Decline of PD-L1 Immunoreactivity with Storage Duration in Formalin-Fixed Paraffin-Embedded Breast Cancer Specimens: Implications for Diagnostic Accuracy and Immunotherapy Eligibility in Triple-Negative Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Pathological Assessment at Diagnosis
2.3. FFPE Storage and Restaining
2.4. Tissue Fixation and Processing
2.5. PD-L1 Immunohistochemistry (22C3 pharmDx)
Total number of viable tumor cells
2.6. Classification of Storage Duration
- FFPE storage duration was categorized as:
- <1 year
- 1–2 years
- 2–3 years
- ≥3 years
- PD-L1 status changes were classified as:
- Increased staining
- No change
- Decreased staining (CPS decreased to <10)
2.7. Association with Clinicopathologic Factors
2.8. Survival Analysis
2.9. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Neoadjuvant Chemotherapy and pCR
3.3. Change in PD-L1 Status After Storage
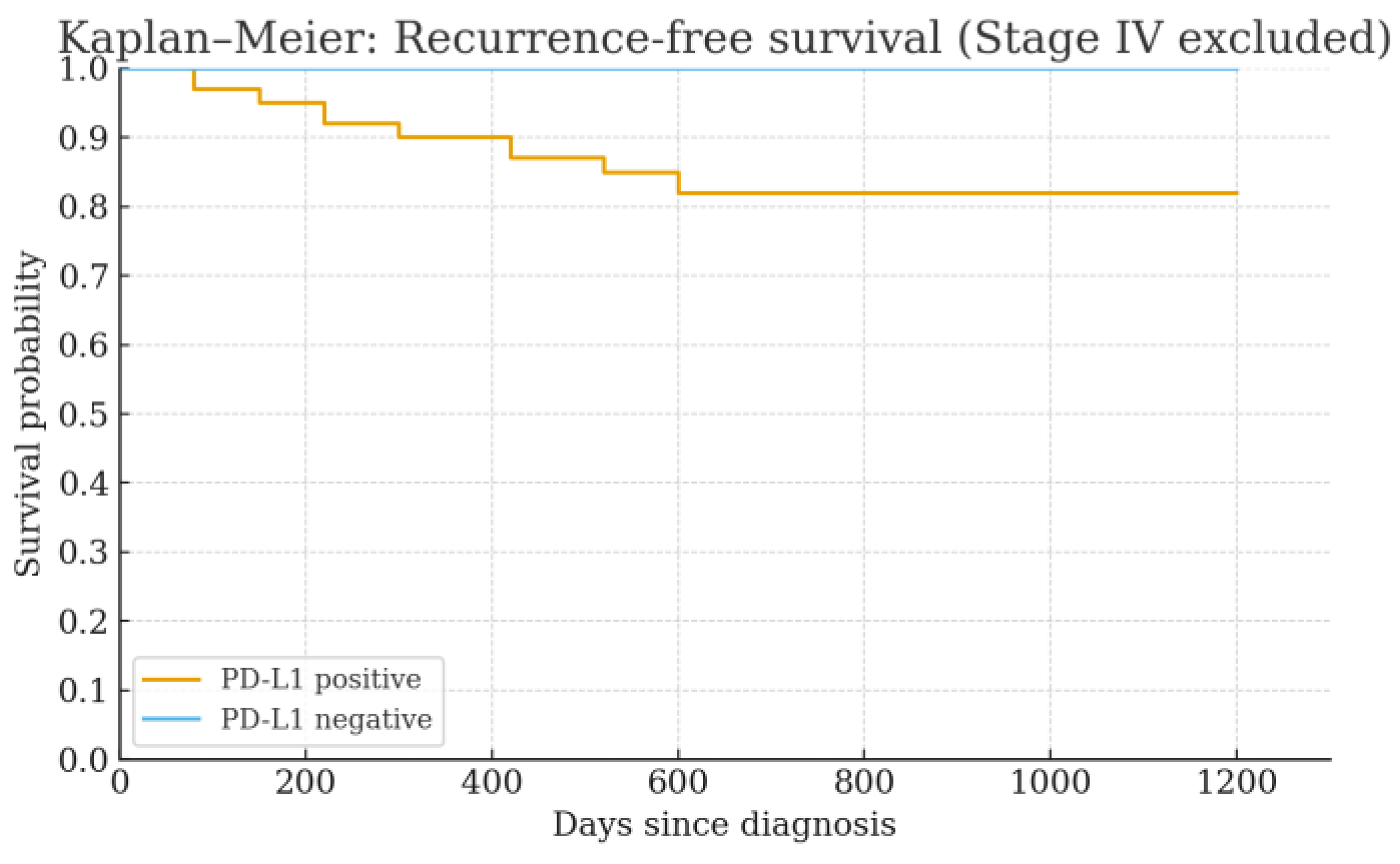
3.4. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FFPE | Formalin-fixed paraffin-embedded |
| PD-L1 | Programmed death-ligand 1 |
| IHC | Immunohistochemistry |
| ICI | Immune checkpoint inhibitor |
| TNBC | Triple-negative breast cancer |
| CPS | Combined Positive Score |
| pCR | Pathologic complete response |
| NAC | Neoadjuvant chemotherapy |
| ER | Estrogen receptor |
| PgR | Progesterone receptor |
| HER2 | human epidermal growth factor receptor 2 |
| NG | Nuclear grade |
| RFS | Recurrence-free survival |
| OS | Overall survival |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.; Feuer, E.J.; et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, A.; Tokunaga, E.; Masuda, N.; Shien, T.; Kawabata, K.; MIyashita, M. Clinicopathological features of young patients (<35 years of age) with breast cancer in a Japanese Breast Cancer Society supported study. Breast Cancer 2014, 21, 643–650. [Google Scholar]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Lee, A.H.; Robertson, J.F.; Ellis, I.O. Prognostic markers in triple-negative breast cancer. Cancer 2007, 109, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; O’Neill, R.; Shah, R.; Langseliu, O.; Su, Y.; Frick, C.; Fink, H.; Bardot, A.; M. Walsh, P.; R. Woods, R.; et al. Metastatic recurrence in women diagnosed with non-metastatic breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2024, 26, 171. [Google Scholar] [CrossRef]
- Biswas, T.; Efird, J.T.; Prasad, S.; Jindal, C.; Walker, P.R. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget 2017, 8, 112712–112719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007, 13 Pt 1, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Citron, M.L.; Berry, D.A.; Cirrincione, C.; Hudis, C.; Winer, E.P.; Gradishar, W.J.; Davidson, N.E.; Martino, S.; Livingston, R.; Ingle, J.N.; et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 2003, 21, 1431–1439, Erratum in J. Clin. Oncol. 2003, 21, 2226. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. IMpassion130 Trial Investigators. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Diéras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Nguyen-Duc, A.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800, Erratum in Nat. Med. 2002, 8, 1039. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 511–520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Engel, K.B.; Moore, H.M. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch. Pathol. Lab. Med. 2011, 135, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Grillo, F.; Bruzzone, M.; Pigozzi, S.; Prosapio, S.; Migliora, P.; Fiocca, R.; Mastracci, L. Immunohistochemistry on old archival paraffin blocks: Is there an expiry date? J. Clin. Pathol. 2017, 70, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Grillo, F.; Pigozzi, S.; Ceriolo, P.; Calamaro, P.; Fiocca, R.; Mastracci, L. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem. Cell Biol. 2015, 144, 93–99. [Google Scholar] [CrossRef] [PubMed]
- RLott, J.; Tunnicliff, B.; Mackay, C. Steele, The effects of prolonged formalin fixation on the immunohistochemical demonstration of antigens. J. Histotechnol. 1986, 9, 173–177. [Google Scholar]
- Agilent Technologies. PD-L1 IHC 22C3 pharmDx TNBC Interpretation Manual. Available online: https://www.agilent.com/cs/library/usermanuals/public/29389_22c3_pharmdx_tnbc_interpretation_manual_kn355.pdf (accessed on 6 March 2025).
- Werner, M.; Chott, A.; Fabiano, A.; Battifora, H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am. J. Surg. Pathol. 2000, 24, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–526. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rimm, D.L.; Han, G.; Taube, J.M.; Yi, E.S.; Bridge, J.A.; Flieder, D.B.; Homer, R.; West, W.W.; Wu, H.; Roden, A.C.; et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017, 3, 1051–1058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schalper, K.A.; Velcheti, V.; Carvajal, D.; Wimberly, H.; Brown, J.; Pusztai, L.; Rimm, D.L. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin. Cancer Res. 2014, 20, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gatti-Mays, M.E.; Kalinsky, K.; Korde, L.A.; Sharon, E.; Amiri-Kordestani, L.; Bear, H.; McArthur, H.L.; Frank, E.; Perlmutter, J.; et al. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol. 2019, 5, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fitzgibbons, P.L.; Bradley, L.A.; Fatheree, L.A.; Alsabeh, R.; Fulton, R.S.; Goldsmith, J.D.; Haas, T.S.; Karabakhtsian, R.G.; Loykasek, P.A.; Marolt, M.J.; et al. College of American Pathologists Pathology Laboratory Quality Center Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology Laboratory Quality Center. Arch. Pathol. Lab. Med. 2014, 138, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Shohdy, K.S.; Elghazawy, H.; Salib, M.M.; Almeldin, D.; Kassem, L. Programmed death-ligand 1 (PD-L1) expression predicts response to neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Biomarkers 2022, 27, 764–772. [Google Scholar] [CrossRef]
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, D.R.; Rimm, D.L. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol. Res. 2015, 3, 326–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, J.; Wang, X.; Cai, L.; Jia, Z.; Liu, C.; Sun, X.; Wu, S.; Ding, C.; Zhang, Z.; Liu, Y. Effect of storage time of paraffin sections on the expression of PD-L1 (SP142) in invasive breast cancer. Diagn. Pathol. 2023, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.I.; Gaule, P.; Rimm, D.L. Tissue Age Affects Antigenicity and Scoring for the 22C3 Immunohistochemistry Companion Diagnostic Test. Mod. Pathol. 2023, 36, 100159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haragan, A.; Liebler, D.C.; Das, D.M.; Soper, M.D.; Morrison, R.D.; Slebos, R.J.C.; Ackermann, B.L.; Fill, J.A.; Schade, A.E.; Gosney, J.R.; et al. Accelerated instability testing reveals quantitative mass spectrometry overcomes specimen storage limitations associated with PD-L1 immunohistochemistry. Lab. Investig. 2020, 100, 874–886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
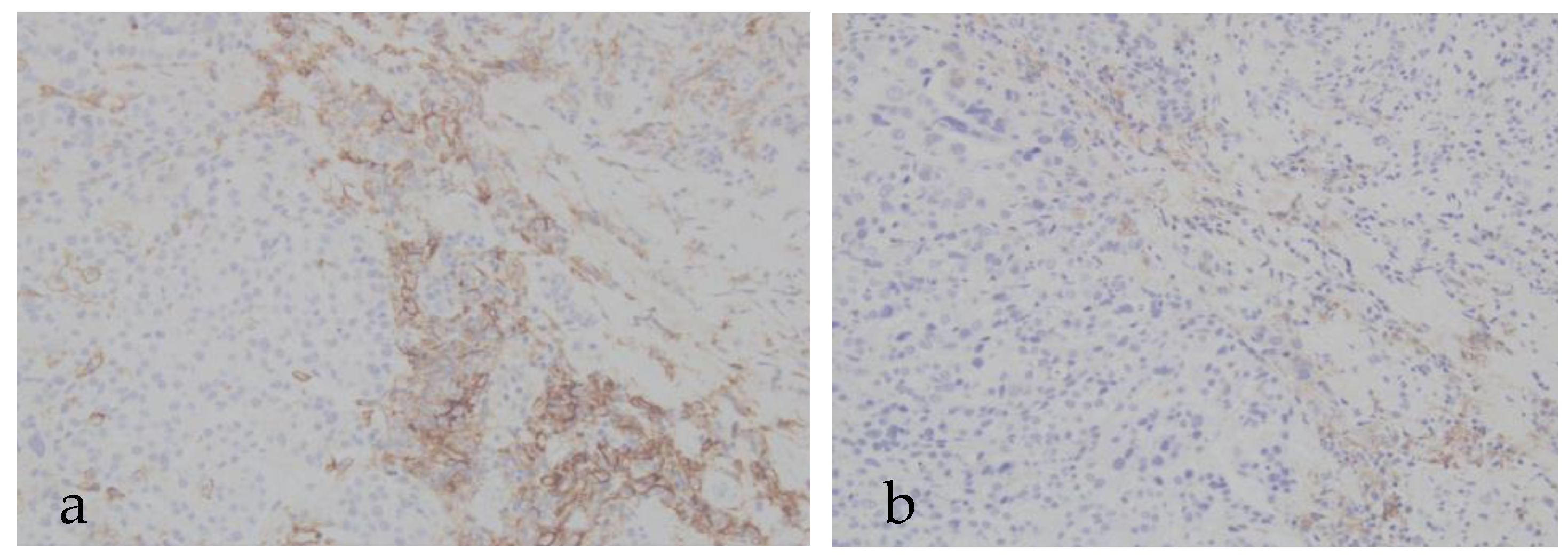
| Negative | Positive | |
|---|---|---|
| Number of cases (n) | 22 | 41 |
| Age (years) (mean) | 42–87 (63.3) | 42–93 (67.4) |
| Observation period (days) (median) | 94–1334 (869) | 63–1298 (574) |
| FFPE elapsed years ≥ 3 (cases) | 9 (41%) | 8 (20%) |
| 2–3 years | 9 (41%) | 16 (39%) |
| 1–2 years | 2 (9%) | 9 (22%) |
| <1 year | 2 (9%) | 8 (20%) |
| Ki67 (median) | 7–90 (25.2%) | 20–99 (48.2%) |
| NG 1 | 9 (41%) | 6 (15%) |
| 2 | 6 (27%) | 8 (20%) |
| 3 | 6 (27%) | 26 (63%) |
| Unknown | 1 (5%) | 1 (2%) |
| p53 wild type | 11 (50%) | 15 (37%) |
| mutant | 7 (32%) | 20 (49%) |
| null | 0 | 3 (7%) |
| equivocal | 2 (9%) | 0 |
| unknown | 2 (9%) | 3 (7%) |
| Stage I | 11 (50%) | 8 (20%) |
| II | 6 (27%) | 20 (49%) |
| III | 2 (9%) | 9 (22%) |
| IV | 3 (14%) | 4 (10%) |
| Neoadjuvant chemotherapy: Yes | 11 (50%) | 15 (37%) |
| No | 11 (50%) | 26 ** (63%) |
| Number of recurrence cases * | 0 | 5 (14%) |
| Number of deaths * | 0 | 3 (8%) |
| Negative (n = 11) | Positive (n = 15) | Fisher’s Exact Test | |
|---|---|---|---|
| pCR | 0 (0%) | 5 (33%) | p-value |
| non-pCR | 11 (100%) | 10 (67%) | 0.0527 |
| Negative (n = 11) | Positive (n = 15) | |||||
|---|---|---|---|---|---|---|
| Regimen | n = 11 | non-pCR | pCR | n = 15 | non-pCR | pCR |
| Dose Dense | 8 | 8 | 0 | 9 | 6 | 3 |
| Triweekly | 2 | 2 | 0 | 1 | 1 | 0 |
| ICI combination | 0 | 0 | 0 | 2 | 0 | 2 |
| Bevacizumab + Paclitaxel | 1 | 1 | 0 | 3 | 3 | 0 |
| Negative (n = 22) | Positive (n = 41) | |
|---|---|---|
| Increased staining | 0 (0%) | 0 (0%) |
| No change | 22 (100%) | 34 (83%) |
| Decreased staining | 0 (0%) | 7 (17%) |
| Negative (n = 22) | Positive (n = 41) | ||||||
|---|---|---|---|---|---|---|---|
| After Storage | n | No Change | Decreased | n | No Change | Decreased | Negative vs. Positive |
| <3 years | 9 | 9 (100%) | 0 | 8 | 4 (50%) | 4 (50%) | p = 0.0294 |
| 2–3 years | 9 | 9 (100%) | 0 | 16 | 14 (88%) | 2 (13%) | p = 0.520 |
| 1–2 years | 2 | 2 (100%) | 0 | 9 | 8 (89%) | 1 (11%) | p = 1.000 |
| >1 year | 2 | 2 (100%) | 0 | 8 | 8 (100%) | 0 | p = 1.000 |
| Total | 22 | 22 (100%) | 0 | 41 | 34 (83%) | 7 (17%) | |
| N/A | p = 0.015 | Cochran-Armitage trend test | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagihara, K.; Nagata, K.; Yamakawa, T.; Kato, S.; Tamura, M.; Yoshida, M. Decline of PD-L1 Immunoreactivity with Storage Duration in Formalin-Fixed Paraffin-Embedded Breast Cancer Specimens: Implications for Diagnostic Accuracy and Immunotherapy Eligibility in Triple-Negative Breast Cancer. Cancers 2025, 17, 3103. https://doi.org/10.3390/cancers17193103
Yanagihara K, Nagata K, Yamakawa T, Kato S, Tamura M, Yoshida M. Decline of PD-L1 Immunoreactivity with Storage Duration in Formalin-Fixed Paraffin-Embedded Breast Cancer Specimens: Implications for Diagnostic Accuracy and Immunotherapy Eligibility in Triple-Negative Breast Cancer. Cancers. 2025; 17(19):3103. https://doi.org/10.3390/cancers17193103
Chicago/Turabian StyleYanagihara, Keiko, Koji Nagata, Tamami Yamakawa, Sena Kato, Miki Tamura, and Masato Yoshida. 2025. "Decline of PD-L1 Immunoreactivity with Storage Duration in Formalin-Fixed Paraffin-Embedded Breast Cancer Specimens: Implications for Diagnostic Accuracy and Immunotherapy Eligibility in Triple-Negative Breast Cancer" Cancers 17, no. 19: 3103. https://doi.org/10.3390/cancers17193103
APA StyleYanagihara, K., Nagata, K., Yamakawa, T., Kato, S., Tamura, M., & Yoshida, M. (2025). Decline of PD-L1 Immunoreactivity with Storage Duration in Formalin-Fixed Paraffin-Embedded Breast Cancer Specimens: Implications for Diagnostic Accuracy and Immunotherapy Eligibility in Triple-Negative Breast Cancer. Cancers, 17(19), 3103. https://doi.org/10.3390/cancers17193103








