Integration of Next-Generation Sequencing in Measurable Residual Disease Monitoring in Acute Myeloid Leukemia and Myelodysplastic Neoplasm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
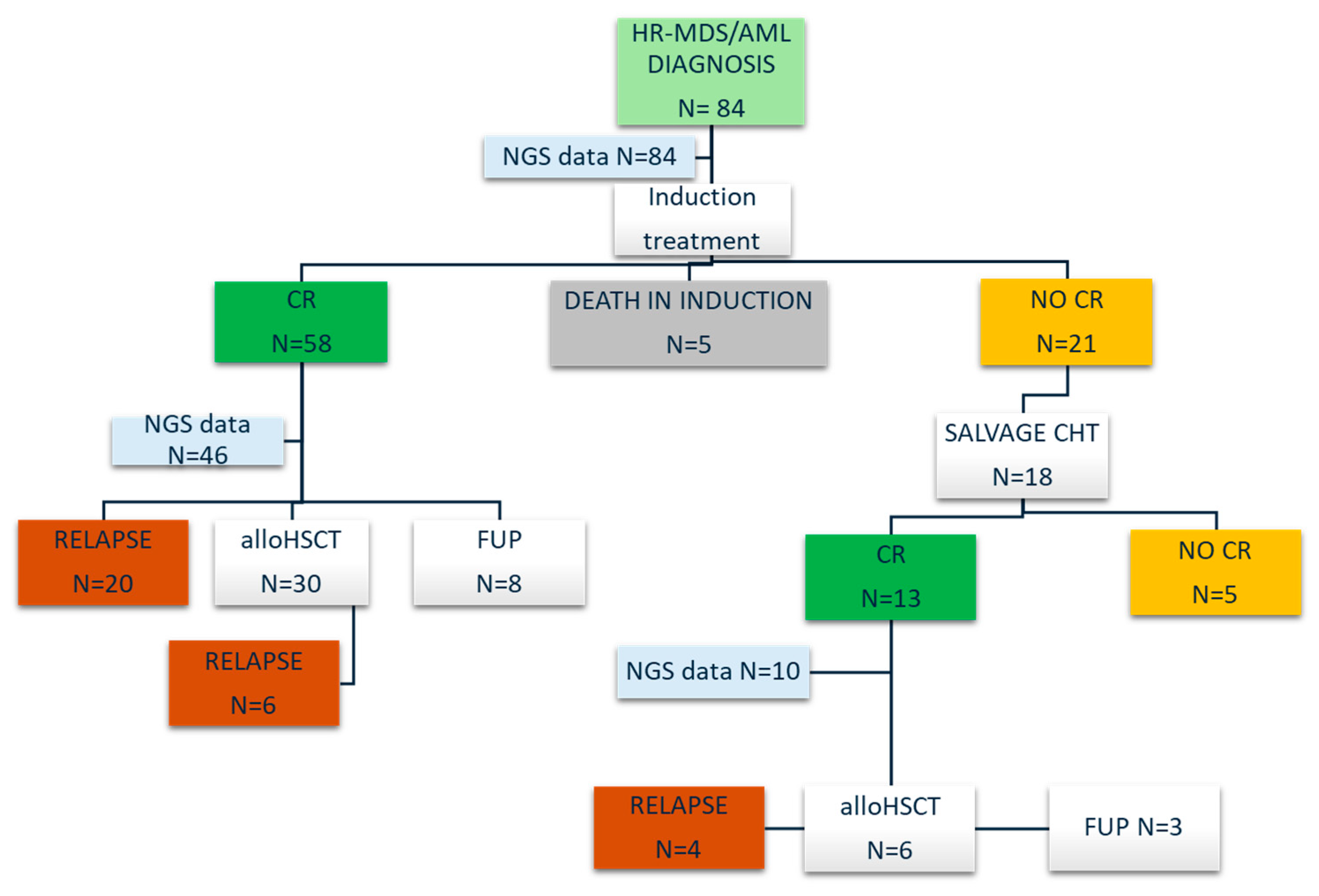
3. Results
3.1. Patient Population
3.2. Clinical Outcomes
3.3. NGS Results and MRD Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Adès, L.; Itzykson, R.; Fenaux, P. Myelodysplastic Syndromes. Lancet 2014, 383, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Dombret, H.; Itzykson, R. How and When to Decide between Epigenetic Therapy and Chemotherapy in Patients with Aml. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Leone, G.; Piciocchi, A.; Fianchi, L.; Santarone, S.; Candoni, A.; Criscuolo, M.; Masciulli, A.; Cerqui, E.; Molteni, A.; et al. Feasibility of Allogeneic Stem-Cell Transplantation after Azacitidine Bridge in Higher-Risk Myelodysplastic Syndromes and Low Blast Count Acute Myeloid Leukemia: Results of the Bmt-Aza Prospective Study. Ann. Oncol. 2017, 28, 1547–1553. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk Aml. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef]
- Balsat, M.; Renneville, A.; Thomas, X.; de Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.-P.; Nibourel, O.; Berthon, C.; et al. Postinduction Minimal Residual Disease Predicts Outcome and Benefit from Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia with Npm1 Mutation: A Study by the Acute Leukemia French Association Group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.A.L.; O’BRien, M.A.; Hills, R.K.; Daly, S.B.; Wheatley, K.; Burnett, A.K. Minimal Residual Disease Monitoring by Quantitative Rt-Pcr in Core Binding Factor Aml Allows Risk Stratification and Predicts Relapse: Results of the United Kingdom Mrc Aml-15 Trial. Blood 2012, 120, 2826–2835. [Google Scholar] [CrossRef]
- Chen, X.; Xie, H.; Wood, B.L.; Walter, R.B.; Pagel, J.M.; Becker, P.S.; Sandhu, V.K.; Abkowitz, J.L.; Appelbaum, F.R.; Estey, E.H. Relation of Clinical Response and Minimal Residual Disease and Their Prognostic Impact on Outcome in Acute Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 1258–1264. [Google Scholar] [CrossRef]
- Buccisano, F.; Maurillo, L.; Del Principe, M.I.; Del Poeta, G.; Sconocchia, G.; Lo-Coco, F.; Arcese, W.; Amadori, S.; Venditti, A. Prognostic and Therapeutic Implications of Minimal Residual Disease Detection in Acute Myeloid Leukemia. Blood 2012, 119, 332–341. [Google Scholar] [CrossRef]
- Zhang, L.; Deeb, G.; Deeb, K.K.; Vale, C.; Barclift, D.P.; Papadantonakis, N. Measurable (Minimal) Residual Disease in Myelodysplastic Neoplasms (Mds): Current State and Perspectives. Cancers 2024, 16, 1503. [Google Scholar] [CrossRef]
- Cazzola, M.; Della Porta, M.G.; Malcovati, L. The Genetic Basis of Myelodysplasia and Its Clinical Relevance. Blood 2013, 122, 4021–4034. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and Biological Implications of Driver Mutations in Myelodysplastic Syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’lAughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. Tp53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical Effect of Point Mutations in Myelodysplastic Syndromes. N. Engl. J. Med. 2011, 364, 2496–2506. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.-H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Gallì, A.; Bacigalupo, A.; Zibellini, S.; Bernardi, M.; Rizzo, E.; Allione, B.; van Lint, M.T.; Pioltelli, P.; Marenco, P.; et al. Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients with Myelodysplastic Syndromes Treated with Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 3627–3637. [Google Scholar] [CrossRef]
- Khoury, R.; Raffoul, C.; Khater, C.; Hanna, C. Precision Medicine in Hematologic Malignancies: Evolving Concepts and Clinical Applications. Biomedicines 2025, 13, 1654. [Google Scholar] [CrossRef]
- Blachly, J.S.; Walter, R.B.; Hourigan, C.S. Hourigan. The Present and Future of Measurable Residual Disease Testing in Acute Myeloid Leukemia. Haematologica 2022, 107, 2810–2822. [Google Scholar] [CrossRef]
- Bejar, R.; Lord, A.; Stevenson, K.; Bar-Natan, M.; Pérez-Ladaga, A.; Zaneveld, J.; Wang, H.; Caughey, B.; Stojanov, P.; Getz, G.; et al. Tet2 Mutations Predict Response to Hypomethylating Agents in Myelodysplastic Syndrome Patients. Blood 2014, 124, 2705–2712. [Google Scholar] [CrossRef]
- Klco, J.M.; Miller, C.A.; Griffith, M.; Petti, A.; Spencer, D.H.; Ketkar-Kulkarni, S.; Wartman, L.D.; Christopher, M.; Lamprecht, T.L.; Helton, N.M.; et al. Association between Mutation Clearance after Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA 2015, 314, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Kantarjian, H.M.; Wang, F.; Yan, Y.; Bueso-Ramos, C.; Sasaki, K.; Issa, G.C.; Wang, S.; Jorgensen, J.; Song, X.; et al. Clearance of Somatic Mutations at Remission and the Risk of Relapse in Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 1788–1797. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef]
- Steensma, D.P. How I Use Molecular Genetic Tests to Evaluate Patients Who Have or May Have Myelodysplastic Syndromes. Blood 2018, 132, 1657–1663, Erratum in Blood 2018, 132, 2419. [Google Scholar] [CrossRef]
- Zhou, Y.; Wood, B.L. Methods of Detection of Measurable Residual Disease in Aml. Curr. Hematol. Malig. Rep. 2017, 12, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Getta, B.M.; Devlin, S.M.; Levine, R.L.; Arcila, M.E.; Mohanty, A.S.; Zehir, A.; Tallman, M.S.; Giralt, S.A.; Roshal, M. Multicolor Flow Cytometry and Multigene Next-Generation Sequencing Are Complementary and Highly Predictive for Relapse in Acute Myeloid Leukemia after Allogeneic Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Gaidzik, V.I.; Weber, D.; Paschka, P.; Kaumanns, A.; Krieger, S.; Corbacioglu, A.; Krönke, J.; Kapp-Schwoerer, S.; Krämer, D.; Horst, H.-A.; et al. Dnmt3a Mutant Transcript Levels Persist in Remission and Do Not Predict Outcome in Patients with Acute Myeloid Leukemia. Leukemia 2018, 32, 30–37. [Google Scholar] [CrossRef]
- Pløen, G.G.; Nederby, L.; Guldberg, P.; Hansen, M.; Ebbesen, L.H.; Jensen, U.B.; Hokland, P.; Aggerholm, A. Persistence of Dnmt3a Mutations at Long-Term Remission in Adult Patients with Aml. Br. J. Haematol. 2014, 167, 478–486. [Google Scholar] [CrossRef]
- Rothenberg-Thurley, M.; Amler, S.; Goerlich, D.; Köhnke, T.; Konstandin, N.P.; Schneider, S.; Sauerland, M.C.; Herold, T.; Hubmann, M.; Ksienzyk, B.; et al. Persistence of Pre-Leukemic Clones During First Remission and Risk of Relapse in Acute Myeloid Leukemia. Leukemia 2018, 32, 1598–1608. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of Aml in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the Eln. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Feller, N.; A van der Pol, M.; van Stijn, A.; Weijers, G.W.D.; Westra, A.H.; Evertse, B.W.; Ossenkoppele, G.J.; Schuurhuis, G.J. Mrd Parameters Using Immunophenotypic Detection Methods Are Highly Reliable in Predicting Survival in Acute Myeloid Leukaemia. Leukemia 2004, 18, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Hokland, P.; Ommen, H.B.; Nyvold, C.G.; Roug, A.S. Sensitivity of Minimal Residual Disease in Acute Myeloid Leukaemia in First Remission--Methodologies in Relation to Their Clinical Situation. Br. J. Haematol. 2012, 158, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, Y.; Kang, D.; Kim, H.S.; Lee, J.-M.; Kim, M.; Cho, B.-S. Prognostic Value of Measurable Residual Disease Monitoring by Next-Generation Sequencing before and after Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia. Blood Cancer J. 2021, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Gabdoulline, R.; Liebich, A.; Klement, P.; Schiller, J.; Kandziora, C.; Hambach, L.; Stadler, M.; Koenecke, C.; Flintrop, M.; et al. Measurable Residual Disease Monitoring by Ngs before Allogeneic Hematopoietic Cell Transplantation in Aml. Blood 2018, 132, 1703–1713. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Kim, T.; Jung, S.-H.; Ahn, S.-Y.; Jung, S.-Y.; Song, G.-Y.; Kim, M.; Yang, D.-H.; Lee, J.-J.; Choi, S.; et al. Allogeneic Transplant Can Abrogate the Risk of Relapse in the Patients of First Remission Acute Myeloid Leukemia with Detectable Measurable Residual Disease by Next-Generation Sequencing. Bone Marrow Transpl. 2021, 56, 1159–1170. [Google Scholar] [CrossRef]
- Heuser, M.; Heida, B.; Büttner, K.; Wienecke, C.P.; Teich, K.; Funke, C.; Brandes, M.; Klement, P.; Liebich, A.; Wichmann, M.; et al. Posttransplantation Mrd Monitoring in Patients with Aml by Next-Generation Sequencing Using Dta and Non-Dta Mutations. Blood Adv. 2021, 5, 2294–2304. [Google Scholar] [CrossRef]
- Kim, T.; Moon, J.H.; Ahn, J.-S.; Kim, Y.-K.; Lee, S.-S.; Ahn, S.-Y.; Jung, S.-H.; Yang, D.-H.; Lee, J.-J.; Choi, S.H.; et al. Next-Generation Sequencing-Based Posttransplant Monitoring of Acute Myeloid Leukemia Identifies Patients at High Risk of Relapse. Blood 2018, 132, 1604–1613. [Google Scholar] [CrossRef]
- Wienecke, C.P.; Heida, B.; Venturini, L.; Gabdoulline, R.; Krüger, K.; Teich, K.; Büttner, K.; Wichmann, M.; Puppe, W.; Neziri, B.; et al. Clonal Relapse Dynamics in Acute Myeloid Leukemia Following Allogeneic Hematopoietic Cell Transplantation. Blood 2024, 144, 296–307. [Google Scholar] [CrossRef]
- Dillon, L.W.; Gui, G.; Page, K.M.; Ravindra, N.; Wong, Z.C.; Andrew, G.; Mukherjee, D.; Zeger, S.L.; El Chaer, F.; Spellman, S.; et al. DNA Sequencing to Detect Residual Disease in Adults with Acute Myeloid Leukemia Prior to Hematopoietic Cell Transplant. JAMA 2023, 329, 745–755. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Yu, G.; Wang, Y.; Shao, R.; Du, X.; Xu, N.; Lin, D.; Zhao, W.; Zhang, X.; et al. Sorafenib Plus Triplet Therapy with Venetoclax, Azacitidine and Homoharringtonine for Refractory/Relapsed Acute Myeloid Leukemia with Flt3-Itd: A Multicenter Phase 2 Study. J. Intern. Med. 2024, 295, 216–228. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Loghavi, S.; Zeng, Z.; Tanaka, T.; Kim, Y.J.; Uryu, H.; Turkalj, S.; Jakobsen, N.A.; Luskin, M.R.; Duose, D.Y.; et al. A Phase Ib/Ii Study of Ivosidenib with Venetoclax ± Azacitidine in Idh1-Mutated Myeloid Malignancies. Blood Cancer Discov. 2023, 4, 276–293. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in Idh1-Mutated Relapsed or Refractory Aml. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Stein, E.M.; Dinardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in Mutant Idh2 Relapsed or Refractory Acute Myeloid Leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef]
- de Botton, S.; Fenaux, P.; Yee, K.; Récher, C.; Wei, A.H.; Montesinos, P.; Taussig, D.C.; Pigneux, A.; Braun, T.; Curti, A.; et al. Olutasidenib (Ft-2102) Induces Durable Complete Remissions in Patients with Relapsed or Refractory Idh1-Mutated Aml. Blood Adv. 2023, 7, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.M.; Baer, M.R.; Yang, J.; Prebet, T.; Lee, S.; Schiller, G.J.; Dinner, S.N.; Pigneux, A.; Montesinos, P.; Wang, E.S.; et al. Olutasidenib Alone or with Azacitidine in Idh1-Mutated Acute Myeloid Leukaemia and Myelodysplastic Syndrome: Phase 1 Results of a Phase 1/2 Trial. Lancet Haematol. 2023, 10, e46–e58. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Aldoss, I.; DiPersio, J.; Cuglievan, B.; Stone, R.; Arellano, M.; Thirman, M.J.; Patel, M.R.; Dickens, D.S.; Shenoy, S.; et al. The Menin Inhibitor Revumenib in Kmt2a-Rearranged or Npm1-Mutant Leukaemia. Nature 2023, 615, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Searle, E.; Abdul-Hay, M.; Abedin, S.; Aldoss, I.; Piérola, A.A.; Alonso-Dominguez, J.M.; Chevallier, P.; Cost, C.; Daskalakis, N.; et al. A First-in-Human Phase 1 Study of the Menin-Kmt2a (Mll1) Inhibitor Jnj-75276617 in Adult Patients with Relapsed/Refractory Acute Leukemia Harboring Kmt2a or Npm1 Alterations. Blood 2023, 142, 57. [Google Scholar] [CrossRef]
- Issa, G.C.; Cuglievan, B.; DiNardo, C.D.; Short, N.J.; McCall, D.; Gibson, A.; Nunez, C.; Garcia, M.B.; Roth, M.; Bidikian, A.; et al. Early Results of the Phase I/Ii Study Investigating the All-Oral Combination of the Menin Inhibitor Revumenib (Sndx-5613) with Decitabine/Cedazuridine (Astx727) and Venetoclax in Acute Myeloid Leukemia (Save). Blood 2023, 142 (Suppl. S1), 58. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory Flt3-Mutated Aml. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Numan, Y.; Rahman, Z.A.; Grenet, J.; Boisclair, S.; Bewersdorf, J.P.; Collins, C.; Barth, D.; Fraga, M.; Bixby, D.L.; Zeidan, A.M.; et al. Gilteritinib Clinical Activity in Relapsed/Refractory Flt3 Mutated Acute Myeloid Leukemia Previously Treated with Flt3 Inhibitors. Am. J. Hematol. 2022, 97, 322–328. [Google Scholar] [CrossRef]
- Potter, N.; Miraki-Moud, F.; Ermini, L.; Titley, I.; Vijayaraghavan, G.; Papaemmanuil, E.; Campbell, P.; Gribben, J.; Taussig, D.; Greaves, M. Single Cell Analysis of Clonal Architecture in Acute Myeloid Leukaemia. Leukemia 2018, 33, 1113–1123. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. New Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Othman, J.; Tiong, I.S.; O’Nions, J.; Dennis, M.; Mokretar, K.; Ivey, A.; Austin, M.; Latif, A.L.; Amer, M.; Chan, W.Y.; et al. Molecular MRD is strongly prognostic in patients with NPM1-mutated AML receiving venetoclax-based nonintensive therapy. Blood 2024, 143, 336–341. [Google Scholar] [CrossRef]
- Chua, C.C.; Hammond, D.; Kent, A.; Tiong, I.S.; Konopleva, M.Y.; Pollyea, D.A.; DiNardo, C.D.; Wei, A.H. Treatment-free remission after ceasing venetoclax-based therapy in patients with acute myeloid leukemia. Blood Adv. 2022, 6, 3879–3883. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Fukushima, K.; Hosen, N.; Chi, S.; Haeno, H.; Yoshimoto, G.; Arai, H.; Ikeda, D.; Usuki, K.; Takahashi, N.; et al. NGS Profile and the Mathematical Prediction Model for Venetoclax Combination Therapy in HM-Screen-Japan 02 Study. Blood 2023, 142 (Suppl. S1), 5761. [Google Scholar] [CrossRef]




| All Patients N = 84 | Patients Evaluable for NGS MRD N = 56 | ||||
|---|---|---|---|---|---|
| N° | % | N° | % | ||
| Baseline clinical and biological data | |||||
| Age | median (range) | 63 (29–86) | 61 (29–83) | ||
| Sex | male | 50 | 60% | 33 | 59% |
| female | 34 | 40% | 23 | 41% | |
| WBC × 109/L | median (range) | 7.4 (0.6–393) | 5.0 (0.6–393) | ||
| PLTS × 109/L | median (range) | 70 (10–288) | 81 (13–288) | ||
| Diagnosis | AML | 75 | 88% | 49 | 88% |
| MDS-IB1 | 1 | 1% | 0 | 0% | |
| MDS-IB2 | 8 | 9% | 7 | 13% | |
| ELN 2022 group risk | favorable | 18 | 24% | 15 | 31% |
| intermediate | 19 | 25% | 11 | 22% | |
| adverse | 38 | 51% | 23 | 47% | |
| Therapy-related | no | 74 | 88% | 53 | 95% |
| yes | 10 | 12% | 3 | 5% | |
| Cytogenetics | normal | 49 | 58% | 35 | 62% |
| abnormal | 35 | 42% | 21 | 38% | |
| Conventional MRD marker | present | 22 | 26% | 16 | 29% |
| absent | 62 | 74% | 40 | 71% | |
| NGS mutation at diagnosis | present | 80 | 95% | 52 | 93% |
| absent | 4 | 5% | 4 | 7% | |
| Number of somatic mutations by NGS at diagnosis | median (range) | 3 (1–8) | 3 (1–8) | ||
| Treatment and outcome | |||||
| Induction treatment | intensive CHT | 65 | 77% | 48 | 86% |
| HMA-VEN | 19 | 23% | 8 | 14% | |
| Response to treatment | CR after I induction | 59 | 70% | 47 | 84% |
| CR after salvage treatment | 12 | 14% | 9 | 16% | |
| refractory | 8 | 10% | - | - | |
| death in induction | 5 | 6% | - | - | |
| Relapse | no | 41 | 58% | 30 | 54% |
| yes | 30 | 42% | 26 | 46% | |
| Death | no | 38 | 45% | 27 | 48% |
| yes | 45 | 54% | 28 | 50% | |
| Variables | HR | 95.0% CI | ||
|---|---|---|---|---|
| Lower | Upper | p Value | ||
| MRD positivity by NGS | 2.48 | 1.12 | 5.52 | 0.025 |
| MRD positivity by MFC | 2.58 | 1.02 | 6.48 | 0.043 |
| ELN 2022 risk adverse vs. intermediate vs. favorable | 1.66 | 0.95 | 2.90 | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisà, E.; Dogliotti, I.; Lia, G.; Cerrano, M.; Audisio, E.; Lanzarone, G.; Brunello, L.; Caravelli, D.; Carnevale Schianca, F.; Berrino, E.; et al. Integration of Next-Generation Sequencing in Measurable Residual Disease Monitoring in Acute Myeloid Leukemia and Myelodysplastic Neoplasm. Cancers 2025, 17, 2874. https://doi.org/10.3390/cancers17172874
Crisà E, Dogliotti I, Lia G, Cerrano M, Audisio E, Lanzarone G, Brunello L, Caravelli D, Carnevale Schianca F, Berrino E, et al. Integration of Next-Generation Sequencing in Measurable Residual Disease Monitoring in Acute Myeloid Leukemia and Myelodysplastic Neoplasm. Cancers. 2025; 17(17):2874. https://doi.org/10.3390/cancers17172874
Chicago/Turabian StyleCrisà, Elena, Irene Dogliotti, Giuseppe Lia, Marco Cerrano, Ernesta Audisio, Giuseppe Lanzarone, Lucia Brunello, Daniela Caravelli, Fabrizio Carnevale Schianca, Enrico Berrino, and et al. 2025. "Integration of Next-Generation Sequencing in Measurable Residual Disease Monitoring in Acute Myeloid Leukemia and Myelodysplastic Neoplasm" Cancers 17, no. 17: 2874. https://doi.org/10.3390/cancers17172874
APA StyleCrisà, E., Dogliotti, I., Lia, G., Cerrano, M., Audisio, E., Lanzarone, G., Brunello, L., Caravelli, D., Carnevale Schianca, F., Berrino, E., Bellomo, S. E., Bartolini, A., Riera, L., Francia di Celle, P., Gaidano, G., Lunghi, M., Giaccone, L., & Bruno, B. (2025). Integration of Next-Generation Sequencing in Measurable Residual Disease Monitoring in Acute Myeloid Leukemia and Myelodysplastic Neoplasm. Cancers, 17(17), 2874. https://doi.org/10.3390/cancers17172874








