Failure to Rescue and Lung Resections for Lung Cancer: Measuring Quality from the Operation Room to the Intensive Care Unit
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
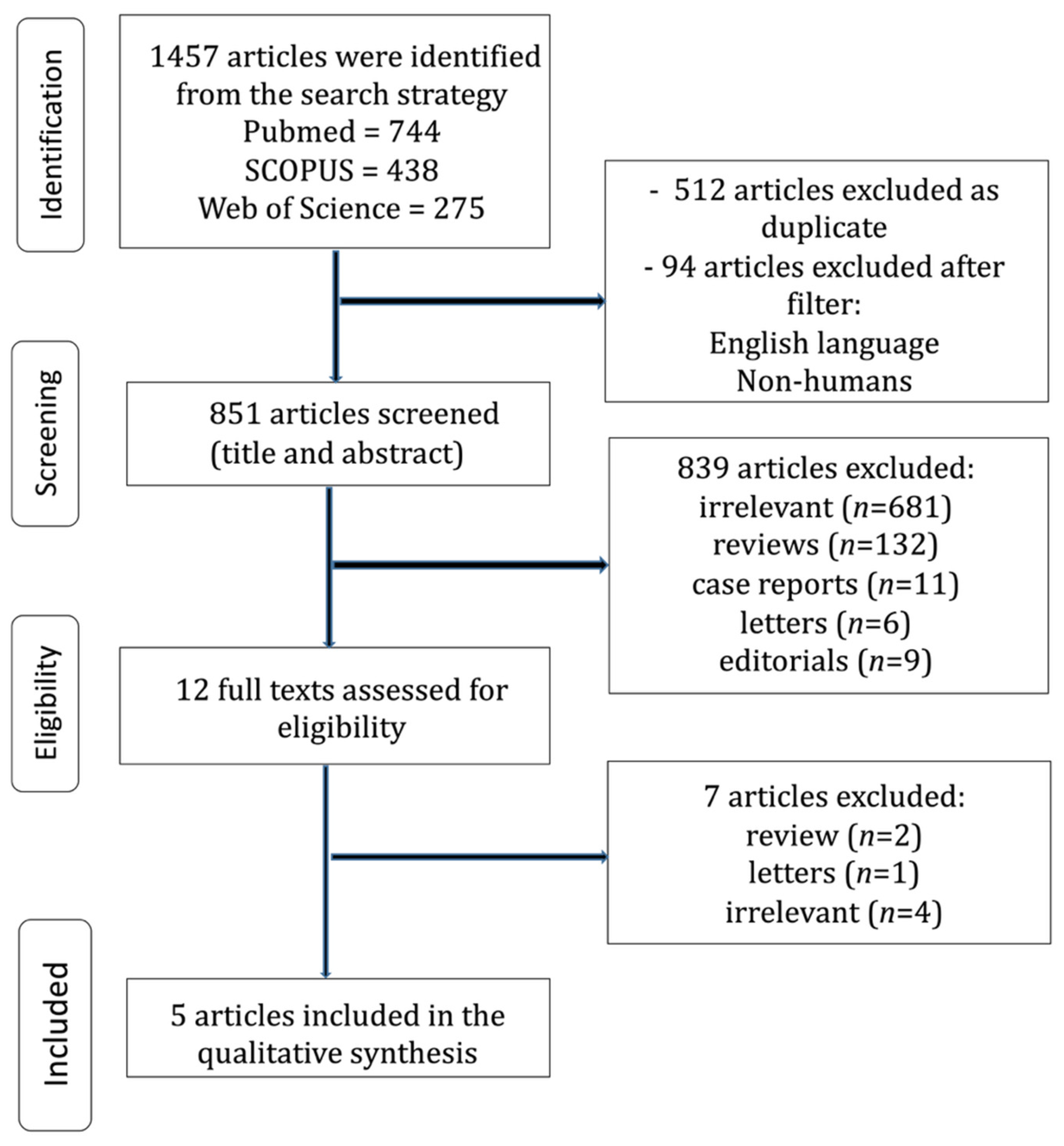
2.1. Search and Articles Selection Strategy
2.2. Data Extraction and Quality Assessment
3. Results
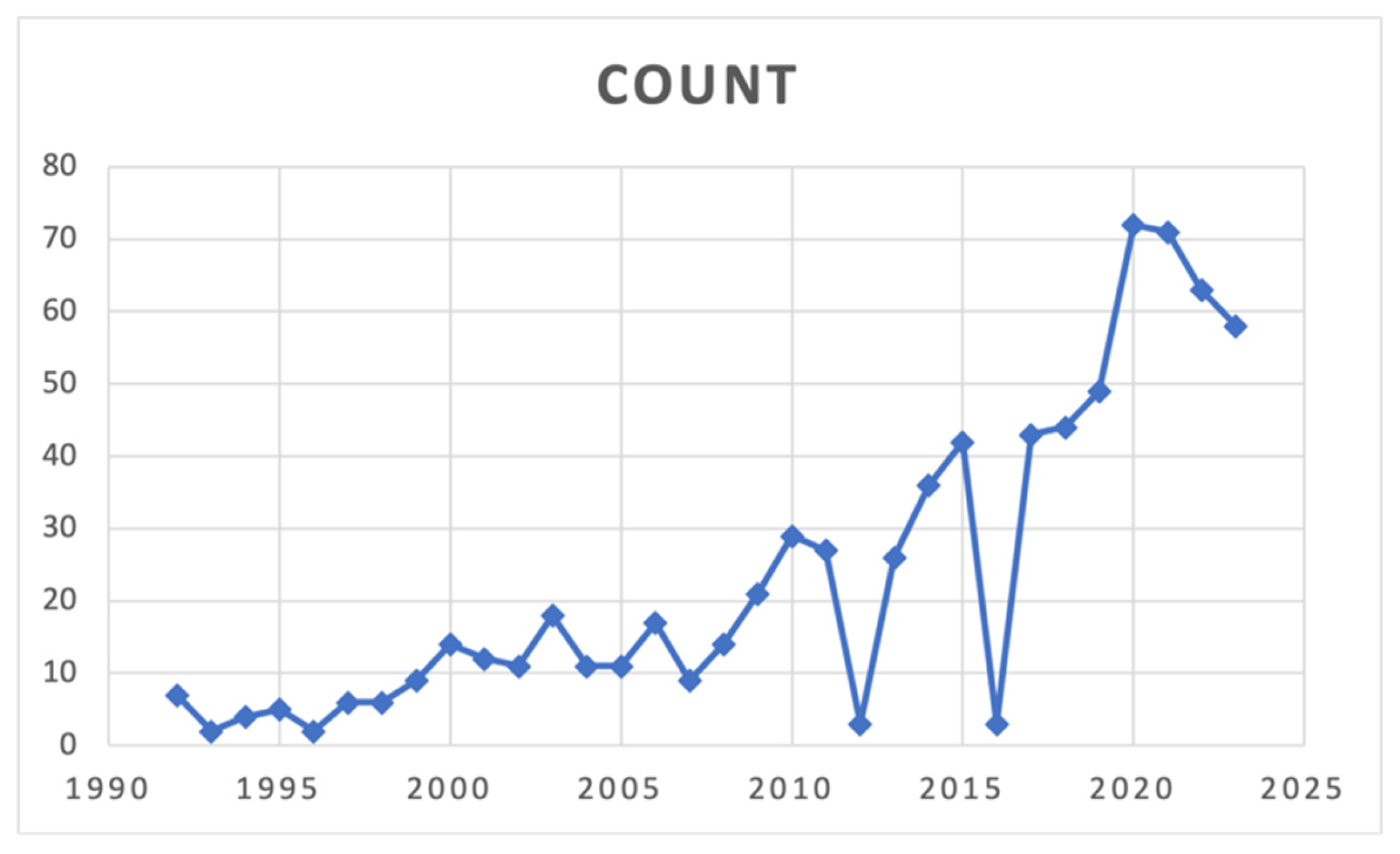
3.1. Search Strategy and Patient Demographics
3.2. Historical Evolution of the FTR Definition
3.3. Factors Affecting FTR
3.3.1. Patient-Level Factors
3.3.2. Surgical Complexity
3.3.3. System-Level Challenges
3.3.4. Institutional Readiness
3.4. Preventive Measures to Minimize Failure-to-Rescue in Lung Cancer Care
- 1.
- Enhanced Care Coordination
- 2.
- Advanced Staff Training and Education
- 3.
- Integration of Advanced Monitoring Technologies
- 4.
- Implementation of Standardized Rapid Response Protocols
- 5.
- Patient and Family Engagement
- 6.
- Continuous Quality Improvement
3.5. Impact of Failure-to-Rescue on Lung Cancer Patients
- 1.
- Increased Morbidity and Mortality
- 2.
- Prolonged Hospitalization and Delayed Recovery
- 3.
- Reduced Quality of Life
- 4.
- Psychological and Emotional Distress
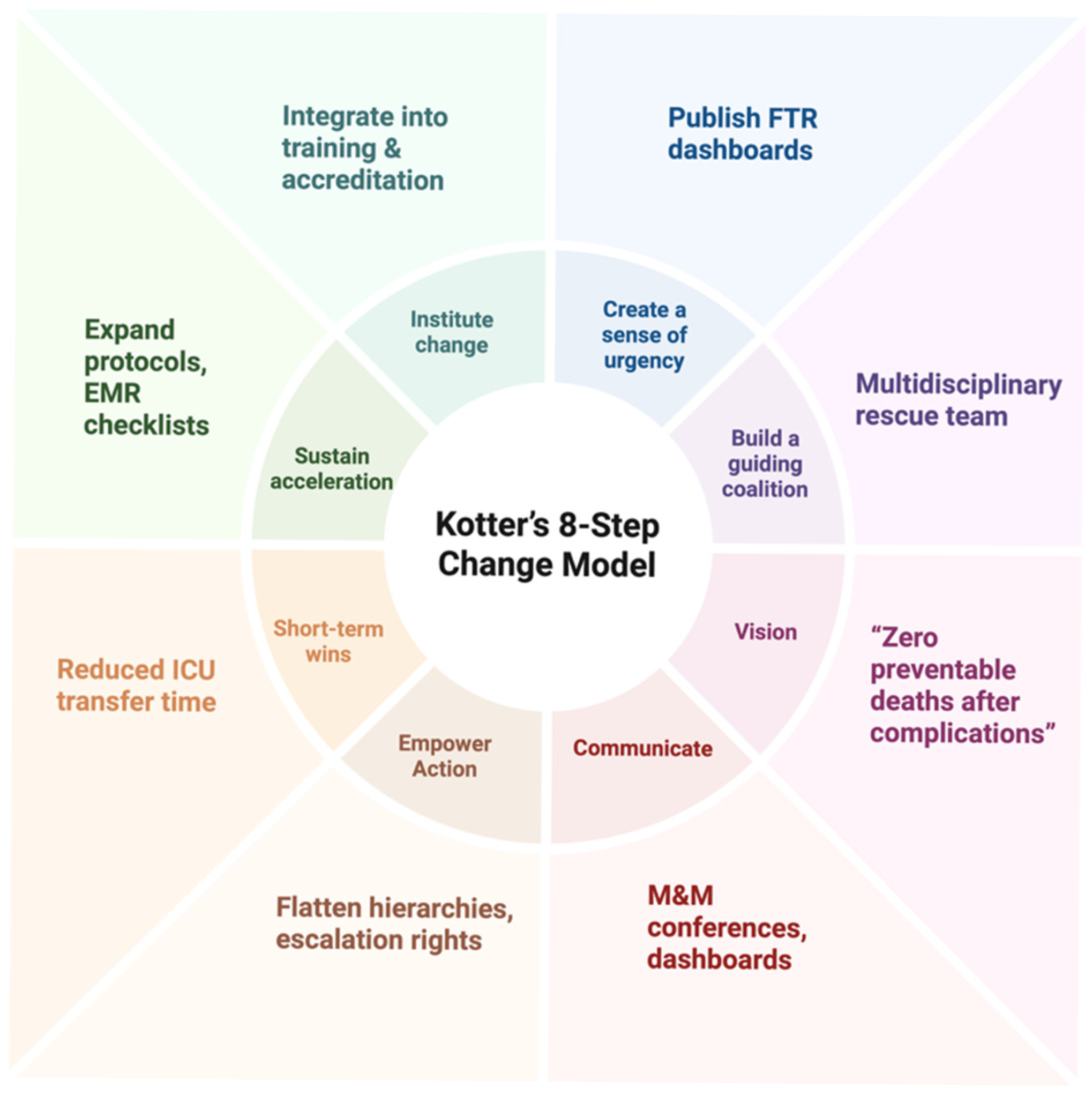
3.6. A Change-Driven Rescue Framework: An 8-Step Roadmap for Reducing Failure-to-Rescue After Lung Cancer Surgery
- 1.
- Establish a Sense of Urgency: Recognize FTR as a Quality Crisis
- 2.
- Build a Guiding Coalition: Form a Multidisciplinary Rescue Team
- 3.
- Develop a Unified Vision and Protocolized Rescue Pathway
- 4.
- Communicate the Vision for Buy-In
- 5.
- Empower Others to Act on the Vision: Break Down Barriers
- 6.
- Generate Short-Term Wins: Track and Celebrate Early Successes
- 7.
- Consolidate Gains and Produce More Change: Refine and Expand
- 8.
- Anchor New Approaches in the Culture: Make Rescue a Shared Value
3.7. Building a Culture of Reflection: Continuous Review and Adaptation in Lung Cancer Surgery Pathways
- (1)
- Creation of a Thoracic Outcomes Intelligence Unit (TOIU)
- (2)
- Multidisciplinary Complication Review Boards (MCRBs)
- (3)
- Failure Pattern Mapping (FPM)
- (4)
- Real-Time Benchmarking Dashboards
- (5)
- Thoracic Quality Symposia (TQS)
- (6)
- Incorporation of Evolving Evidence into Practice Loops
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 28 January 2023).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Boffa, D.J.; Allen, M.S.; Grab, J.D.; Gaissert, H.A.; Harpole, D.H.; Wright, C.D. Data from The Society of Thoracic Surgeons General Thoracic Surgery Database: The surgical management of primary lung tumors. J. Thorac. Cardiovasc. Surg. 2008, 135, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Luo, J.; Liu, L.; Wu, Q.; Zhou, R.; Li, L.; Zhang, C. Risk factors for postoperative pneumonia and prognosis in lung cancer patients after surgery: A retrospective study. Medicine 2021, 100, e25295. [Google Scholar] [CrossRef]
- Farjah, F.; Grau-Sepulveda, M.V.; Gaissert, H.; Block, M.; Grogan, E.; Brown, L.M.; Kosinski, A.S.; Kozower, B.D. Volume pledge is not associated with better short-term outcomes after lung cancer resection. J. Clin. Oncol. 2020, 38, 3518–3527. [Google Scholar] [CrossRef]
- Grenda, T.R.; Revels, S.L.; Yin, H.; Birkmeyer, J.D.; Wong, S.L. Lung cancer resection at hospitals with high versus low mortality rates. JAMA Surg. 2015, 150, 1034–1040. [Google Scholar] [CrossRef]
- Andalib, A.; Ramana-Kumar, A.V.; Bartlett, G.; Franco, E.L.; Ferri, L.E. Influence of postoperative infectious complications on long-term survival of lung cancer patients: A population-based cohort study. J. Thorac. Oncol. 2013, 8, 554–561. [Google Scholar] [CrossRef]
- Nathan, H.; Yin, H.; Wong, S.L. Postoperative complications and long-term survival after complex cancer resection. Ann. Surg. Oncol. 2017, 24, 638–644. [Google Scholar] [CrossRef]
- Silber, J.H.; Williams, S.V.; Krakauer, H.; Schwartz, S. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med. Care 1992, 30, 615–629. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Xanthopoulos, A.; Zotos, P.-A.; Rad, A.A.; Tatsios, E.; Bareka, M.; Briasoulis, A.; Triposkiadis, F.; Skoularigis, J.; Athanasiou, T. The Emerging Role of “Failure to Rescue” as the Primary Quality Metric for Cardiovascular Surgery and Critical Care. J. Clin. Med. 2023, 12, 4876. [Google Scholar] [CrossRef]
- Alabbadi, S.; Roach, A.; Chikwe, J.; Egorova, N.N. National trend in failure to rescue after cardiac surgeries. J. Thorac. Cardiovasc. Surg. 2023, 166, 1157–1165.e6. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Chen, X.; Zhang, Y.; Li, Y.; Hu, X. Effects of Web-Based Acceptance and Commitment Therapy on Health-Related Outcomes Among Patients With Lung Cancer: A Feasibility Randomized Controlled Trial. Psycho-Oncology 2024, 33, e70045. [Google Scholar] [CrossRef]
- Panakkal, N.; Lekshmi, A.; Saraswathy, V.V.; Sujathan, K. Effective lung cancer control: An unaccomplished challenge in cancer research. Cytojournal 2023, 20, 16. [Google Scholar] [CrossRef]
- Liu, R.; Wang, S.; Tian, F.; Yi, L. SIR-3DCNN: A Framework of Multivariate Time Series Classification for Lung Cancer Detection. IEEE Trans. Instrum. Meas. 2025, 74, 2526413. [Google Scholar] [CrossRef]
- Pasquali, S.K.; He, X.; Jacobs, J.P.; Jacobs, M.L.; O’BRien, S.M.; Gaynor, J.W. Evaluation of failure to rescue as a quality metric in pediatric heart surgery: An analysis of the STS Congenital Heart Surgery Database. Ann. Thorac. Surg. 2012, 94, 573–580. [Google Scholar] [CrossRef]
- Kotter, J.P. Leading Change; Harvard Business School Press: Boston, MA, USA, 1996. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Bernard, A.; Cottenet, J.; Pagès, P.-B.; Quantin, C. Mortality and failure-to-rescue major complication trends after lung cancer surgery between 2005 and 2020: A nationwide population-based study. BMJ Open 2023, 13, e075463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farjah, F.; Backhus, L.; Cheng, A.; Englum, B.; Kim, S.; Saha-Chaudhuri, P.; Wood, D.E.; Mulligan, M.S.; Varghese, T.K. Failure to rescue and pulmonary resection for lung cancer. J. Thorac. Cardiovasc. Surg. 2015, 149, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, M.T.; Rivas, C.; Novoa, N.; Jiménez, M.F.; the Spanish Group of Video-assisted Thoracic Surgery (GEVATS) Failure to rescue following anatomical lung resection. Analysis of a prospective nationwide database. Front. Surg. 2023, 10, 1077046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Kapula, N.; Yang, C.-F.J.; Manapat, P.; Elliott, I.A.; Guenthart, B.A.; Lui, N.S.; Backhus, L.M.; Berry, M.F.; Shrager, J.B.; et al. Comparison of failure to rescue in younger versus elderly patients following lung cancer resection. JTCVS Open 2023, 16, 855–872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Sahaf, M.; Lim, E. The association between surgical volume, survival and quality of care. J. Thorac. Dis. 2015, 7, S152–S155. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Biere, S.S.A.Y.; Van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Rosen, A.K.; Rivard, P.; Zhao, S.; Loveland, S.; Tsilimingras, D.; Christiansen, C.L.; Elixhauser, A.; Romano, P.S. Evaluating the patient safety indicators: How well do they perform on Veterans Health Administration data? Med. Care 2005, 43, 873–884. [Google Scholar] [CrossRef]
- Silber, J.H.; Romano, P.S.; Rosen, A.K.; Wang, Y.; Even-Shoshan, O.; Volpp, K.G. Failure-torescue: Comparing definitions to measure quality of care. Med. Care 2007, 45, 918–925. [Google Scholar] [CrossRef]
- Shiloach, M.; Frencher, S.K., Jr.; Steeger, J.E.; Rowell, K.S.; Bartzokis, K.; Tomeh, M.G.; Richards, K.E.; Ko, C.Y.; Hall, B.L. Toward robust information: Data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J. Am. Coll. Surg. 2010, 210, 6–16. [Google Scholar] [CrossRef]
- Magee, M.J.; Wright, C.D.; McDonald, D.; Fernandez, F.G.; Kozower, B.D. External validation of the Society of Thoracic Surgeons General Thoracic Surgery Database. Ann. Thorac. Surg. 2013, 96, 1734–1739, discussion: 1738–1739. [Google Scholar] [CrossRef]
- Kurlansky, P.A.; O’bRien, S.M.; Vassileva, C.M.; Lobdell, K.W.; Edwards, F.H.; Jacobs, J.P.; von Ballmoos, M.W.; Paone, G.; Edgerton, J.R.; Thourani, V.H.; et al. Failure to Rescue: A New Society of Thoracic Surgeons Quality Metric for Cardiac Surgery. Ann. Thorac. Surg. 2022, 113, 1935–1942. [Google Scholar] [CrossRef]
- Dewan, K.C.; Navale, S.M.; Hirji, S.A.; Koroukian, S.M.; Dewan, K.S.; Svensson, L.G.; Gillinov, A.M.; Roselli, E.E.; Johnston, D.; Bakaeen, F.; et al. The role of frailty in failure to rescue after cardiovascular surgery. Ann. Thorac. Surg. 2021, 111, 472–478. [Google Scholar] [CrossRef]
- Hatchimonji, J.S.M.; Kaufman, E.J.M.; Sharoky, C.E.M.; Ma, L.; Whitlock, A.E.G.; Holena, D.N.M. Failure to rescue in surgical patients: A review for acute care surgeons. J. Trauma Acute Care Surg. 2019, 87, 699–706. [Google Scholar] [CrossRef]
- Sheetz, K.H.; Dimick, J.B.; Ghaferi, A.A. Impact of hospital characteristics on failure to rescue following major surgery. Ann. Surg. 2016, 263, 692–697. [Google Scholar] [CrossRef]
- Ward, S.T.; Dimick, J.B.; Zhang, W.M.; Campbell, D.A.; Ghaferi, A.A. Association between hospital staffing models and failure to rescue. Ann. Surg. 2019, 270, 91–94. [Google Scholar] [CrossRef]
- Ghaferi, A.A.; Birkmeyer, J.D.; Dimick, J.B. Hospital volume and failure to rescue with high-risk surgery. Med. Care 2011, 49, 1076–1081. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Dimick, J.B.; Birkmeyer, J.D.; Ghaferi, A.A. Understanding the volume-outcome effect in cardiovascular surgery: The role of failure to rescue. JAMA Surg. 2014, 149, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Espin, S.; Indar, A.; Gross, M.; Labricciosa, A.; D’ARpino, M. Processes and tools to improve teamwork and communication in surgical settings: A narrative review. BMJ Open Qual. 2020, 9, e000937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Q.; Zheng, X.; Xu, L.; Chen, Q.; Zhou, F.; Peng, L. The effectiveness of education strategies for nurses to recognise and manage clinical deterioration: A systematic review. Nurse Educ. Today 2023, 126, 105838. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.L.; Søreide, E.; Hillman, K.; Hansen, B.S. Succeeding with rapid response systems—A never-ending process: A systematic review of how health-care professionals perceive facilitators and barriers within the limbs of the RRS. Resuscitation 2019, 144, 75–90. [Google Scholar] [CrossRef]
- Winters, B.D.; Rosen, M.; Sharma, R.; Zhang, A.; Bass, E.B. Failure To Rescue—Rapid Response Systems: Rapid Review. In Making Healthcare Safer IV: A Continuous Updating of Patient Safety Harms and Practices [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2023; March 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK603026/ (accessed on 31 July 2025).
- Diaz-Castrillon, C.E.; Serna-Gallegos, D.; Arnaoutakis, G.; Szeto, W.Y.; Sá, M.P.; Sezer, A.; Sultan, I. The burden of major complications on failure to rescue after surgery for acute type A aortic dissection: Analysis of more than 19,000 patients. J. Thorac. Cardiovasc. Surg. 2025, 169, 1415–1426.e11. [Google Scholar] [CrossRef]
- Rosero, E.B.; Romito, B.T.; Joshi, G.P. Failure to rescue: A quality indicator for postoperative care. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 575–589. [Google Scholar] [CrossRef]
- Pinto, A.; Faiz, O.; Davis, R.; Almoudaris, A.; Vincent, C. Surgical complications and their impact on patients’ psychosocial well-being: A systematic review and meta-analysis. BMJ Open 2016, 6, e007224. [Google Scholar] [CrossRef]
| Study ID, Year | Country/Database | Study Design | Definition of FTR | Study Population, n | Adverse Events, % | Mortality, % | FTR, % | Statistical Notes | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Grenda 2015 [6] | USA, NCDB | R | Death after major complication (e.g., pneumonia, ARDS, PE, MI, sepsis) | 645 | 15.6–23.3 | 1.4–6 | 8.7–25.9 | Logistic regression; p-values for hospital-level predictors | 7 |
| Bernard 2023 [19] | France, National Database | R | Death after severe cardiopulmonary complication (pneumonia, PE, ARDS) | 157,566 | 27.8 | Reduced from 3.8% in 2005 to 2.9% in 2020 | Reduced from 12.2% in 2005 to 7.1% in 2020 | Multivariable analysis, CI reported | 7 |
| Farjah 2015 [20] | USA, STS Database | R | Death after ≥ 1 postoperative major complication (STS defined) | 30,000 | 36–42 | 0.7–3.2 | 1.7–6.8 | Adjusted OR for hospital volume; CI reported | 7 |
| Gómez-Hernández 2023 [21] | Spain, National Database | R | In-hospital mortality after Clavien-Dindo grade ≥ IIIb complication | 3533 | 10.2 | 1.7 | 16.3 | Kaplan-Meier and logistic regression, p-values | 7 |
| Wang 2023 [22] | USA, ACS NSQIP | R | Death following ≥ 1 major complication (NSQIP-defined: pneumonia, sepsis, unplanned intubation, etc.) | 40,934 | 10.1 | N/A | 5.6–12 | OR per decade age (1.55, p < 0.001), subgroup p-values reported | 7 |
| Category | Risk Factor | Description |
|---|---|---|
| Surgical Complexity | Procedure Type and Extent of Resection | Complex procedures and extensive resections (e.g., pneumonectomy) increase complication risk and reduce the likelihood of successful rescue. |
| Patient Comorbidities | Coexisting conditions (e.g., cardiac disease, poor pulmonary reserve) raise the risk of adverse outcomes postoperatively. | |
| Postoperative Monitoring | Inadequate Surveillance | Delayed detection of complications due to insufficient monitoring protocols or clinical vigilance can lead to missed rescue opportunities. |
| Care Coordination | Poor Multidisciplinary Communication | Lack of effective handoffs or collaboration between surgeons, anesthesiologists, intensivists, and nurses delays appropriate interventions. |
| Resource Allocation | Staffing Shortages and Inadequate ICU Resources | Limited staff, overwhelmed ICUs, and a lack of essential equipment impair the ability to intervene quickly and appropriately after complications arise. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotos, P.-A.; Androutsopoulou, V.; Scarci, M.; Minervini, F.; Cioffi, U.; Xanthopoulos, A.; Athanasiou, T.; Magouliotis, D.E. Failure to Rescue and Lung Resections for Lung Cancer: Measuring Quality from the Operation Room to the Intensive Care Unit. Cancers 2025, 17, 2784. https://doi.org/10.3390/cancers17172784
Zotos P-A, Androutsopoulou V, Scarci M, Minervini F, Cioffi U, Xanthopoulos A, Athanasiou T, Magouliotis DE. Failure to Rescue and Lung Resections for Lung Cancer: Measuring Quality from the Operation Room to the Intensive Care Unit. Cancers. 2025; 17(17):2784. https://doi.org/10.3390/cancers17172784
Chicago/Turabian StyleZotos, Prokopis-Andreas, Vasiliki Androutsopoulou, Marco Scarci, Fabrizio Minervini, Ugo Cioffi, Andrew Xanthopoulos, Thanos Athanasiou, and Dimitrios E. Magouliotis. 2025. "Failure to Rescue and Lung Resections for Lung Cancer: Measuring Quality from the Operation Room to the Intensive Care Unit" Cancers 17, no. 17: 2784. https://doi.org/10.3390/cancers17172784
APA StyleZotos, P.-A., Androutsopoulou, V., Scarci, M., Minervini, F., Cioffi, U., Xanthopoulos, A., Athanasiou, T., & Magouliotis, D. E. (2025). Failure to Rescue and Lung Resections for Lung Cancer: Measuring Quality from the Operation Room to the Intensive Care Unit. Cancers, 17(17), 2784. https://doi.org/10.3390/cancers17172784











