Survival Outcomes in the Canadian Merkel Cell Carcinoma Population Between 2000 and 2018 and Descriptive Comparison with the American Joint Committee on Cancer 8th Edition Staging System—A Study from the Pan-Canadian Merkel Cell Collaborative
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Study Population
2.3. Inclusion and Exclusion Criteria
2.4. Data Collection
2.5. Data Variables
2.6. Statistical Analyses
3. Results
3.1. Canadian Patient Characteristics
3.2. Overall Survival Among Canadian Patients
3.3. DFS and CSS Among Canadian Patients
3.4. Overall Survival Among Canadian Versus AJCC 8th Edition Patients
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| CHUM | Centre hospitalier de l’Université de Montréal |
| CI | Confidence interval |
| CSS | Cancer-specific survival |
| DFS | Disease-free survival |
| IQR | Interquartile range |
| MCC | Merkel cell carcinoma |
| NCCN | National Comprehensive Cancer Network |
| NCDB | National Cancer Data Base |
| OS | Overall survival |
| PMH | Princess Margaret Hospital |
| SD | Standard deviation |
| UHN | University Health Network |
| UV | Ultraviolet |
References
- Tothill, R.; Estall, V.; Rischin, D. Merkel Cell Carcinoma: Emerging Biology, Current Approaches, and Future Directions. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e519–e526. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, S.T.; Price, E.L.; Chan, A.-W.; Hanna, T.P.; Limacher, J.J.; Nessim, C.; Shiers, J.E.; Tron, V.; Wright, F.C.; Drucker, A.M. Incidence, Mortality and Survival of Merkel Cell Carcinoma: A Systematic Review of Population-Based Studies. Br. J. Dermatol. 2024, 190, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.; Walradt, T.; Markarov, V.; Blom, A.; Riaz, N.; Doumani, R.; Stafstrom, K.; Moshiri, A.; Yelistratova, L.; Levinsohn, J.; et al. Mutational Landscape of MCPyV-Positive and MCPyV-Negative Merkel Cell Carcinomas with Implications for Immunotherapy. Oncotarget 2016, 7, 3403–3415. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Liu, K.X.; Shin, K.; Thakuria, M.; Schoenfeld, J.D.; Tishler, R.B.; Silk, A.W.; Yoon, C.H.; Fite, E.; Margalit, D.N. Changes in Merkel Cell Oncoprotein Antibodies after Radiation Therapy in Curatively Treated Merkel Cell Carcinoma and Association with Recurrence. Cancer 2024, 130, 4267–4275. [Google Scholar] [CrossRef]
- Stang, A.; Becker, J.C.; Nghiem, P.; Ferlay, J. The Association between Geographic Location and Incidence of Merkel Cell Carcinoma in Comparison to Melanoma: An International Assessment. Eur. J. Cancer 2018, 94, 47–60. [Google Scholar] [CrossRef]
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef]
- Ahmed, S.; Shahid, R.K. Disparity in Cancer Care: A Canadian Perspective. Curr. Oncol. Tor. Ont 2012, 19, e376–e382. [Google Scholar] [CrossRef]
- Weir, H.K.; Bryant, H.; Turner, D.; Coleman, M.P.; Mariotto, A.B.; Spika, D.; Matz, M.; Harewood, R.; Tucker, T.C.; Allemani, C. Population-Based Cancer Survival in Canada and the United States by Socioeconomic Status: Findings from the CONCORD-2 Study. J. Regist. Manag. 2022, 49, 23–33. [Google Scholar]
- Garneski, K.M.; Warcola, A.H.; Feng, Q.; Kiviat, N.B.; Leonard, J.H.; Nghiem, P. Merkel Cell Polyomavirus Is More Frequently Present in North American than Australian Merkel Cell Carcinoma Tumors. J. Investig. Dermatol. 2009, 129, 246–248. [Google Scholar] [CrossRef]
- Elm, E.V.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Guddati, A.K. Clinical Endpoints in Oncology—A Primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar] [PubMed]
- Etzioni, R.; Gulati, R.; Lin, D.W. Measures of Survival Benefit in Cancer Drug Development and Their Limitations. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 122–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlag, P.M. The ‘Sentinel Node’ Concept: More Questions Raised than Answers Provided? Oncologist 1998, 3, vi–vii. [Google Scholar] [CrossRef][Green Version]
- Noguchi, M. Therapeutic Relevance of Breast Cancer Micrometastases in Sentinel Lymph Nodes. Br. J. Surg. 2002, 89, 1505–1515. [Google Scholar] [CrossRef]
- Nagase, K.; Narisawa, Y. Immunotherapy for Merkel Cell Carcinoma. Curr. Treat. Options Oncol. 2018, 19, 57. [Google Scholar] [CrossRef]
- Ma, J.; Brewer, J. Merkel Cell Carcinoma in Immunosuppressed Patients. Cancers 2014, 6, 1328–1350. [Google Scholar] [CrossRef]
- Winge-Main, A.; Robsahm, T.E.; Nyakas, M.; Festervoll, G.; Torkilseng, E.; Thybo, S.; Pati, S.; Carroll, R. Long-Term Outcomes of Stage IIB–IV Melanoma Patients: Nationwide Data from Norway. Future Oncol. 2023, 19, 205–215. [Google Scholar] [CrossRef]
- Li, Y.; Hua, R.; He, J.; Zhang, H. Survival Contradiction in Stage II, IIIA, And IIIB Colon Cancer: A Surveillance, Epidemiology, and End Result-Based Analysis. Evid. Based Complement. Alternat. Med. 2022, 2022, 4088117. [Google Scholar] [CrossRef]
- Lee, R.; Mandala, M.; Long, G.V.; Eggermont, A.M.M.; Van Akkooi, A.C.J.; Sandhu, S.; Garbe, C.; Lorigan, P. Adjuvant Therapy for Stage II Melanoma: The Need for Further Studies. Eur. J. Cancer 2023, 189, 112914. [Google Scholar] [CrossRef]
- Poklepovic, A.S.; Luke, J.J. Considering Adjuvant Therapy for Stage II Melanoma. Cancer 2020, 126, 1166–1174. [Google Scholar] [CrossRef]
- Wong, S.Q.; Waldeck, K.; Vergara, I.A.; Schröder, J.; Madore, J.; Wilmott, J.S.; Colebatch, A.J.; De Paoli-Iseppi, R.; Li, J.; Lupat, R.; et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015, 75, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel Cell Carcinoma. Nat. Rev. Dis. Primer 2017, 3, 17077. [Google Scholar] [CrossRef]
- Fang, L.C.; Lemos, B.; Douglas, J.; Iyer, J.; Nghiem, P. Radiation Monotherapy as Regional Treatment for Lymph Node-positive Merkel Cell Carcinoma. Cancer 2010, 116, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Lodde, G.C.; Leiter, U.; Gesierich, A.; Eigentler, T.; Hauschild, A.; Pföhler, C.; Gambichler, T.; Herbst, R.; Meier, F.; Hassel, J.C.; et al. Clinical Course of Merkel Cell Carcinoma: A DeCOG Multicenter Study of 1049 Patients. Eur. J. Cancer 2025, 221, 115406. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2018, 16, 742–774. [Google Scholar] [CrossRef]
- Lugowska, I.; Becker, J.C.; Ascierto, P.A.; Veness, M.; Blom, A.; Lebbe, C.; Migliano, E.; Hamming-Vrieze, O.; Goebeler, M.; Kneitz, H.; et al. Merkel-Cell Carcinoma: ESMO–EURACAN Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. ESMO Open 2024, 9, 102977. [Google Scholar] [CrossRef]
- Chan, I.S.; Bhatia, S.; Kaufman, H.L.; Lipson, E.J. Immunotherapy for Merkel Cell Carcinoma: A Turning Point in Patient Care. J. Immunother. Cancer 2018, 6, 23. [Google Scholar] [CrossRef]
- Angeles, C.V.; Sabel, M.S. Immunotherapy for Merkel Cell Carcinoma. J. Surg. Oncol. 2021, 123, 775–781. [Google Scholar] [CrossRef]
- Duarte-Bateman, D.; Shen, A.; Bullock, T.; Sadeghi, P.; Escandón, J.M.; Dedkova, E.; Gastman, B.R. Best Practices in Surgical and Nonsurgical Management of Head and Neck Merkel Cell Carcinoma: An Update. Mol. Carcinog. 2023, 62, 101–112. [Google Scholar] [CrossRef]
- Femia, D.; Prinzi, N.; Anichini, A.; Mortarini, R.; Nichetti, F.; Corti, F.; Torchio, M.; Peverelli, G.; Pagani, F.; Maurichi, A.; et al. Treatment of Advanced Merkel Cell Carcinoma: Current Therapeutic Options and Novel Immunotherapy Approaches. Target. Oncol. 2018, 13, 567–582. [Google Scholar] [CrossRef]
- Topalian, S.L.; Bhatia, S.; Amin, A.; Kudchadkar, R.R.; Sharfman, W.H.; Lebbé, C.; Delord, J.-P.; Dunn, L.A.; Shinohara, M.M.; Kulikauskas, R.; et al. Neoadjuvant Nivolumab for Patients with Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020, 38, 2476–2487. [Google Scholar] [CrossRef]

| Total | ||
| N = 899 | ||
| Sex | ||
| Male | 61.1% (549) | |
| Female | 38.8% (349) | |
| Missing | 0.1% (1) | |
| Age at diagnosis (mean ± sd) | ||
| 75 (11) | ||
| Charlson Comorbidity Index | ||
| 0–1 | 6.6% (59) | |
| 2–3 | 23.4% (210) | |
| ≥4 | 68.7% (618) | |
| Missing | 1.3% (12) | |
| Immunosuppressed | ||
| No | 87.1% (783) | |
| Yes | 10.2% (92) | |
| Missing | 2.7% (24) | |
| Geography | p value | |
| East | 57.0% (512) | <0.001 |
| Central | 20.4% (183) | <0.001 |
| West | 22.7% (204) | <0.001 |
| Hospital | p value | |
| TOH | 9.2% (83) | <0.001 |
| CHUM | 4.3% (39) | <0.001 |
| Sunnybrook | 5.8% (52) | <0.001 |
| PMH/UHN/Mount Sinai | 11.6% (104) | <0.001 |
| Memorial | 2.3% (21) | <0.001 |
| Manitoba | 5.6% (50) | <0.001 |
| Alberta | 14.8% (133) | <0.001 |
| Sherbrooke | 1.7% (15) | <0.001 |
| BC | 22.7% (204) | <0.001 |
| Queen’s | 6.7% (60) | <0.001 |
| McMaster | 11.7% (105) | <0.001 |
| McGill | 3.7% (33) | <0.001 |
| Site of Primary Tumour | p value | |
| Head & neck | 44.6% (401) | <0.001 |
| Upper limbs/shoulder | 21.2% (191) | <0.001 |
| Lower limbs/hips | 16.7% (150) | <0.001 |
| Trunk/back | 7.3% (66) | <0.001 |
| Unknown primary | 9.8% (88) | <0.001 |
| Missing | 0.3% (3) | <0.001 |
| Stage at Diagnosis | p value | |
| Stage 1 | 36.4% (327) | <0.001 |
| Stage 2 | 21.7% (195) | <0.001 |
| Stage 3 | 33.9% (305) | <0.001 |
| Stage 4 | 8.0% (72) | <0.001 |
| Substage at Diagnosis | p value | |
| Stage 1 | 36.4% (327) | <0.001 |
| Stage 2A | 19.0% (171) | <0.001 |
| Stage 2B | 1.7% (15) | <0.001 |
| Stage 3A | 16.9% (152) | <0.001 |
| Stage 3B | 16.7% (150) | <0.001 |
| Stage 4 | 8.0% (72) | <0.001 |
| Missing | 1.3% (12) | <0.001 |
| T Stage at Diagnosis | p value | |
| T0 | 9.8% (88) | <0.001 |
| T1 | 45.3% (407) | <0.001 |
| T2 | 27.0% (243) | <0.001 |
| T3 | 7.9% (71) | <0.001 |
| T4 | 4.9% (44) | <0.001 |
| Missing | 5.1% (46) | <0.001 |
| N Stage at Diagnosis | p value | |
| N0 (clinical or pathological) | 59.2% (532) | <0.001 |
| N1a | 9.2% (83) | <0.001 |
| N1b | 26.1% (235) | <0.001 |
| N2/3 | 3.4% (31) | <0.001 |
| Missing | 2.0% (18) | <0.001 |
| Treatment Received | ||
| None | 7.9% (71) | |
| Surgery alone | 31.4% (282) | |
| Surgery + radiation | 39.3% (353) | |
| Radiation alone | 19.0% (171) | |
| Other 2.4% | −22 | |
| Median Follow-Up Period (years) (IQR) | ||
| 1.9 (0.8–4.3) | ||
| Canadian Population | AJCC 8th Edition Population | ||||
|---|---|---|---|---|---|
| Deaths/Total Population | 5-Year OS | 95% CI | 5-Year OS | 95% CI | |
| Overall survival (N0 Population) by T stage | |||||
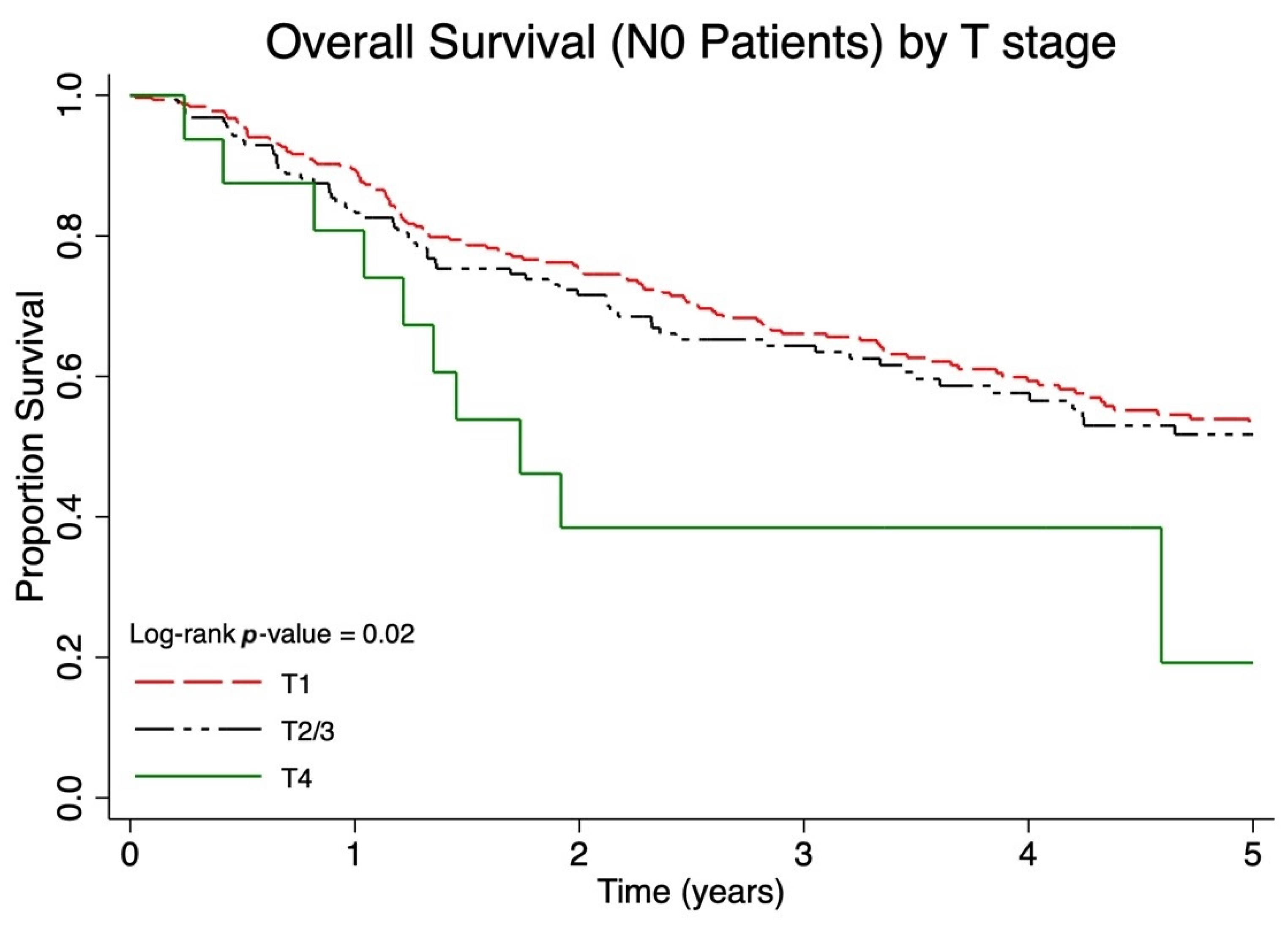
| T1N0 | 112/324 | 56.4% | 50.1–62.2% | 55.6% | 54.1–57.5% |
| T2/3N0 | 62/171 | 53.9% | 45.1–62.0% | 41.1% | 38.8–43.7% |
| T4N0 | 10/16 | 25.9% | 7.2–50.0% | 31.8% | 24.8–38.9% |
| Overall survival (any T) by N stage | |||||
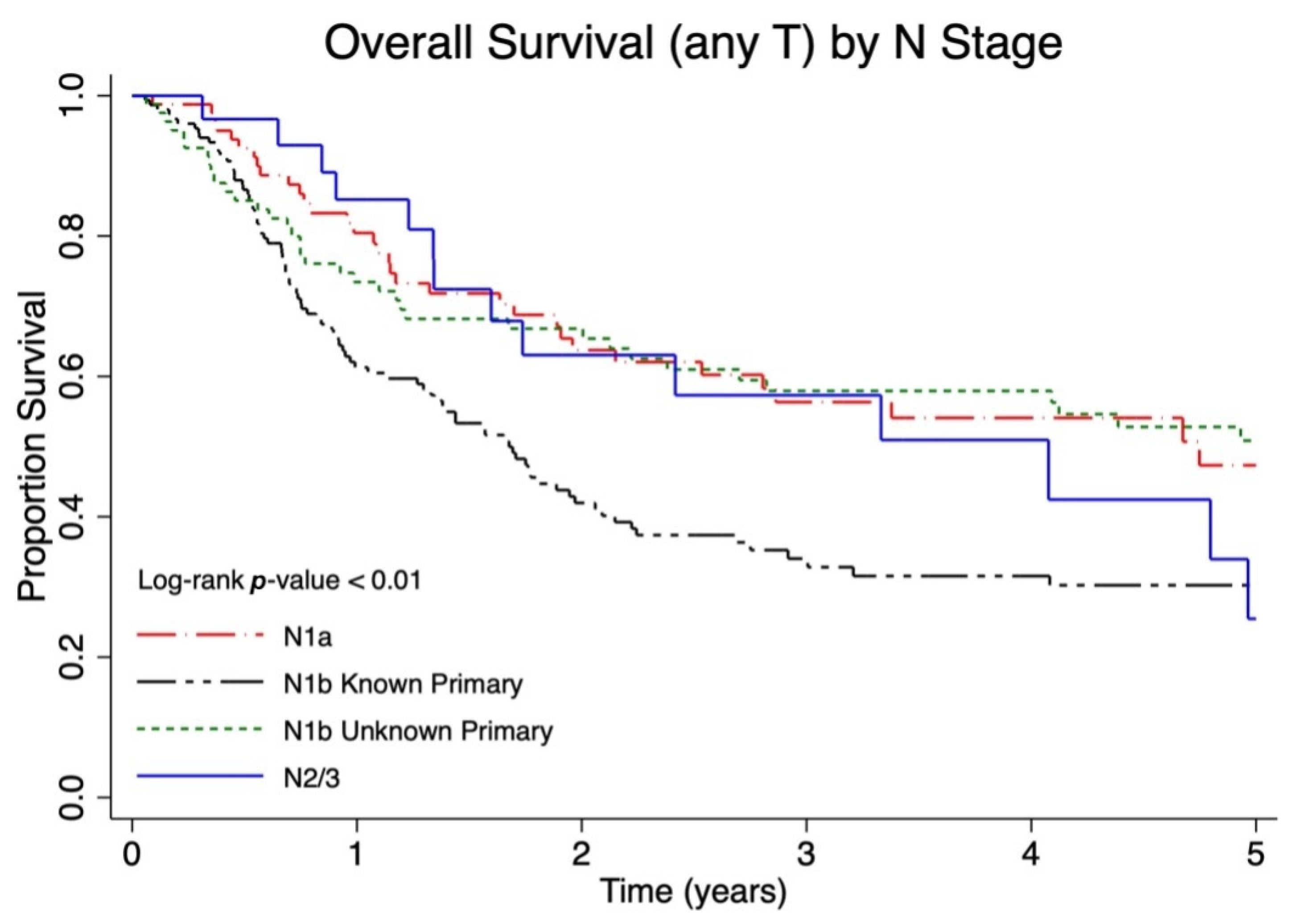
| T anyN1a | 33/82 | 48.4% | 35.8–59% | 39.7% | 36.7–42.7% |
| T anyN1b known primary | 89/153 | 32.6% | 24.8–40.6% | 26.8% | 23.3–30.4% |
| T0N1b unknown primary | 36/82 | 49.7% | 37.6–60.6% | 42.2% | 35.7–48.5% |
| N2/3 | 14/30 | 40.4% | 21.1–59.1% | 41.4% | 25.0–57.0% |
| Overall survival by disease extent | |||||
| Local disease | 59/188 | 54.3% | 49.4–59.0% | 50.6% | 49.2–52.0% |
| Nodal disease | 135/304 | 46.2% | 40.0–52.2% | 35.4% | 33.0–37.6% |
| Metastatic disease | 56/71 | 13.9% | 6.8–23.3% | 13.5% | 11.0–16.3% |
| Overall survival by substage | |||||
| Stage I | 112/326 | 56.8% | 50.5–62.5% | 62.8% | 59.6–65.8% |
| Stage IIA | 61/169 | 54.0% | 45.1–62.0% | 54.6% | 49.3–59.7% |
| Stage IIB | 9/15 | 28.0% | 7.7–53.1% | 34.8% | 25.6–44.1% |
| Stage IIIA | 58/151 | 52.7% | 43.5–61.0% | 40.3% | 37.5–43% |
| Stage IIIB | 75/150 | 40.2% | 31.7–48.7% | 26.8% | 23.4–30.4% |
| Stage IV | 56/71 | 13.9% | 6.8–23.3% | 13.5% | 11.0–16.3% |
| OS | DFS | CSS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths/ Total Population | 5-Year OS | 95% CI | Deaths/ Total Population | 5-Year DFS | 95% CI | Deaths/ Total Population | 5-Year CSS | 95% CI | |
| Stage I | 112/326 | 56.8% | 50.5–62.5% | 79/326 | 66.6% | 60.2–72.2% | 18/326 | 91.5% | 86.9–94.6% |
| Stage IIA | 61/169 | 54.0% | 45.1–62.0% | 52/169 | 58.7% | 49.6–66.7% | 15/169 | 86.3% | 78.0–91.5% |
| Stage IIB | 9/15 | 28.0% | 7.7–53.1% | 7/15 | 36.4% | 11.2–62.9% | 2/15 | 77.8% | 36.5–93.9% |
| Stage IIIA | 58/151 | 52.7% | 43.5–61.0% | 41/151 | 63.9% | 54.3–72.0% | 21/151 | 79.8% | 70.7–86.3% |
| Stage IIIB | 75/150 | 40.2% | 31.7–48.7% | 72/150 | 40.0% | 31.2–48.6% | 34/150 | 65.3% | 55.4–73.5% |
| Stage IV | 56/71 | 13.9% | 6.8–23.3% | 31/71 | 41.0% | 27.9–53.8% | 27/71 | 46.5% | 32.4–59.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dingley, B.; Delisle, M.; Light, A.; Apte, S.; Mallick, R.; Hamilton, T.; Stuart, H.; Talbot, M.; McKinnon, G.; Jost, E.; et al. Survival Outcomes in the Canadian Merkel Cell Carcinoma Population Between 2000 and 2018 and Descriptive Comparison with the American Joint Committee on Cancer 8th Edition Staging System—A Study from the Pan-Canadian Merkel Cell Collaborative. Cancers 2025, 17, 3238. https://doi.org/10.3390/cancers17193238
Dingley B, Delisle M, Light A, Apte S, Mallick R, Hamilton T, Stuart H, Talbot M, McKinnon G, Jost E, et al. Survival Outcomes in the Canadian Merkel Cell Carcinoma Population Between 2000 and 2018 and Descriptive Comparison with the American Joint Committee on Cancer 8th Edition Staging System—A Study from the Pan-Canadian Merkel Cell Collaborative. Cancers. 2025; 17(19):3238. https://doi.org/10.3390/cancers17193238
Chicago/Turabian StyleDingley, Brittany, Megan Delisle, Anne Light, Sameer Apte, Ranjeeta Mallick, Trevor Hamilton, Heather Stuart, Martha Talbot, Gregory McKinnon, Evan Jost, and et al. 2025. "Survival Outcomes in the Canadian Merkel Cell Carcinoma Population Between 2000 and 2018 and Descriptive Comparison with the American Joint Committee on Cancer 8th Edition Staging System—A Study from the Pan-Canadian Merkel Cell Collaborative" Cancers 17, no. 19: 3238. https://doi.org/10.3390/cancers17193238
APA StyleDingley, B., Delisle, M., Light, A., Apte, S., Mallick, R., Hamilton, T., Stuart, H., Talbot, M., McKinnon, G., Jost, E., Thiboutot, E., Francescutti, V., Samman, S., Easson, A. M., Schellenberg, A., Merchant, S., La, J., Vanderbeck, K., Wright, F. C., ... Nessim, C. (2025). Survival Outcomes in the Canadian Merkel Cell Carcinoma Population Between 2000 and 2018 and Descriptive Comparison with the American Joint Committee on Cancer 8th Edition Staging System—A Study from the Pan-Canadian Merkel Cell Collaborative. Cancers, 17(19), 3238. https://doi.org/10.3390/cancers17193238








