.; Qin, W.; Wu, Q.Y.; Huang, X.L.; Peng, K.; Lao, Q.; Ye, X.P.; Zhu, G.Z.; Li, T.M.; et al. Prognostic value of geriatric nutritional risk index and prognostic nutritional index in hepatocellular carcinoma. Clin. Nutr. ESPEN 2024, 59, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Grinstead, C.; Yoon, S.L. Geriatric Nutritional Risk Index (GNRI) and Survival in Pancreatic Cancer: A Retrospective Study. Nutrients 2025, 17, 509. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Nafiu, O.O.; Ghaferi, A.; Tremper, K.K.; Shanks, A.; Kheterpal, S. Independent predictors and outcomes of unanticipated early postoperative tracheal intubation after nonemergent, noncardiac surgery. Anesthesiology 2011, 115, 44–53. [Google Scholar] [CrossRef]
- Mosquera, C.; Koutlas, N.J.; Edwards, K.C.; Strickland, A.; Vohra, N.A.; Zervos, E.E.; Fitzgerald, T.L. Impact of malnutrition on gastrointestinal surgical patients. J. Surg. Res. 2016, 205, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Zamora, M.D.; Gordillo-Brenes, A.; Banderas-Bravo, E.; Arboleda-Sánchez, J.A.; Hinojosa-Pérez, R.; Aguilar-Alonso, E.; Herruzo-Aviles, Á.; Curiel-Balsera, E.; Sánchez-Rodríguez, Á.; Rivera-Fernández, R. Prolonged Mechanical Ventilation as a Predictor of Mortality After Cardiac Surgery. Respir. Care 2018, 63, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Brandecker, S.; Rana, S.; Potthoff, A.L.; Eichhorn, L.; Bode, C.; Schmeel, F.C.; Radbruch, A.; Schäfer, N.; Herrlinger, U.; et al. Postoperative prolonged mechanical ventilation correlates to poor survival in patients with surgically treated spinal metastasis. Front. Oncol. 2022, 12, 940790. [Google Scholar] [CrossRef]
- Lessing, Y.; Pencovich, N.; Nevo, N.; Lubezky, N.; Goykhman, Y.; Nakache, R.; Lahat, G.; Klausner, J.M.; Nachmany, I. Early reoperation following pancreaticoduodenectomy: Impact on morbidity, mortality, and long-term survival. World J. Surg. Oncol. 2019, 17, 26. [Google Scholar] [CrossRef]
- Henry, A.C.; van Dongen, J.C.; van Goor, I.; Smits, F.J.; Nagelhout, A.; Besselink, M.G.; Busch, O.R.; Bonsing, B.A.; Bosscha, K.; van Dam, R.M.; et al. Impact of complications after resection of pancreatic cancer on disease recurrence and survival, and mediation effect of adjuvant chemotherapy: Nationwide, observational cohort study. BJS Open 2023, 7, zrac174. [Google Scholar] [CrossRef]
- Higashi, T.; Murase, K.; Yokoi, R.; Kuno, M.; Fukada, M.; Tajima, J.Y.; Kiyama, S.; Tanaka, Y.; Okumura, N.; Matsuhashi, N. Association of Pre-operative Geriatric Nutritional Risk Index With Complete Adjuvant Chemotherapy and Prognosis Post-pancreatectomy. Anticancer. Res. 2024, 44, 427–434. [Google Scholar] [CrossRef]
- Wu, W.; He, J.; Cameron, J.L.; Makary, M.; Soares, K.; Ahuja, N.; Rezaee, N.; Herman, J.; Zheng, L.; Laheru, D.; et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 2873–2881. [Google Scholar] [CrossRef]
- Wentz, S.C.; Zhao, Z.G.; Shyr, Y.; Shi, C.J.; Merchant, N.B.; Washington, K.; Xia, F.; Chakravarthy, A.B. Lymph node ratio and preoperative CA 19-9 levels predict overall survival and recurrence-free survival in patients with resected pancreatic adenocarcinoma. World J. Gastrointest. Oncol. 2012, 4, 207–215. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, J.H.; Jung, K.U.; Lee, S.R. Prognostic value of carcinoembryonic antigen and carbohydrate antigen 19-9 in periampullary cancer patients receiving pancreaticoduodenectomy. Asian J. Surg. 2021, 44, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Boyev, A.; Prakash, L.R.; Chiang, Y.J.; Newhook, T.E.; Bruno, M.L.; Arvide, E.M.; Dewhurst, W.L.; Kim, M.P.; Ikoma, N.; Lee, J.E.; et al. Elevated CA 19-9 is associated with worse survival in patients with resected ampullary adenocarcinoma. Surg. Oncol. 2023, 51, 101994. [Google Scholar] [CrossRef] [PubMed]
- Uijterwijk, B.A.; Lemmers, D.H.; Ghidini, M.; Wilmink, H.; Zaniboni, A.; Salvia, R.; Kito Fusai, G.; Groot Koerkamp, B.; Koek, S.; Ghorbani, P.; et al. The Five Periampullary Cancers, not Just Different Siblings but Different Families: An International Multicenter Cohort Study. Ann. Surg. Oncol. 2024, 31, 6157–6169. [Google Scholar] [CrossRef]
- Russell, T.B.; Labib, P.L.; Denson, J.; Ausania, F.; Pando, E.; Roberts, K.J.; Kausar, A.; Mavroeidis, V.K.; Marangoni, G.; Thomasset, S.C.; et al. Predictors of actual five-year survival and recurrence after pancreatoduodenectomy for ampullary adenocarcinoma: Results from an international multicentre retrospective cohort study. HPB 2023, 25, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, H.J.; Cho, C.W.; Yun, S.S.; Lee, D.S. Factors influencing patterns of recurrence following pancreaticoduodenectomy for patients with distal bile duct cancer and ampulla of Vater cancer. Ann. Hepatobiliary Pancreat. Surg. 2022, 26, 138–143. [Google Scholar] [CrossRef]
- Perioperative total parenteral nutrition in surgical patients. N. Engl. J. Med. 1991, 325, 525–532. [CrossRef]
- Bozzetti, F.; Gavazzi, C.; Miceli, R.; Rossi, N.; Mariani, L.; Cozzaglio, L.; Bonfanti, G.; Piacenza, S. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: A randomized, clinical trial. JPEN J. Parenter. Enter. Nutr. 2000, 24, 7–14. [Google Scholar] [CrossRef]
- Serón-Arbeloa, C.; Labarta-Monzón, L.; Puzo-Foncillas, J.; Mallor-Bonet, T.; Lafita-López, A.; Bueno-Vidales, N.; Montoro-Huguet, M. Malnutrition Screening and Assessment. Nutrients 2022, 14, 2392. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.N.; Zhou, L.S.; Zhang, S.; Li, J.X.; Xu, C.J. Performance of nutritional and inflammatory markers in patients with pancreatic cancer. World J. Clin. Oncol. 2024, 15, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Qiu, Z.; Zhang, L.; Ma, W.; Zi, L.; Wang, K.; Kuang, T.; Zhao, K.; Wang, W. High intramuscular adipose tissue content associated with prognosis and postoperative complications of cancers. J. Cachexia Sarcopenia Muscle 2023, 14, 2509–2519. [Google Scholar] [CrossRef]

| Match (n = 371) | ||||
|---|---|---|---|---|
| Characteristics | Inferior GNRI Status GNRI < 82 (n = 53) | Moderate GNRI Status 82 ≤ GNRI < 98 (n = 159) | Superior GNRI Status GNRI ≥ 98 (n = 159) | p-Value |
| Tumor Type | ||||
| Pancreatic cancer, n (%) | 16 (30.19%) | 48 (30.19%) | 48 (30.19%) | 1 |
| Distal CBD cancer, n (%) | 12 (22.64%) | 37 (23.27%) | 34 (21.38%) | 0.921 |
| Ampullary cancer, n (%) | 22 (41.51%) | 63 (39.62%) | 63 (39.62%) | 0.967 |
| Duodenal/gastric cancer, n (%) | 3 (5.66%) | 11 (6.92%) | 14 (8.81%) | 0.697 |
| Age, year ± SD | 69.81 ± 10.26 | 68.03 ± 10.11 | 65.82 ± 10.29 | 0.983 |
| Gender | 0.904 | |||
| Male, n (%) | 28 (52.83%) | 82 (51.57%) | 86 (54.09%) | |
| Female, n (%) | 25 (47.17%) | 77 (48.43%) | 73 (45.91%) | |
| BMI, mean ± SD | 21.5 ± 2.48 | 22.33 ± 2.49 | 23.02 ± 2.63 | <0.001 * |
| Pre-OP biliary drainage, n (%) | 28 (52.83%) | 90 (56.6%) | 65 (40.88%) | 0.017 * |
| Underlying disease | ||||
| HTN, n (%) | 19 (35.85%) | 68 (42.77%) | 55 (34.59%) | 0.301 |
| DM, n (%) | 20 (37.74%) | 51 (32.08%) | 44 (27.67%) | 0.362 |
| Lab data, mean ± SD | ||||
| CA19-9 (U/mL), mean ± SD | 1284.97 ± 5067.47 | 660.36 ± 2177.19 | 492.14 ± 2798.76 | <0.001 * |
| ALB (g/dL), mean ± SD | 2.53 ± 0.32 | 3.37 ± 0.3 | 4.17 ± 0.28 | <0.001 * |
| T-BIL(mg/dL), mean ± SD | 3.44 ± 3.81 | 3.46 ± 3.92 | 2.21 ± 3.3 | <0.001 * |
| Surgery Type | ||||
| Open, n (%) | 52 (98.11%) | 155 (97.48%) | 155 (97.48%) | 0.821 |
| MIS, n (%) | 1 (1.89%) | 4 (2.52%) | 4 (2.52%) | |
| ASA physical status | 0.955 | |||
| I–II, n (%) | 10 (18.87%) | 32 (20.13%) | 30 (18.87%) | |
| III–IV, n (%) | 43 (81.13%) | 127 (79.87%) | 129 (81.13%) | |
| OP time (minutes), mean ± SD | 524.95 ± 146.68 | 515.29 ± 132.66 | 511.52 ± 155.08 | 0.516 |
| OP blood loss (mL), mean ± SD | 604.34 ± 688.43 | 492.58 ± 861.86 | 473.02 ± 733.84 | 0.058 |
| LN number, mean ± SD | 19.36 ± 11.65 | 20.01 ± 10.81 | 19.42 ± 10.54 | 0.747 |
| positive LN number, mean ± SD | 1.9 ± 3.66 | 1.46 ± 2.23 | 1.33 ± 2.18 | 0.503 |
| Tumor size (mm), mean ± SD | 32.9 ± 23.92 | 32.97 ± 23.04 | 28.09 ± 14.66 | 0.180 |
| Tumor margin | 0.713 | |||
| R0 resection, n (%) | 44 (83.02%) | 138 (86.79%) | 139 (87.42%) | |
| Not R0 resection, n (%) | 9 (16.98%) | 21 (13.21%) | 20 (12.58%) | |
| Pathological staging | 0.192 | |||
| Stage I | 10 (18.87%) | 29 (18.24%) | 40 (25.16%) | |
| Stage II | 28 (52.83%) | 77 (48.43%) | 65 (40.88%) | |
| Stage III | 6 (11.32%) | 31 (19.50%) | 27 (16.98%) | |
| Stage IV | 4 (7.55%) | 3 (1.89%) | 6 (3.77%) | |
| Perioperative chemotherapy in 3 months | ||||
| NACT | 1 (1.89%) | 2 (1.26%) | 7 (4.40%) | 0.207 |
| ACT | 14 (26.42%) | 60 (37.74%) | 48 (30.19%) | 0.2 |
| Match (n = 371) | ||||
|---|---|---|---|---|
| Prognostic Outcomes | Inferior GNRI Status GNRI < 82 (n = 53) | Moderate GNRI Status 82 ≤ GNRI < 98 (n = 159) | Superior GNRI Status GNRI ≥ 98 (n = 159) | p-Value |
| Post-operative stays, day(s) ± SD | 24.51 ± 11.43 | 23.57 ± 9.35 | 27.36 ± 12.7 | 0.057 |
| Length of ICU stay, day(s) ± SD | 7.22 ± 8.79 | 4.73 ± 5.05 | 4.1 ± 4.59 | 0.001 * |
| Length of ventilator use, day(s) ± SD | 5.02 ± 7.44 | 2.9 ± 4.24 | 2.81 ± 3.19 | 0.019 * |
| Reintubation, n (%) | 8 (15.09%) | 7 (4.4%) | 6 (3.77%) | 0.025 * |
| Readmission to ICU, n (%) | 9 (16.98%) | 11 (6.92%) | 9 (5.66%) | 0.337 |
| TPN use, n (%) | 37 (69.81%) | 74 (46.54%) | 66 (41.51%) | 0.002 * |
| ≥Grade 3 Complication, n (%) | 14 (26.42%) | 19 (11.95%) | 17 (10.69%) | 0.112 |
| Reoperation, n (%) | 10 (18.87%) | 8 (5.03%) | 7 (4.4%) | 0.001 * |
| 30-day Mortality, n (%) | 6 (11.32%) | 7 (4.4%) | 3 (1.89%) | 0.014 * |
| 90-day Mortality, n (%) | 13 (24.53%) | 11 (6.92%) | 8 (5.03%) | <0.001 * |
| 1-Year Mortality, n (%) | 23 (43.4%) | 41 (25.79%) | 23 (14.47%) | <0.001 * |
| Variables | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| ALL | |||||
| Age (years) § | 1.011 (0.998–1.024) | 0.0917 | |||
| Sex (female/male) | 0.878 (0.671–1.148) | 0.343 | |||
| BMI (kg/m2) § | 0.955 (0.905–1.008) | 0.098 | |||
| ASA physical status (III–IV/I–II) | 1.234 (0.87–1.751) | 0.239 | |||
| Operative time (h) § | 1.116 (1.06–1.175) | <0.001 * | 1.092 (1.024–1.164) | 0.007 * | |
| Lymph node positive (positive/negative) | 2.419 (1.835–3.188) | <0.001 * | 2.407 (1.699–3.41) | <0.001 * | |
| Operative blood loss (100 mL) § | 1.052 (1.037–1.067) | <0.001 * | 1.041 (1.022–1.059) | <0.001 * | |
| Tumor size (mm) § | 1.007 (1.001–1.012) | 0.013 * | 1.007 (1.001–1.013) | 0.031 * | |
| Tumor margin (not R0 resection/R0 resection) | 1.613 (1.111–2.34) | 0.012 * | 1.133 (0.733–1.744) | 0.578 | |
| CA19-9 (50 U/mL) § | 1.002 (1–1.004) | 0.014 * | 1.001 (0.999–1.003) | 0.365 | |
| GNRI | GNRI ≥ 98 | reference | reference | ||
| GNRI = 82–98 | 1.548 (1.146–2.091) | 0.004 * | 1.768 (1.246–2.511) | 0.001 * | |
| GNRI < 82 | 2.869 (1.957–4.205) | <0.001 * | 2.65 (1.706–4.116) | <0.001 * | |
| Perioperative chemotherapy in 3 months | |||||
| NACT | 0.792 (0.252–2.487) | 0.690 | |||
| ACT | 1.346 (1.018–1.778) | 0.037 * | 0.71 (0.499–1.011) | 0.058 | |
| Pancreatic cancer (N = 112) | |||||
| Age (years) § | 1.024 (1.002–1.046) | 0.034 * | 1.024 (1.001–1.048) | 0.041 * | |
| Sex (female/male) | 1.026 (0.633–1.662) | 0.918 | |||
| BMI (kg/m2) § | 0.966 (0.882–1.058) | 0.459 | |||
| ASA physical status (III–IV/I–II) | 0.956 (0.595–1.534) | 0.851 | |||
| Operative time (h) § | 1.081 (0.975–1.199) | 0.139 | |||
| Lymph node positive (positive/negative) | 1.868 (1.154–3.024) | 0.011 * | 1.715 (1.046–2.814) | 0.033 * | |
| Operative blood loss (100 mL) § | 1.036 (1.017–1.056) | 0.001 * | 1.043 (1.022–1.064) | <0.001 * | |
| Tumor size (mm) § | 1.004 (0.996–1.012) | 0.283 | |||
| Tumor margin (not R0 resection/R0 resection) | 1.132 (0.68–1.885) | 0.633 | |||
| CA19-9 (50 U/mL) § | 1 (0.997–1.003) | 0.908 | |||
| GNRI | GNRI ≥ 98 | reference | reference | ||
| GNRI = 82–98 | 1.373 (0.835–2.259) | 0.212 | 1.401 (0.823–2.382) | 0.214 | |
| GNRI < 82 | 2.981 (1.531–5.803) | 0.001 * | 2.697 (1.357–5.358) | 0.005 * | |
| Perioperative chemotherapy in 3 months | |||||
| NACT | 0.493 (0.12–2.017) | 0.325 | |||
| ACT | 0.934 (0.595–1.466) | 0.765 | |||
| Other Malignancy(N = 259) | |||||
| Age (years) § | 1.022 (1.004–1.04) | 0.018 * | 1.009 (0.988–1.03) | 0.404 | |
| Sex (female/male) | 0.982 (0.702–1.372) | 0.914 | |||
| BMI (kg/m2) § | 0.957 (0.896–1.022) | 0.187 | |||
| ASA physical status (III–IV/I–II) | 2.283 (1.277–4.082) | 0.005 * | 2.089 (1.064–4.104) | 0.033 * | |
| Operative time (h) § | 1.101 (1.033–1.173) | 0.003 * | 1.082 (0.999–1.173) | 0.054 | |
| Lymph node positive (positive/negative) | 2.44 (1.734–3.434) | <0.001 * | 2.417 (1.667–3.503) | <0.001 * | |
| Operative blood loss (100 mL) § | 1.083 (1.046–1.121) | <0.001 * | 1.061 (1.019–1.106) | 0.004 * | |
| Tumor size (mm) § | 1.005 (0.997–1.013) | 0.197 | 1 (1–1) | 0.142 | |
| Tumor margin (not R0 resection/R0 resection) | 1.635 (0.921–2.902) | 0.093 | |||
| CA19-9 (50 U/mL) § | 1.003 (1.001–1.006) | 0.006 * | 1.002 (0.999–1.005) | 0.142 | |
| GNRI | GNRI ≥ 98 | reference | reference | ||
| GNRI = 82–98 | 1.616 (1.106–2.362) | 0.013 * | 1.6 (1.058–2.419) | 0.026 * | |
| GNRI < 82 | 3.035 (1.888–4.879) | <0.001 * | 2.385 (1.397–4.072) | <0.001 * | |
| Perioperative chemotherapy in 3 months | |||||
| NACT | 0.976 (0.136–7.017) | 0.980 | |||
| ACT | 1.273 (0.872–1.859) | 0.211 | |||
| Variables | Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| ALL | |||||
| Age (years) § | 0.98 (0.966–0.995) | 0.001 * | 0.978 (0.963–0.994) | 0.006 * | |
| Sex (female/male) | 0.934 (0.662–1.318) | 0.699 | |||
| BMI (kg/m2) § | 0.949 (0.883–1.02) | 0.157 | |||
| ASA physical status (III–IV/I–II) | 0.688 (0.463–1.021) | 0.063 | |||
| Operative time (h) § | 1.137 (1.065–1.213) | <0.001 * | 1.159 (1.079–1.245) | <0.001 * | |
| Lymph node positive (positive/negative) | 4.434 (3.028–6.493) | <0.001 * | 4.583 (3.019–6.956) | <0.001 * | |
| Operative blood loss (100 mL) § | 1.023 (0.995–1.052) | 0.106 | |||
| Tumor size (mm) § | 1.005 (0.998–1.013) | 0.168 | |||
| Tumor margin (not R0 resection/R0 resection) | 0.88 (0.496–1.562) | 0.662 | |||
| CA19-9 (50 U/mL) § | 1.001 (0.999–1.004) | 0.168 | |||
| GNRI | GNRI ≥ 98 | reference | reference | ||
| GNRI = 82–98 | 1.688 (1.161–2.454) | 0.006 * | 1.865 (1.265–2.751) | 0.002 * | |
| GNRI < 82 | 1.679 (0.962–2.929) | 0.068 | 1.681 (0.955–2.957) | 0.072 | |
| Perioperative chemotherapy in 3 months | |||||
| NACT | 0.61 (0.151–2.471) | 0.489 | |||
| ACT | 2.029 (1.434–2.872) | <0.001 * | 0.846 (0.573–1.249) | 0.4 | |
| Pancreatic cancer (N = 112) | |||||
| Age (years) § | 0.981 (0.957–1.006) | 0.139 | |||
| Sex (female/male) | 1.53 (0.847–2.764) | 0.159 | |||
| BMI (kg/m2) § | 0.981 (0.827–1.047) | 0.232 | |||
| ASA physical status (III–IV/I–II) | 0.445 (0.25–0.793) | 0.006 * | 0.438 (0.244–0.786) | 0.006 * | |
| Operative time (h) § | 1.136 (0.998–1.293) | 0.054 | |||
| Lymph node positive (positive/negative) | 4.87 (2.26–10.492) | <0.001 * | 4.651 (2.146–10.081) | <0.001 * | |
| Operative blood loss (100 mL) § | 1 (0.963–1.039) | 0.986 | |||
| Tumor size (mm) § | 0.997 (0.981–1.012) | 0.682 | |||
| Tumor margin (not R0 resection/R0 resection) | 0.625 (0292–1.339) | 0.227 | |||
| CA19-9 (50 U/mL) § | 0.999 (0.995–1.004) | 0.805 | |||
| GNRI | GNRI ≥ 98 | reference | reference | ||
| GNRI = 82–98 | 2.163 (1.162–4.027) | 0.015 * | 1.922 (1.03–3.586) | 0.04 * | |
| GNRI < 82 | 1.044 (0.347–3.144) | 0.939 | 0.736 (0.244–2.217) | 0.586 | |
| Perioperative chemotherapy in 3 months | |||||
| NACT | 0.268 (0.037–1.947) | 0.193 | |||
| ACT | 1.779 (0.963–3.29) | 0.066 | |||
| Other Malignancy(N = 259) | |||||
| Age (years) § | 0.988 (0.967–1.008) | 0.231 | |||
| Sex (female/male) | 0.904 (0.587–1.391) | 0.646 | |||
| BMI (kg/m2) § | 0.961 (0.88–1.051) | 0.387 | |||
| ASA physical status (III–IV/I–II) | 1.31 (0.693–2.473) | 0.406 | |||
| Operative time (h) § | 1.113 (1.026–1.208) | 0.01 * | 1.144 (1.05–1.247) | 0.002 * | |
| Lymph node positive (positive/negative) | 3.907 (2.49–6.129) | <0.001 * | 4.035 (2.413–6.747) | <0.001 * | |
| Operative blood loss (100 mL) | 1.043 (0.989–1.1) | 0.119 | |||
| Tumor size (mm) § | 1.007 (0.998–1.017) | 0.138 | |||
| Tumor margin (not R0 resection/R0 resection) | 0.88 (0.356–2.177) | 0.783 | |||
| CA19-9 (50 U/mL) § | 1.003 (1–1.006) | 0.027 * | 1.001 (0.998–1.004) | 0.385 | |
| GNRI | GNRI ≥ 98 | reference | reference | ||
| GNRI = 82–98 | 1.471 (0.918–2.357) | 0.109 | 1.438 (0.88–2.349) | 0.147 | |
| GNRI < 82 | 2.022 (1.056–3.87) | 0.034 * | 1.692 (0.861–3.326) | 0.127 | |
| Perioperative chemotherapy in 3 months | |||||
| NACT | 1.143 (0.159–8.239) | 0.894 | |||
| ACT | 1.788 (1.132–2.823) | 0.013 * | 0.891 (0.527–1.504) | 0.665 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-Y.; Li, W.-F.; Liu, Y.-W.; Liu, Y.-Y.; Yeh, C.-H.; Lin, Y.-H.; Cheng, J.-Y.; Yin, S.-M. The Prognostic Role of Geriatric Nutritional Risk Index in Periampullary Cancer Patients Undergoing Pancreaticoduodenectomy: A Propensity Score-Matched Survival Study. Cancers 2025, 17, 3273. https://doi.org/10.3390/cancers17193273
Li C-Y, Li W-F, Liu Y-W, Liu Y-Y, Yeh C-H, Lin Y-H, Cheng J-Y, Yin S-M. The Prognostic Role of Geriatric Nutritional Risk Index in Periampullary Cancer Patients Undergoing Pancreaticoduodenectomy: A Propensity Score-Matched Survival Study. Cancers. 2025; 17(19):3273. https://doi.org/10.3390/cancers17193273
Chicago/Turabian StyleLi, Chih-Ying, Wei-Feng Li, Yueh-Wei Liu, Yu-Yin Liu, Cheng-Hsi Yeh, Yu-Hung Lin, Jen-Yu Cheng, and Shih-Min Yin. 2025. "The Prognostic Role of Geriatric Nutritional Risk Index in Periampullary Cancer Patients Undergoing Pancreaticoduodenectomy: A Propensity Score-Matched Survival Study" Cancers 17, no. 19: 3273. https://doi.org/10.3390/cancers17193273
APA StyleLi, C.-Y., Li, W.-F., Liu, Y.-W., Liu, Y.-Y., Yeh, C.-H., Lin, Y.-H., Cheng, J.-Y., & Yin, S.-M. (2025). The Prognostic Role of Geriatric Nutritional Risk Index in Periampullary Cancer Patients Undergoing Pancreaticoduodenectomy: A Propensity Score-Matched Survival Study. Cancers, 17(19), 3273. https://doi.org/10.3390/cancers17193273






