Initial Treatment and Outcomes of Complete Hydatidiform Mole in Women 40 Years or Older: A Multicenter Cohort Study
Simple Summary
Abstract
1. Introduction
2. Methods
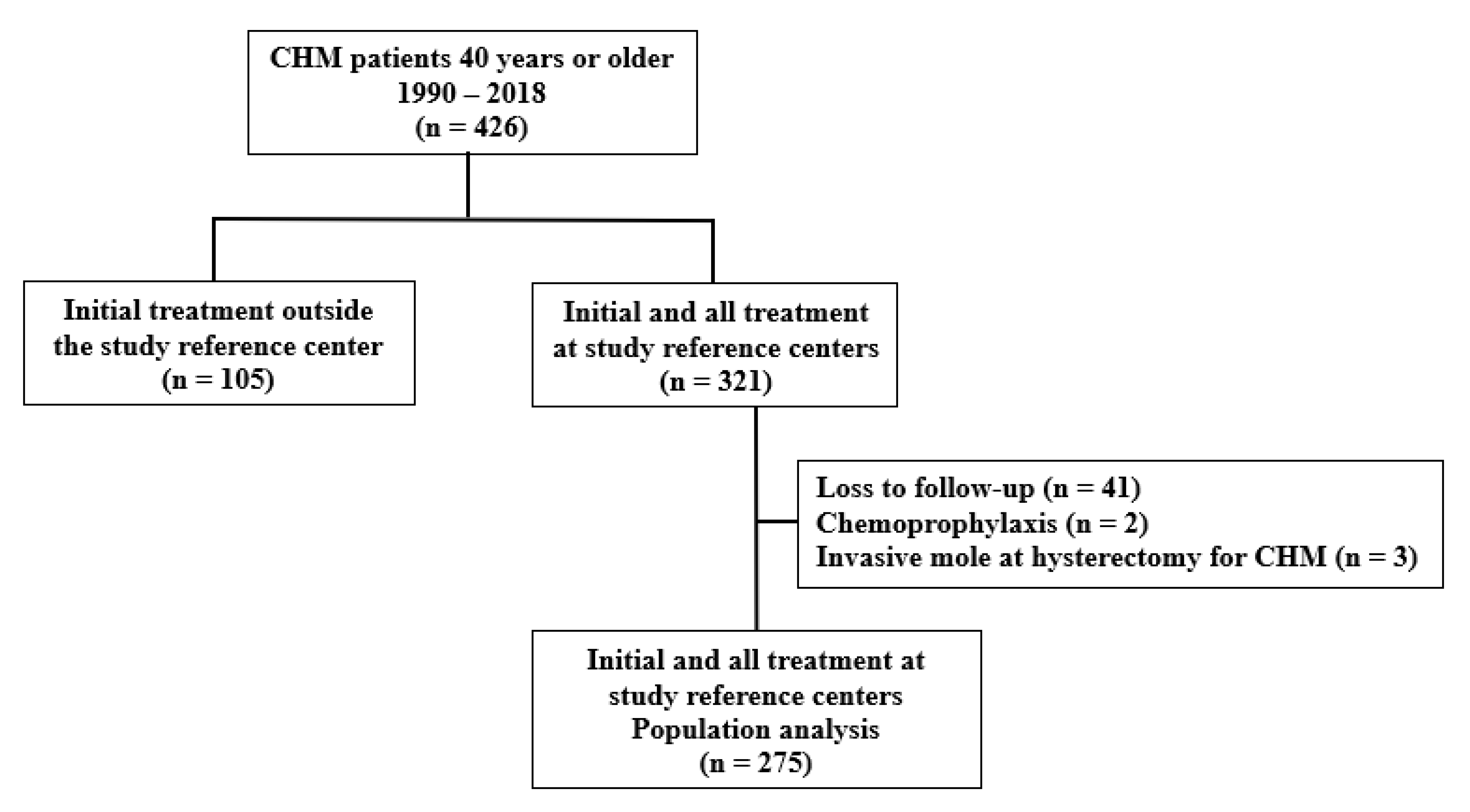
2.1. Study Design and Setting
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection and Study Variables
2.4. Outcomes
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szulman, A.; Surti, U. The syndromes of hydatidiform mole. Am. J. Obstet. Gynecol. 1978, 132, 20–27. [Google Scholar] [CrossRef]
- Royal College of Obstetricians & Gynaecologists. Management of Gestational Trophoblastic Disease: Green-top Guideline No. 38. BJOG 2021, 128, e1–e27. [Google Scholar] [CrossRef]
- Savage, P.M.; Sita-Lumsden, A.; Dickson, S.; Iyer, R.; Everard, J.; Coleman, R.; Fisher, R.A.; Short, D.; Casalboni, S.; Catalano, K.; et al. The relationship of maternal age to molar pregnancy incidence, risks for chemotherapy and subsequent pregnancy outcome. J. Obstet. Gynaecol. 2013, 33, 406–411. [Google Scholar] [CrossRef]
- Seckl, M.J.; Fisher, R.A.; Salerno, G.; Rees, H.; Paradinas, F.J.; Foskett, M.; Newlands, E.S. Choriocarcinoma and partial hydatidiform moles. Lancet. 2000, 356, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Soper, J.T. Gestational Trophoblastic Disease: Current Evaluation and Management. Obstet. Gynecol. 2021, 137, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Foskett, M.; Fisher, R.A.; Rees, H.; Seckl, M.; Newlands, E. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. BJOG 2002, 109, 99–102. [Google Scholar] [CrossRef]
- Lok, C.; Frijstein, M.; van Trommel, N. Clinical presentation and diagnosis of Gestational Trophoblastic Disease. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021, 74, 42–52. [Google Scholar] [CrossRef]
- Lok, C.; van Trommel, N.; Braicu, E.I.; Planchamp, F.; Berkowitz, R.; Seckl, M.; on behalf of the EOTTD-ESGO-GCIG-ISSTD Guideline Committee; Baas, I.O.; Bergamini, A.; Abreu, M.H.; et al. Practical Guidelines for the Treatment of Gestational Trophoblastic Disease: Collaboration of the European Organisation for the Treatment of Trophoblastic Disease (EOTTD)–European Society of Gynaecologic Oncology (ESGO)–Gynecologic Cancer InterGroup (GCIG)–International Society for the Study of Trophoblastic Diseases (ISSTD). J. Clin. Oncol. 2025, 43, 2119–2128. [Google Scholar] [CrossRef]
- Ngan, H.Y.S.; Seckl, M.J.; Berkowitz, R.S.; Xiang, Y.; Golfier, F.; Sekharan, P.K.; Braga, A.; Garrett, A. Diagnosis and management of gestational trophoblastic disease: 2025 update. Int. J. Gynecol. Obstet. 2025, 171, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Chen, Q.; Lu, W. Comparison of different therapeutic strategies for complete hydatidiform mole in women at least 40 years old: A retrospective cohort study. BMC Cancer. 2017, 17, 733. [Google Scholar] [CrossRef]
- Giorgione, V.; Bergamini, A.; Cioffi, R.; Pella, F.; Rabaiotti, E.; Petrone, M.; Candiani, M.; Mangili, G. Role of Surgery in the Management of Hydatidiform Mole in Elderly Patients. Int. J. Gynecol. Cancer 2017, 27, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Makrydimas, G.; Agnantis, N.J.; Zagorianakou, N.; Rees, H.; Fisher, R.A. Updated diagnostic criteria for partial and complete hydatidiform moles in early pregnancy. Anticancer Res. 2003, 23, 1723–1728. [Google Scholar]
- Chilosi, M.; Piazzola, E.; Lestani, M.; Benedetti, A.; Guasparri, I.; Granchelli, G.; Aldovini, D.; Leonardi, E.; Pizzolo, G.; Doglioni, C.; et al. Differential expression of p57kip2, a maternally imprinted cdk inhibitor, in normal human placenta and gestational trophoblastic disease. Lab. Investig. 1998, 78, 269–276. [Google Scholar]
- FIGO Oncology Committee. FIGO staging for gestational trophoblastic neoplasia 2000. FIGO Oncology Committee. Int. J. Gynaecol Obstet. 2022, 77, 285–287. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Fertil. Steril. 2014, 101, 633–634. [CrossRef]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Braga, A.; Canelas, A.C.; Torres, B.; Maesta, I.; Pedrotti, L.G.; Bessel, M.; Esteves, A.P.V.d.S.; Junior, J.A.; Filho, J.R.; Elias, K.M.; et al. Neutrophil/lymphocyte ratio and other blood cell component counts are not associated with the development of postmolar gestational trophoblastic neoplasia. PLoS ONE 2022, 17, e0277892. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, Y.; Huang, W.; Tong, B.; Lu, W. Total hysterectomy versus uterine evacuation for preventing post-molar gestational trophoblastic neoplasia in patients who are at least 40 years old: A systematic review and meta-analysis. BMC Cancer 2019, 19, 13. [Google Scholar] [CrossRef]
- Maestá, I.; Growdon, W.B.; Goldstein, D.P.; Bernstein, M.R.; Horowitz, N.S.; Rudge, M.V.C.; Berkowitz, R.S. Prognostic factors associated with time to hCG remission in patients with low-risk postmolar gestational trophoblastic neoplasia. Gynecol. Oncol. 2013, 130, 312–316. [Google Scholar] [CrossRef]
- Maestá, I.; Nitecki, R.; Horowitz, N.S.; Goldstein, D.P.; Moreira, M.d.F.S.; Elias, K.M.; Berkowitz, R.S. Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: The New England Trophoblastic Disease Center experience. Gynecol. Oncol. 2018, 148, 161–167. [Google Scholar] [CrossRef]
- Chapman-Davis, E.; Hoekstra, A.V.; Rademaker, A.W.; Schink, J.C.; Lurain, J.R. Treatment of nonmetastatic and metastatic low-risk gestational trophoblastic neoplasia: Factors associated with resistance to single-agent methotrexate chemotherapy. Gynecol. Oncol. 2012, 125, 572–575. [Google Scholar] [CrossRef]
- Patel, M.; Stuparich, M.; Nahas, S. Total laparoscopic hysterectomy in combination with dilation and evacuation of an 18-week–sized uterus with gestational trophoblastic neoplasia: A novel treatment approach. Am. J. Obstet. Gynecol. 2021, 224, 314–315. [Google Scholar] [CrossRef]
- Bolze, P.-A.; Mathe, M.; Hajri, T.; You, B.; Dabi, Y.; Schott, A.-M.; Patrier, S.; Massardier, J.; Golfier, F. First-line hysterectomy for women with low-risk non-metastatic gestational trophoblastic neoplasia no longer wishing to conceive. Gynecol. Oncol. 2018, 150, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Pickett, C.M.; Seeratan, D.D.; Mol, B.W.J.; Nieboer, T.E.; Johnson, N.; Bonestroo, T.; Aarts, J.W.; Cochrane Gynaecology and Fertility Group. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst. Rev. 2023, 2023, CD003677. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, M.; Wu, C.; Zhu, Q.; Wu, T.; Zhu, X.; Wu, M.; Wang, S. Effect of hysterectomy on ovarian function: A systematic review and meta-analysis. J. Ovarian Res. 2023, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.-S.; Kim, B.G.; Lee, B.K.; Seo, J.; Kim, G.S.; Min, K.; Lee, H.Y.; Byun, Y.S.; Yang, S.-W.; Kim, M.-H.; et al. Association of Early Hysterectomy With Risk of Cardiovascular Disease in Korean Women. JAMA Netw. Open 2023, 6, e2317145. [Google Scholar] [CrossRef]
| Initial Treatment | ||||
|---|---|---|---|---|
| Variable | Overall (n = 275) | Hysterectomy (n = 31) | Evacuation (n = 244) | p |
| Age (years) | 44.0 (42.0–47.0) | 47.0 (45.0–51.0) | 44.0 (41.0–46.0) | 0.001 (1) |
| Gravidity | 3.0 (3.0–5.0) | 5.0 (3.0–6.3) | 3.0 (3.0–5.0) | 0.001 (1) |
| Parity | 2.0 (1.0–3.0) | 2.0 (1.0–4.0) | 2.0 (1.0–2.0) | 0.007 (1) |
| Presenting gynecological disorders | NA | 17/31 (54.8%) | NA | - |
| Uterus fibroids | 12 (38.7%) | |||
| Adenomyosis | 4 (12.9%) | |||
| Cervical intraepithelial neoplasia | 1 (3.2%) | |||
| Gestational age (weeks) | 11.0 (10.0–13.0) | 13.0 (10.0–15.0) | 11.0 (10.0–13.0) | 0.064 (1) |
| Uterine size > GA (a) | 94 (37.2%) | 12 (60.0%) | 82 (35.2%) | 0.028 (2) |
| Vaginal bleeding (b) | 203 (75.7%) | 20 (69.0%) | 183 (76.6%) | 0.367 (2) |
| Theca lutein cysts | 22 (8.0%) | 5 (16.1%) | 17 (9.8%) | 1.000 (2) |
| Pre-evacuation hCG (IU/L) | 235,497 | 373,790 | 215,276 | 0.007 (1) |
| (93,228–494,355) | (243,440–976,112) | (84,469–475,271) | ||
| Hemorrhage | 27 (9.8%) | 3 (9.7%) | 24 (9.8%) | 1.000 (3) |
| Hyperemesis | 84 (30.5%) | 5 (16.1%) | 79 (32.4%) | 0.096 (2) |
| Preeclampsia | 20 (7.3%) | 5 (16.1%) | 15 (6.1%) | 0.059 (2) |
| Hyperthyroidism (c) | 25 (9.4%) | 8 (27.6%) | 17 (7.2%) | 0.001 (2) |
| ARDS | 2 (0.7%) | 0 (0.0%) | 2 (0.8%) | 1.000 (3) |
| ≥2 medical complications at presentation | 33 (12.0%) | 7 (22.6%) | 26 (10.7%) | 0.054 (2) |
| GTN | 76 (27.6%) | 3 (9.6%) | 73 (29.9%) | 0.018 (2) |
| Required chemotherapy for remission | 72 (26.1%) | 2 (6.4%) | 70 (28.6%) | 0.007 (3) |
| Time to remission (*) | 69.0 (51.5–90.0) | 80.0 (70.5–107.5) | 65.0 (49.0–89.0) | 0.002 (1) |
| Variable | RR | 95% CI | p | |
|---|---|---|---|---|
| Hysterectomy | 0.17 | 0.04 | 0.71 | 0.015 |
| ≥2 medical complications | 0.94 | 0.49 | 1.78 | 0.849 |
| >1,000,000 IU/L | 18.42 | 5.24 | 64.78 | <0.001 |
| (750,000–100,000] IU/L | 9.48 | 2.11 | 42.70 | 0.003 |
| (50,000–750,000] IU/L | 9.67 | 2.65 | 35.25 | 0.001 |
| (250,000–500,000] IU/L | 8.06 | 2.37 | 27.34 | 0.001 |
| [100,000–250,000] IU/L | 5.48 | 1.59 | 18.82 | 0.007 |
| hCG preevacuation (Ref: <100,000 IU/L) | 1 | |||
| RR | 95% CI | p | ||
|---|---|---|---|---|
| ≥2 medical complications | 0.97 | 0.51 | 1.87 | 0.938 |
| >1,000,000 IU/L | 18.43 | 5.23 | 64.93 | 0.001 |
| (750,000–100,000] IU/L | 9.47 | 2.10 | 42.62 | 0.003 |
| (50,000–750,000] IU/L | 8.71 | 2.35 | 32.27 | 0.001 |
| (250,000–500,000] IU/L | 7.73 | 2.27 | 26.32 | 0.001 |
| [100,000–250,000] IU/L | 4.81 | 1.38 | 16.73 | 0.014 |
| hCG pre-evacuation (Ref: <100,000 IU/L) | 1 | |||
| Hysterectomy | 0.089 | 0.012 | 0.640 | 0.016 |
| N | % | |
|---|---|---|
| Occurrence of complications | 14 | 45.1 |
| Type of complication | ||
| Blood transfusion–Grade II | 5 | 35.7 |
| Bladder injury/intraoperative repair–Grade IIIa | 2 | 14.3 |
| Postoperative paralytic ileus–Grade I | 1 | 7.14 |
| Changed procedure *–Grade I | 1 | 7.14 |
| Pulmonary embolism–Grade II | 1 | 7.14 |
| Atelectasis/lung physiotherapy–Grade I | 1 | 7.14 |
| Incisional hematoma–Grade I | 1 | 7.14 |
| Vaginal vault granulation tissue #–Grade I | 1 | 7.14 |
| Urinary infection–Grade II | 1 | 7.14 |
| Type of hysterectomy | ||
| Total | 29 | 93.5 |
| Subtotal | 2 | 6.5 |
| Route of hysterectomy | ||
| Laparotomy | 28 | 90.3 |
| Laparoscopy | 3 | 9.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmarais, C.C.F.; Maestá, I.; Sun, S.Y.; de Rezende-Filho, J.; de Araújo Costa, R.A.; Lin, L.H.; Branco-Silva, M.; Horowitz, N.S.; Elias, K.M.; Braga, A.; et al. Initial Treatment and Outcomes of Complete Hydatidiform Mole in Women 40 Years or Older: A Multicenter Cohort Study. Cancers 2025, 17, 3125. https://doi.org/10.3390/cancers17193125
Desmarais CCF, Maestá I, Sun SY, de Rezende-Filho J, de Araújo Costa RA, Lin LH, Branco-Silva M, Horowitz NS, Elias KM, Braga A, et al. Initial Treatment and Outcomes of Complete Hydatidiform Mole in Women 40 Years or Older: A Multicenter Cohort Study. Cancers. 2025; 17(19):3125. https://doi.org/10.3390/cancers17193125
Chicago/Turabian StyleDesmarais, Cecília Canêdo Freitas, Izildinha Maestá, Sue Yazaki Sun, Jorge de Rezende-Filho, Roberto Antonio de Araújo Costa, Lawrence Hsu Lin, Mariza Branco-Silva, Neil S. Horowitz, Kevin M. Elias, Antonio Braga, and et al. 2025. "Initial Treatment and Outcomes of Complete Hydatidiform Mole in Women 40 Years or Older: A Multicenter Cohort Study" Cancers 17, no. 19: 3125. https://doi.org/10.3390/cancers17193125
APA StyleDesmarais, C. C. F., Maestá, I., Sun, S. Y., de Rezende-Filho, J., de Araújo Costa, R. A., Lin, L. H., Branco-Silva, M., Horowitz, N. S., Elias, K. M., Braga, A., & Berkowitz, R. S. (2025). Initial Treatment and Outcomes of Complete Hydatidiform Mole in Women 40 Years or Older: A Multicenter Cohort Study. Cancers, 17(19), 3125. https://doi.org/10.3390/cancers17193125





