Choice of Animal Models to Investigate Cell Migration and Invasion in Glioblastoma
Simple Summary
Abstract
1. Introduction
2. Animal Models of Glioblastoma
2.1. Advantages of Animal Models over In Vitro Models
2.2. Types of Animals Used to Model Glioblastoma
2.2.1. Rodents (Mice and Rats)
2.2.2. Zebrafish
2.2.3. Other Animal Models (Canine and Non-Human Primate)
2.3. Recent Advances in Glioblastoma Animal Model Techniques
2.3.1. CRISPR-Cas9 Gene Editing
2.3.2. Optogenetics
2.3.3. Immunological Models
3. Animal Models Used to Study Glioblastoma Cell Migration and Invasion
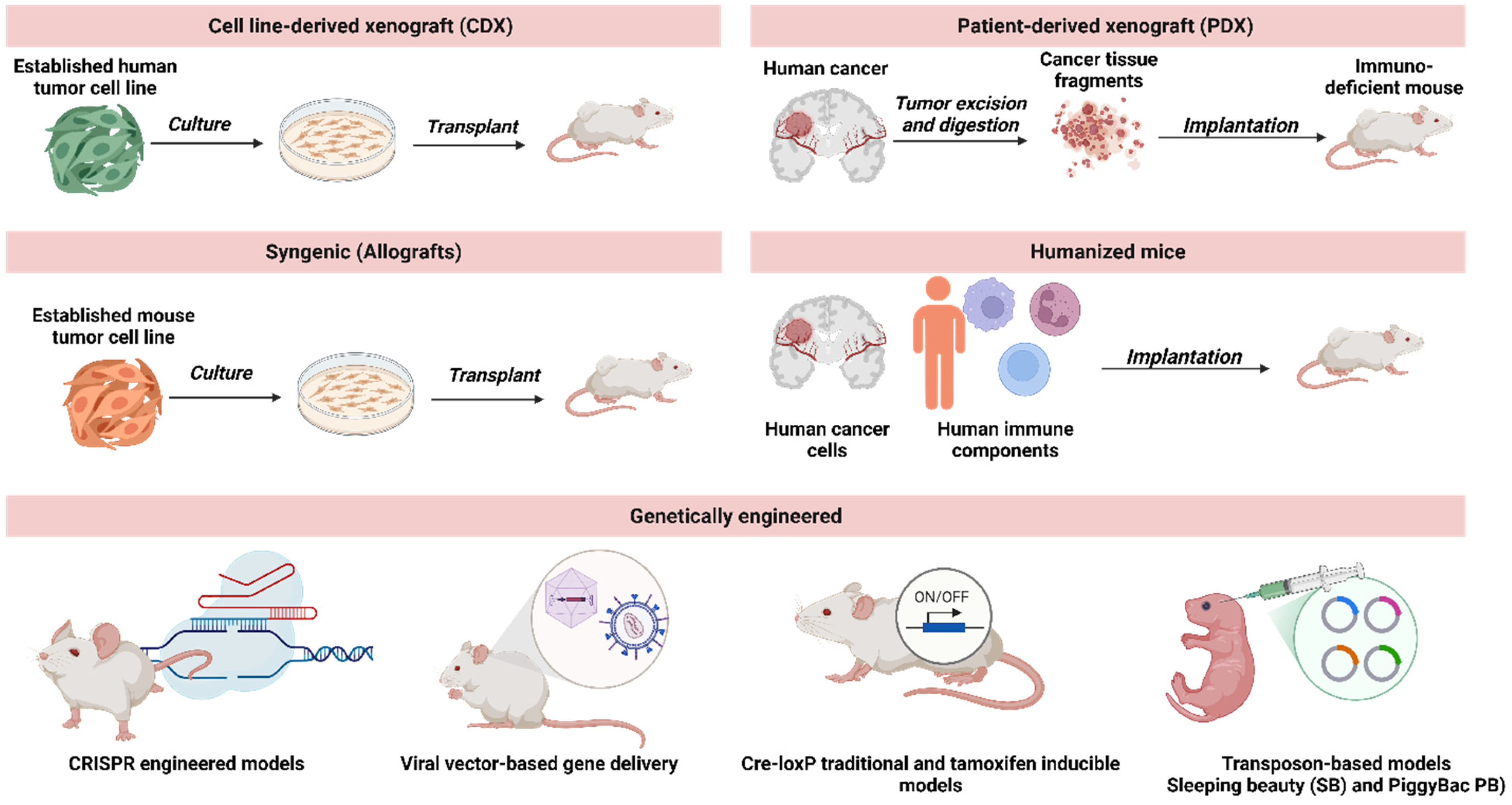
3.1. Transplantation Models
3.1.1. Cell Line-Derived Xenograft (CDX)
3.1.2. Patient-Derived Xenograft (PDX)
3.1.3. Allografts
3.2. Genetic Engineering: Transgenic and Knockout Mouse Models
3.3. Explant Models
3.4. Quantitative Comparisons of Invasion Rates Across Models
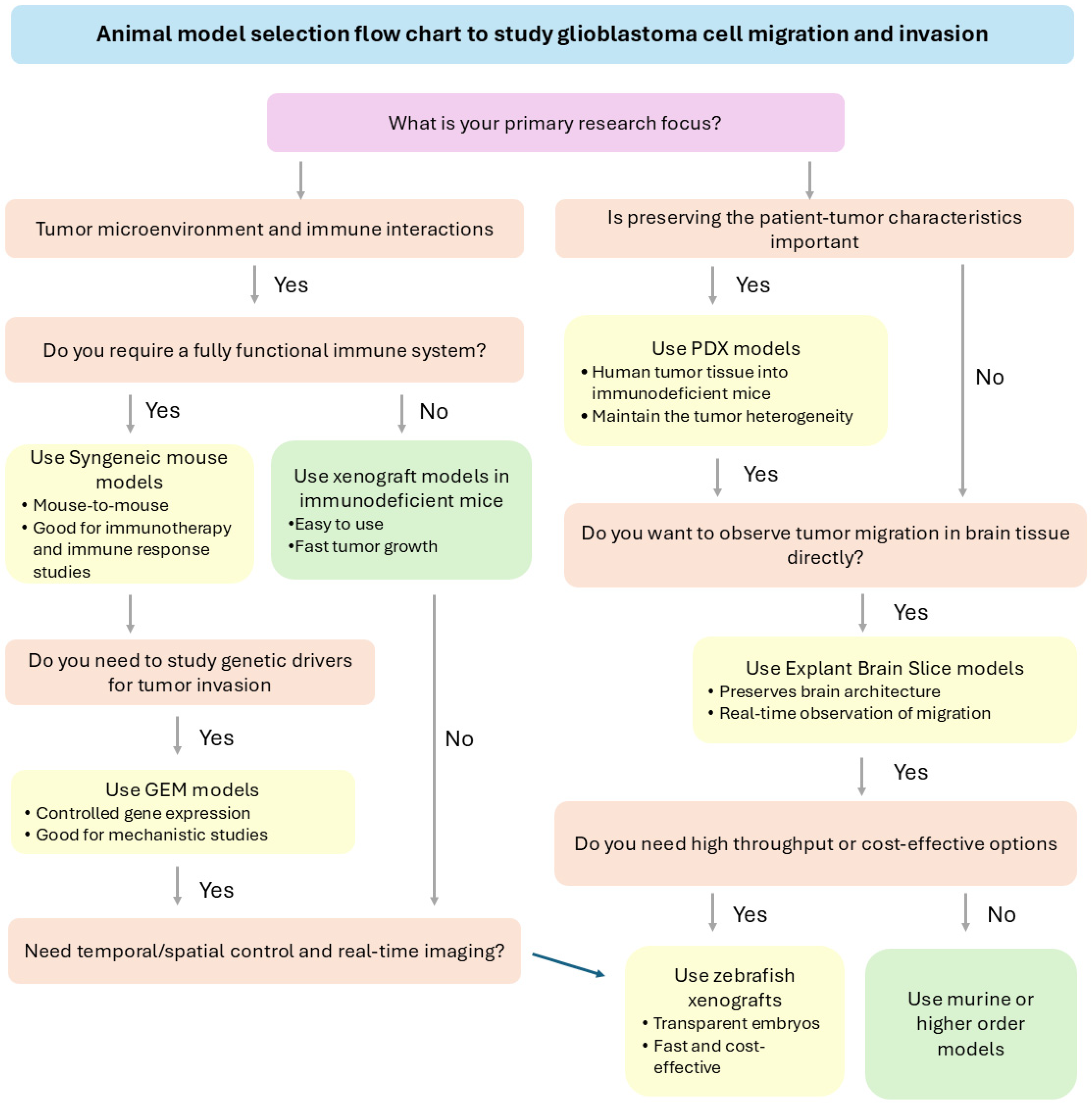
3.5. Strategic Selection of Animal Models Based on Research Objectives
3.6. Methodological Considerations for In Vivo Invasion and Migration Studies
4. Comparative Analysis Between Results Derived from Animal Models and Clinical Patient Data
5. Comparison of Animal Models with Advanced In Vitro Platforms for Glioblastoma Research
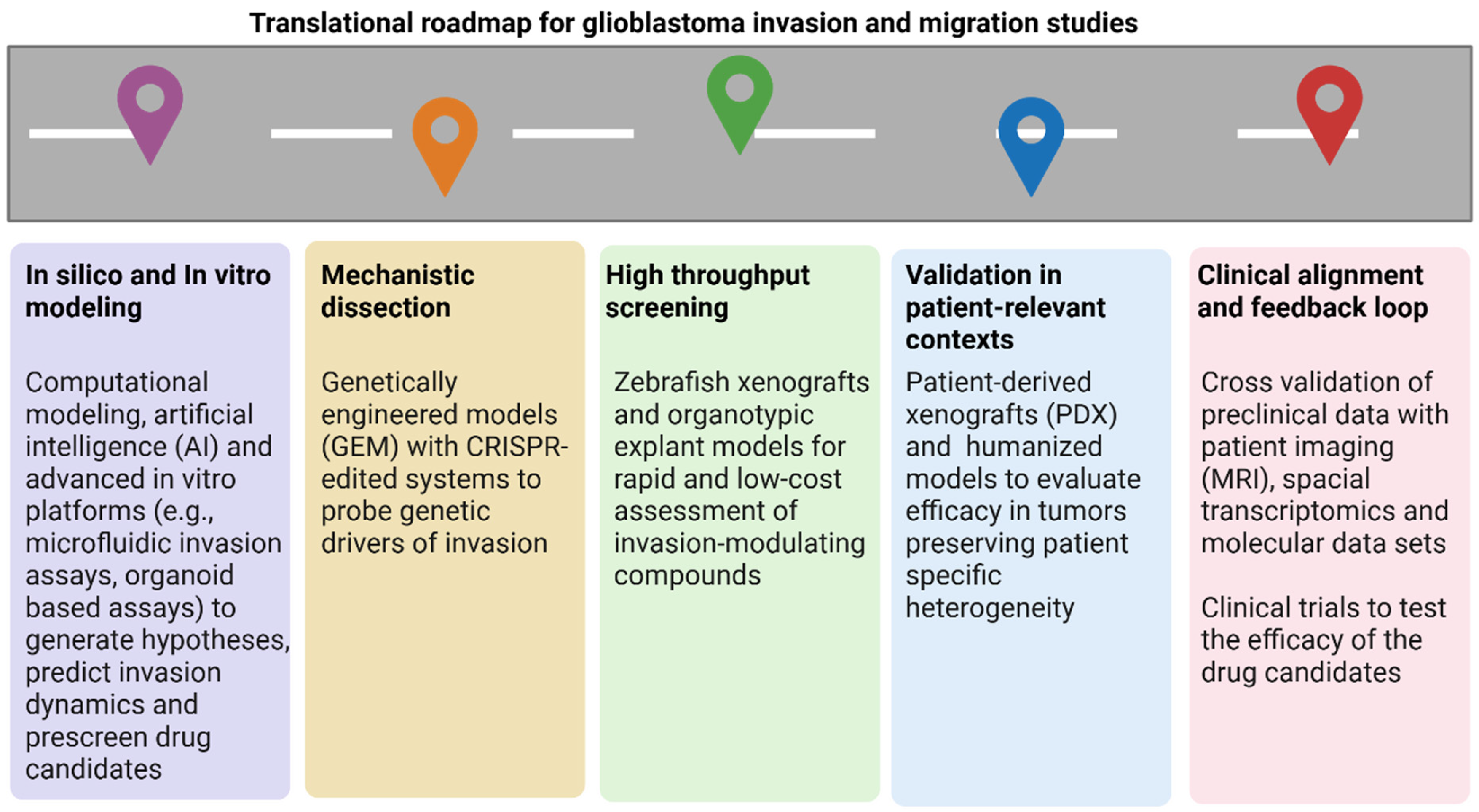
6. Challenges and Future Directions
6.1. Limitations and Challenges Associated with Using Animal Models for Migration Studies
6.2. Future Directions and Potential Advancements in the Field
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013, 15 (Suppl. S2), ii1–ii56. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016—2020. Neuro-Oncology 2023, 25 (Suppl. S4), iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Cantidio, F.S.; Gil, G.O.B.; Queiroz, I.N.; Regalin, M. Glioblastoma—Treatment and obstacles. Rep. Pract. Oncol. Radiother. 2022, 27, 744–753. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Huang, M.; Gong, G.; Deng, Y.; Long, X.; Long, W.; Liu, Q.; Zhao, W.; Chen, R. Crosstalk between cancer cells and the nervous system. Med. Adv. 2023, 1, 173–189. [Google Scholar] [CrossRef]
- Mair, D.B.; Ames, H.M.; Li, R. Mechanisms of invasion and motility of high-grade gliomas in the brain. Mol. Biol. Cell 2018, 29, 2509–2515. [Google Scholar] [CrossRef]
- Bakhshinyan, D.; Savage, N.; Salim, S.K.; Venugopal, C.; Singh, S.K. The Strange Case of Jekyll and Hyde: Parallels Between Neural Stem Cells and Glioblastoma-Initiating Cells. Front. Oncol. 2020, 10, 603738. [Google Scholar] [CrossRef]
- Giese, A.; Bjerkvig, R.; Berens, M.E.; Westphal, M. Cost of migration: Invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef]
- Noorani, I. Genetically Engineered Mouse Models of Gliomas: Technological Developments for Translational Discoveries. Cancers 2019, 11, 1335. [Google Scholar] [CrossRef]
- Sahu, U.; Barth, R.F.; Otani, Y.; McCormack, R.; Kaur, B. Rat and Mouse Brain Tumor Models for Experimental Neuro-Oncology Research. J. Neuropathol. Exp. Neurol. 2022, 81, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Pliakopanou, A.; Antonopoulos, I.; Darzenta, N.; Serifi, I.; Simos, Y.V.; Katsenos, A.P.; Bellos, S.; Alexiou, G.A.; Kyritsis, A.P.; Leonardos, I.; et al. Glioblastoma research on zebrafish xenograft models: A systematic review. Clin. Transl. Oncol. 2024, 26, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Alcaniz, J.; Winkler, L.; Dahlmann, M.; Becker, M.; Orthmann, A.; Haybaeck, J.; Krassnig, S.; Skofler, C.; Kratzsch, T.; Kuhn, S.A.; et al. Clinically relevant glioblastoma patient-derived xenograft models to guide drug development and identify molecular signatures. Front. Oncol. 2023, 13, 1129627. [Google Scholar] [CrossRef] [PubMed]
- Greene, H.S.N.; Arnold, H. The Homologous and Heterologous Transplantation of Brain and Brain Tumors. J. Neurosurg. 1945, 2, 315–331. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Perkins, G. Animal models for studying tumor microenvironment (TME) and resistance to lymphocytic infiltration. Cancer Biol. Ther. 2018, 19, 745–754. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Blackwell, T.S. Bioluminescence imaging. Proc. Am. Thorac. Soc. 2005, 2, 537–540. [Google Scholar] [CrossRef]
- Głodek, J.; Adamiak, Z.; Przeworski, A. Magnetic Resonance Imaging of Reptiles, Rodents, and Lagomorphs for Clinical Diagnosis and Animal Research. Comp. Med. 2016, 66, 216–219. [Google Scholar]
- Cherry, S.R.; Gambhir, S.S. Use of positron emission tomography in animal research. ILAR J. 2001, 42, 219–232. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, Fso63. [Google Scholar] [CrossRef]
- Justus, C.R.; Marie, M.A.; Sanderlin, E.J.; Yang, L.V. Transwell In Vitro Cell Migration and Invasion Assays. Methods Mol. Biol. 2023, 2644, 349–359. [Google Scholar] [CrossRef]
- Park, T.; Large, N. Real-Time Quantitative Measurement of Tumor Cell Migration and Invasion Following Synthetic mRNA Transfection. J. Vis. Exp. 2023, e64274. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; Large, N.; Park, T. Impedance-based Real-time Measurement of Cancer Cell Migration and Invasion. J. Vis. Exp. 2020, 158, e60997. [Google Scholar] [CrossRef]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014, e51300. [Google Scholar] [CrossRef] [PubMed]
- Rybin, M.J.; Ivan, M.E.; Ayad, N.G.; Zeier, Z. Organoid Models of Glioblastoma and Their Role in Drug Discovery. Front. Cell. Neurosci. 2021, 15, 605255. [Google Scholar] [CrossRef]
- Logun, M.; Wang, X.; Sun, Y.; Bagley, S.J.; Li, N.; Desai, A.; Zhang, D.Y.; Nasrallah, M.P.; Pai, E.L.-L.; Oner, B.S.; et al. Patient-derived glioblastoma organoids as real-time avatars for assessing responses to clinical CAR-T cell therapy. Cell Stem Cell 2025, 32, 181-190.e4. [Google Scholar] [CrossRef]
- Poggi, A.; Villa, F.; Fernadez, J.L.C.; Costa, D.; Zocchi, M.R.; Benelli, R. Three-Dimensional Culture Models to Study Innate Anti-Tumor Immune Response: Advantages and Disadvantages. Cancers 2021, 13, 3417. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Gómez-Oliva, R.; Domínguez-García, S.; Carrascal, L.; Abalos-Martínez, J.; Pardillo-Díaz, R.; Verástegui, C.; Castro, C.; Nunez-Abades, P.; Geribaldi-Doldán, N. Evolution of Experimental Models in the Study of Glioblastoma: Toward Finding Efficient Treatments. Front. Oncol. 2021, 10, 614295. [Google Scholar] [CrossRef]
- Houben, M.P.W.A.; Coebergh, J.W.W.; Birch, J.M.; Tijssen, C.C.; van Duijn, C.M.; McNally, R.J.Q. Space–time clustering patterns of gliomas in the Netherlands suggest an infectious aetiology. Eur. J. Cancer 2005, 41, 2917–2923. [Google Scholar] [CrossRef]
- Candolfi, M.; Curtin, J.F.; Nichols, W.S.; Muhammad, A.G.; King, G.D.; Pluhar, G.E.; McNiel, E.A.; Ohlfest, J.R.; Freese, A.B.; Moore, P.F.; et al. Intracranial glioblastoma models in preclinical neuro-oncology: Neuropathological characterization and tumor progression. J. Neurooncol. 2007, 85, 133–148. [Google Scholar] [CrossRef]
- Bilzer, T.; Reifenberger, G.; Wechsler, W. Chemical induction of brain tumors in rats by nitrosoureas: Molecular biology and neuropathology. Neurotoxicol. Teratol. 1989, 11, 551–556. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hoshino, H.; Kiuchi, Y.; Nakano, G.; Nagamachi, Y. Potential usefulness of a cultured glioma cell line induced by Rous sarcoma virus in B10.A mouse as an immunotherapy model. Jpn. J. Exp. Med. 1989, 59, 173–180. [Google Scholar] [PubMed]
- Guyon, J.; Haidar Ahmad, S.; El Baba, R.; Le Quang, M.; Bikfalvi, A.; Daubon, T.; Herbein, G. Generation of glioblastoma in mice engrafted with human cytomegalovirus-infected astrocytes. Cancer Gene Ther. 2024, 31, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Verma, I.M. Modeling Gliomas Using Two Recombinases. Cancer Res. 2019, 79, 3983–3991. [Google Scholar] [CrossRef] [PubMed]
- Benedykcinska, A.; Ferreira, A.; Lau, J.; Broni, J.; Richard-Loendt, A.; Henriquez, N.V.; Brandner, S. Generation of brain tumours in mice by Cre-mediated recombination of neural progenitors in situ with the tamoxifen metabolite endoxifen. Dis. Models Mech. 2016, 9, 211–220. [Google Scholar] [CrossRef]
- Jaisser, F. Inducible gene expression and gene modification in transgenic mice. J. Am. Soc. Nephrol. 2000, 11 (Suppl. S16), S95–S100. [Google Scholar] [CrossRef]
- Mao, X.Y.; Dai, J.X.; Zhou, H.H.; Liu, Z.Q.; Jin, W.L. Brain tumor modeling using the CRISPR/Cas9 system: State of the art and view to the future. Oncotarget 2016, 7, 33461–33471. [Google Scholar] [CrossRef]
- Wakimoto, H.; Kesari, S.; Farrell, C.J.; Curry, W.T., Jr.; Zaupa, C.; Aghi, M.; Kuroda, T.; Stemmer-Rachamimov, A.; Shah, K.; Liu, T.-C.; et al. Human Glioblastoma–Derived Cancer Stem Cells: Establishment of Invasive Glioma Models and Treatment with Oncolytic Herpes Simplex Virus Vectors. Cancer Res. 2009, 69, 3472–3481. [Google Scholar] [CrossRef]
- Noell, S.; Ritz, R.; Wolburg-Buchholz, K.; Wolburg, H.; Fallier-Becker, P. An Allograft Glioma Model Reveals the Dependence of Aquaporin-4 Expression on the Brain Microenvironment. PLoS ONE 2012, 7, e36555. [Google Scholar] [CrossRef]
- Kim, B.-H.; Lee, H.; Park, C.G.; Jeong, A.J.; Lee, S.-H.; Noh, K.H.; Park, J.B.; Lee, C.-G.; Paek, S.H.; Kim, H.; et al. STAT3 Inhibitor ODZ10117 Suppresses Glioblastoma Malignancy and Prolongs Survival in a Glioblastoma Xenograft Model. Cells 2020, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Labani-Motlagh, A.; Chen, A.; Bohorquez, J.A.; Qin, B.; Dodda, M.; Yang, F.; Ansari, D.; Patel, S.; Ji, H.; et al. Development of a human glioblastoma model using humanized DRAG mice for immunotherapy. Antib. Ther. 2023, 6, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, Z.; Wang, Y.; Fan, X.; Cai, J.; Yao, X.; Liu, L.; Huang, J.; He, J.; Xie, C.; et al. Modulation of gut microbiota to overcome resistance to immune checkpoint blockade in cancer immunotherapy. Curr. Opin. Pharmacol. 2020, 54, 1–10. [Google Scholar] [CrossRef]
- Xiao, J.; Glasgow, E.; Agarwal, S. Zebrafish Xenografts for Drug Discovery and Personalized Medicine. Trends Cancer 2020, 6, 569–579. [Google Scholar] [CrossRef]
- Hason, M.; Bartůněk, P. Zebrafish Models of Cancer-New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]
- Reimunde, P.; Pensado-López, A.; Carreira Crende, M.; Lombao Iglesias, V.; Sánchez, L.; Torrecilla-Parra, M.; Ramírez, C.M.; Anfray, C.; Torres Andón, F. Cellular and Molecular Mechanisms Underlying Glioblastoma and Zebrafish Models for the Discovery of New Treatments. Cancers 2021, 13, 1087. [Google Scholar] [CrossRef]
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef]
- Lonser, R.R.; Walbridge, S.; Vortmeyer, A.O.; Pack, S.D.; Nguyen, T.T.; Gogate, N.; Olson, J.J.; Akbasak, A.; Bobo, R.H.; Goffman, T.; et al. Induction of glioblastoma multiforme in nonhuman primates after therapeutic doses of fractionated whole-brain radiation therapy. J. Neurosurg. 2002, 97, 1378–1389. [Google Scholar] [CrossRef]
- Kang, X.; Wang, Y.; Liu, P.; Huang, B.; Zhou, B.; Lu, S.; Geng, W.; Tang, H. Progresses, Challenges, and Prospects of CRISPR/Cas9 Gene-Editing in Glioma Studies. Cancers 2023, 15, 396. [Google Scholar] [CrossRef]
- Pouyan, A.; Ghorbanlo, M.; Eslami, M.; Jahanshahi, M.; Ziaei, E.; Salami, A.; Mokhtari, K.; Shahpasand, K.; Farahani, N.; Meybodi, T.E.; et al. Glioblastoma multiforme: Insights into pathogenesis, key signaling pathways, and therapeutic strategies. Mol. Cancer 2025, 24, 58. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, X.; Wang, M.; Liu, W.; Zhang, L.; Zhang, Y.; Hu, Z.; Zhou, X.; Jiang, W.; Zou, Q.; et al. Optogenetic-controlled immunotherapeutic designer cells for post-surgical cancer immunotherapy. Nat. Commun. 2022, 13, 6357. [Google Scholar] [CrossRef]
- Taylor, K.R.; Barron, T.; Hui, A.; Spitzer, A.; Yalçin, B.; Ivec, A.E.; Geraghty, A.C.; Hartmann, G.G.; Arzt, M.; Gillespie, S.M.; et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature 2023, 623, 366–374. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tu, J.; Pan, J.Q.; Luo, H.L.; Liu, Y.H.; Wan, J.; Zhang, J.; Wei, P.F.; Jiang, T.; Chen, Y.H.; et al. Light-controlled inhibition of malignant glioma by opsin gene transfer. Cell Death Dis. 2013, 4, e893. [Google Scholar] [CrossRef]
- Camporeze, B.; Manica, B.A.; Bonafé, G.A.; Ferreira, J.J.C.; Diniz, A.L.; de Oliveira, C.T.P.; Mathias Junior, L.R.; de Aguiar, P.H.P.; Ortega, M.M. Optogenetics: The new molecular approach to control functions of neural cells in epilepsy, depression and tumors of the central nervous system. Am. J. Cancer Res. 2018, 8, 1900–1918. [Google Scholar] [PubMed]
- Himes, B.T.; Geiger, P.A.; Ayasoufi, K.; Bhargav, A.G.; Brown, D.A.; Parney, I.F. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 2021, 11, 770561. [Google Scholar] [CrossRef] [PubMed]
- Letchuman, V.; Ampie, L.; Shah, A.H.; Brown, D.A.; Heiss, J.D.; Chittiboina, P. Syngeneic murine glioblastoma models: Reactionary immune changes and immunotherapy intervention outcomes. Neurosurg. Focus 2022, 52, E5. [Google Scholar] [CrossRef]
- Liu, L.; van Schaik, T.A.; Chen, K.-S.; Rossignoli, F.; Borges, P.; Vrbanac, V.; Wakimoto, H.; Shah, K. Establishment and immune phenotyping of patient-derived glioblastoma models in humanized mice. Front. Immunol. 2024, 14, 1324618. [Google Scholar] [CrossRef]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Vandecandelaere, G.; Ramapriyan, R.; Gaffey, M.; Richardson, L.G.; Steuart, S.J.; Tazhibi, M.; Kalaw, A.; Grewal, E.P.; Sun, J.; Curry, W.T.; et al. Pre-Clinical Models for CAR T-Cell Therapy for Glioma. Cells 2024, 13, 1480. [Google Scholar] [CrossRef] [PubMed]
- Coates, K.; Nibbs, R.J.; Fraser, A.R. Going viral: Targeting glioblastoma using oncolytic viruses. Immunother. Adv. 2025, 5, ltaf024. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.F.; Young, J.S.; Amara, D.; Berger, M.S.; Raleigh, D.R.; Aghi, M.K.; Butowski, N.A. Mouse models of glioblastoma for the evaluation of novel therapeutic strategies. Neuro-Oncol. Adv. 2021, 3, vdab100. [Google Scholar] [CrossRef]
- Martens, T.; Laabs, Y.; Günther, H.S.; Kemming, D.; Zhu, Z.; Witte, L.; Hagel, C.; Westphal, M.; Lamszus, K. Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin. Cancer Res. 2008, 14, 5447–5458. [Google Scholar] [CrossRef] [PubMed]
- Mahesparan, R.; Read, T.A.; Lund-Johansen, M.; Skaftnesmo, K.O.; Bjerkvig, R.; Engebraaten, O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003, 105, 49–57. [Google Scholar] [CrossRef]
- Kijima, N.; Hosen, N.; Kagawa, N.; Hashimoto, N.; Kinoshita, M.; Oji, Y.; Sugiyama, H.; Yoshimine, T. Wilms’ tumor 1 is involved in tumorigenicity of glioblastoma by regulating cell proliferation and apoptosis. Anticancer Res. 2014, 34, 61–67. [Google Scholar]
- Anderson, R.C.; Elder, J.B.; Brown, M.D.; Mandigo, C.E.; Parsa, A.T.; Kim, P.D.; Senatus, P.; Anderson, D.E.; Bruce, J.N. Changes in the immunologic phenotype of human malignant glioma cells after passaging in vitro. Clin. Immunol. 2002, 102, 84–95. [Google Scholar] [CrossRef]
- Huszthy, P.C.; Daphu, I.; Niclou, S.P.; Stieber, D.; Nigro, J.M.; Sakariassen, P.; Miletic, H.; Thorsen, F.; Bjerkvig, R. In vivo models of primary brain tumors: Pitfalls and perspectives. Neuro Oncol. 2012, 14, 979–993. [Google Scholar] [CrossRef]
- Ernst, A.; Hofmann, S.; Ahmadi, R.; Becker, N.; Korshunov, A.; Engel, F.; Hartmann, C.; Felsberg, J.; Sabel, M.; Peterziel, H.; et al. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin. Cancer Res. 2009, 15, 6541–6550. [Google Scholar] [CrossRef]
- Clark, M.J.; Homer, N.; O’Connor, B.D.; Chen, Z.; Eskin, A.; Lee, H.; Merriman, B.; Nelson, S.F. U87MG decoded: The genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 2010, 6, e1000832. [Google Scholar] [CrossRef]
- Guillamo, J.S.; Lisovoski, F.; Christov, C.; Le Guérinel, C.; Defer, G.L.; Peschanski, M.; Lefrançois, T. Migration Pathways of Human Glioblastoma Cells Xenografted into the Immunosuppressed Rat Brain. J. Neuro-Oncol. 2001, 52, 205–215. [Google Scholar] [CrossRef]
- Qutaish, M.Q.; Sullivant, K.E.; Burden-Gulley, S.M.; Lu, H.; Roy, D.; Wang, J.; Basilion, J.P.; Brady-Kalnay, S.M.; Wilson, D.L. Cryo-image analysis of tumor cell migration, invasion, and dispersal in a mouse xenograft model of human glioblastoma multiforme. Mol. Imaging Biol. 2012, 14, 572–583. [Google Scholar] [CrossRef]
- Li, W.; Du, Q.; Li, X.; Zheng, X.; Lv, F.; Xi, X.; Huang, G.; Yang, J.; Liu, S. Eriodictyol Inhibits Proliferation, Metastasis and Induces Apoptosis of Glioma Cells via PI3K/Akt/NF-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Lal, S.; La Du, J.; Tanguay, R.L.; Greenwood, J.A. Calpain 2 is required for the invasion of glioblastoma cells in the zebrafish brain microenvironment. J. Neurosci. Res. 2012, 90, 769–781. [Google Scholar] [CrossRef]
- Wehmas, L.C.; Tanguay, R.L.; Punnoose, A.; Greenwood, J.A. Developing a Novel Embryo-Larval Zebrafish Xenograft Assay to Prioritize Human Glioblastoma Therapeutics. Zebrafish 2016, 13, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.T.; Reed-Harris, Y.; Barton, C.L.; La Du, J.; Tanguay, R.; Greenwood, J.A. Quantification of glioblastoma progression in zebrafish xenografts: Adhesion to laminin alpha 5 promotes glioblastoma microtumor formation and inhibits cell invasion. Biochem. Biophys. Res. Commun. 2018, 506, 833–839. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Cai, C.; Zhang, H.; Shen, H.; Han, Y. Patient-derived xenograft models in cancer therapy: Technologies and applications. Signal Transduct. Target. Ther. 2023, 8, 160. [Google Scholar] [CrossRef]
- Li, J.; Gu, A.; Tang, N.; Zengin, G.; Li, M.-Y.; Liu, Y. Patient-derived xenograft models in pan-cancer: From bench to clinic. Interdiscip. Med. 2025, e20250016. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Liu, P.; Li, M.; Luo, F. Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine. Oncol. Lett. 2019, 17, 3–10. [Google Scholar] [CrossRef]
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Asadzadeh Aghdaei, H.; Baghaei, K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J. Transl. Med. 2022, 20, 206. [Google Scholar] [CrossRef]
- Chen, J.; Liao, S.; Xiao, Z.; Pan, Q.; Wang, X.; Shen, K.; Wang, S.; Yang, L.; Guo, F.; Liu, H.F.; et al. The development and improvement of immunodeficient mice and humanized immune system mouse models. Front. Immunol. 2022, 13, 1007579. [Google Scholar] [CrossRef]
- Panaampon, J.; Sasamoto, K.; Kariya, R.; Okada, S. Establishment of Nude Mice Lacking NK Cells and Their Application for Human Tumor Xenografts. Asian Pac. J. Cancer Prev. 2021, 22, 1069–1074. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Jiang, M.; Zhang, T.; Wang, Y.; Wang, Z.; Miao, Y.; Wang, Z.; Li, W. Comparison between NOD/SCID mice and BALB/c mice for patient-derived tumor xenografts model of non-small-cell lung cancer. Cancer Manag. Res. 2018, 10, 6695–6703. [Google Scholar] [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Xia, S.; Lal, B.; Tung, B.; Wang, S.; Goodwin, C.R.; Laterra, J. Tumor microenvironment tenascin-C promotes glioblastoma invasion and negatively regulates tumor proliferation. Neuro Oncol. 2016, 18, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Kumar, S.; Iskander, A.S.; Varma, N.R.; Janic, B.; deCarvalho, A.; Mikkelsen, T.; Frank, J.A.; Ali, M.M.; Knight, R.A.; et al. Subcurative radiation significantly increases cell proliferation, invasion, and migration of primary glioblastoma multiforme in vivo. Chin. J. Cancer 2014, 33, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Niklasson, M.; Bergström, T.; Segerman, A.; Betsholtz, C.; Westermark, B. Tumor-specific migration routes of xenotransplanted human glioblastoma cells in mouse brain. Sci. Rep. 2024, 14, 864. [Google Scholar] [CrossRef]
- Yuzhakova, D.; Kiseleva, E.; Shirmanova, M.; Shcheslavskiy, V.; Sachkova, D.; Snopova, L.; Bederina, E.; Lukina, M.; Dudenkova, V.; Yusubalieva, G.; et al. Highly Invasive Fluorescent/Bioluminescent Patient-Derived Orthotopic Model of Glioblastoma in Mice. Front. Oncol. 2022, 12, 897839. [Google Scholar] [CrossRef]
- Pudelko, L.; Edwards, S.; Balan, M.; Nyqvist, D.; Al-Saadi, J.; Dittmer, J.; Almlöf, I.; Helleday, T.; Bräutigam, L. An orthotopic glioblastoma animal model suitable for high-throughput screenings. Neuro Oncol. 2018, 20, 1475–1484. [Google Scholar] [CrossRef]
- Larsson, S.; Kettunen, P.; Carén, H. Orthotopic Transplantation of Human Paediatric High-Grade Glioma in Zebrafish Larvae. Brain Sci. 2022, 12, 625. [Google Scholar] [CrossRef]
- Almstedt, E.; Rosén, E.; Gloger, M.; Stockgard, R.; Hekmati, N.; Koltowska, K.; Krona, C.; Nelander, S. Real-time evaluation of glioblastoma growth in patient-specific zebrafish xenografts. Neuro Oncol. 2022, 24, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Umans, R.A.; Ten Kate, M.; Pollock, C.; Sontheimer, H. Fishing for Contact: Modeling Perivascular Glioma Invasion in the Zebrafish Brain. ACS Pharmacol. Transl. Sci. 2021, 4, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef]
- Heyer, J.; Kwong, L.N.; Lowe, S.W.; Chin, L. Non-germline genetically engineered mouse models for translational cancer research. Nat. Rev. Cancer 2010, 10, 470–480. [Google Scholar] [CrossRef]
- Assi, H.; Candolfi, M.; Lowenstein, P.R.; Castro, M.G. Rodent Glioma Models: Intracranial Stereotactic Allografts and Xenografts. In Animal Models of Brain Tumors; Martínez Murillo, R., Martínez, A., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 229–243. [Google Scholar] [CrossRef]
- Ahmed, E.N.; Cutmore, L.C.; Marshall, J.F. Syngeneic Mouse Models for Pre-Clinical Evaluation of CAR T Cells. Cancers 2024, 16, 3186. [Google Scholar] [CrossRef]
- Chen, N.; Alieva, M.; van der Most, T.; Klazen, J.A.Z.; Vollmann-Zwerenz, A.; Hau, P.; Vrisekoop, N. Neutrophils Promote Glioblastoma Tumor Cell Migration after Biopsy. Cells 2022, 11, 2196. [Google Scholar] [CrossRef]
- Vajkoczy, P.; Goldbrunner, R.; Farhadi, M.; Vince, G.; Schilling, L.; Tonn, J.C.; Schmiedek, P.; Menger, M.D. Glioma cell migration is associated with glioma-induced angiogenesis in vivo. Int. J. Dev. Neurosci. 1999, 17, 557–563. [Google Scholar] [CrossRef]
- Tatenhorst, L.; Püttmann, S.; Senner, V.; Paulus, W. Genes associated with fast glioma cell migration in vitro and in vivo. Brain Pathol. 2005, 15, 46–54. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Mei, S.; Sun, Y.; Li, J. Acquisition of temozolomide resistance by the rat C6 glioma cell line increases cell migration and side population phenotype. Oncol. Rep. 2019, 42, 2355–2362. [Google Scholar] [CrossRef]
- Wolmarans, E.; Nel, S.; Durandt, C.; Mellet, J.; Pepper, M.S. Side Population: Its Use in the Study of Cellular Heterogeneity and as a Potential Enrichment Tool for Rare Cell Populations. Stem Cells Int. 2018, 2018, 2472137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xie, J.; Liu, G.; He, Y.; Lu, G.; Chen, X. In vivo MRI tracking of cell invasion and migration in a rat glioma model. Mol. Imaging Biol. 2011, 13, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Akella, N.S.; Ding, Q.; Menegazzo, I.; Wang, W.; Gillespie, G.Y.; Grammer, J.R.; Gladson, C.L.; Nabors, L.B. A novel technique to quantify glioma tumor invasion using serial microscopy sections. J. Neurosci. Methods 2006, 153, 183–189. [Google Scholar] [CrossRef] [PubMed]
- García-Vicente, L.; Martínez-Fernández, M.; Borja, M.; Tran, V.; Álvarez-Vázquez, A.; Flores-Hernández, R.; Ding, Y.; González-Sánchez, R.; Granados, A.; McGeever, E.; et al. Single-nucleus RNA sequencing reveals a preclinical model for the most common subtype of glioblastoma. Commun. Biol. 2025, 8, 671. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Parada, L.F.; Holland, E.C.; Charest, A. Genetic modeling of gliomas in mice: New tools to tackle old problems. Glia 2011, 59, 1155–1168. [Google Scholar] [CrossRef]
- Oldrini, B.; Curiel-García, Á.; Marques, C.; Matia, V.; Uluçkan, Ö.; Graña-Castro, O.; Torres-Ruiz, R.; Rodriguez-Perales, S.; Huse, J.T.; Squatrito, M. Somatic genome editing with the RCAS-TVA-CRISPR-Cas9 system for precision tumor modeling. Nat. Commun. 2018, 9, 1466. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Singh, R.K.; Fidler, I.J.; Raz, A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am. J. Pathol. 2007, 170, 793–804. [Google Scholar] [CrossRef]
- Zhu, H.; Acquaviva, J.; Ramachandran, P.; Boskovitz, A.; Woolfenden, S.; Pfannl, R.; Bronson, R.T.; Chen, J.W.; Weissleder, R.; Housman, D.E.; et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 2712–2716. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D.; Bushong, E.A.; Ke, E.; Soda, Y.; Marumoto, T.; Singer, O.; Ellisman, M.H.; Verma, I.M. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 2012, 338, 1080–1084. [Google Scholar] [CrossRef]
- Salam, R.; Saliou, A.; Bielle, F.; Bertrand, M.; Antoniewski, C.; Carpentier, C.; Alentorn, A.; Capelle, L.; Sanson, M.; Huillard, E.; et al. Cellular senescence in malignant cells promotes tumor progression in mouse and patient Glioblastoma. Nat. Commun. 2023, 14, 441. [Google Scholar] [CrossRef]
- Faisal, S.M.; Clewner, J.E.; Stack, B.; Varela, M.L.; Comba, A.; Abbud, G.; Motsch, S.; Castro, M.G.; Lowenstein, P.R. Spatiotemporal Insights into Glioma Oncostream Dynamics: Unraveling Formation, Stability, and Disassembly Pathways. Adv. Sci. 2024, 11, e2309796. [Google Scholar] [CrossRef]
- Kharbanda, K.; Sarkar, C.; Dinda, A.K.; Karak, A.K.; Mathur, M.; Roy, S. Morphological appearance, growth kinetics and glial fibrillary acidic protein (GFAP) expression in primary in vitro explant culture of astrocytic neoplasms. Acta Oncol. 1993, 32, 301–306. [Google Scholar] [CrossRef]
- Soubéran, A.; Cappaï, J.; Chocry, M.; Nuccio, C.; Raujol, J.; Colin, C.; Lafitte, D.; Kovacic, H.; Quillien, V.; Baeza-Kallee, N.; et al. Inhibitor of Apoptosis Proteins Determines Glioblastoma Stem-Like Cell Fate in an Oxygen-Dependent Manner. Stem Cells 2019, 37, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Kallee, N.; Bergès, R.; Soubéran, A.; Colin, C.; Denicolaï, E.; Appay, R.; Tchoghandjian, A.; Figarella-Branger, D. Glycolipids Recognized by A2B5 Antibody Promote Proliferation, Migration, and Clonogenicity in Glioblastoma Cells. Cancers 2019, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Colin, C.; Baeza, N.; Tong, S.; Bouvier, C.; Quilichini, B.; Durbec, P.; Figarella-Branger, D. In vitro identification and functional characterization of glial precursor cells in human gliomas. Neuropathol. Appl. Neurobiol. 2006, 32, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.M.; Odde, D.J. Identifying the Mechanism of Glioblastoma Cell Migration in Mouse Brain Slices. Microsc. Microanal. 2023, 29 (Suppl. S1), 1066–1067. [Google Scholar] [CrossRef]
- Zepecki, J.P.; Snyder, K.M.; Moreno, M.M.; Fajardo, E.; Fiser, A.; Ness, J.; Sarkar, A.; Toms, S.A.; Tapinos, N. Regulation of human glioma cell migration, tumor growth, and stemness gene expression using a Lck targeted inhibitor. Oncogene 2019, 38, 1734–1750. [Google Scholar] [CrossRef]
- Minami, N.; Maeda, Y.; Shibao, S.; Arima, Y.; Ohka, F.; Kondo, Y.; Maruyama, K.; Kusuhara, M.; Sasayama, T.; Kohmura, E.; et al. Organotypic brain explant culture as a drug evaluation system for malignant brain tumors. Cancer Med. 2017, 6, 2635–2645. [Google Scholar] [CrossRef]
- Olson, B.; Li, Y.; Lin, Y.; Liu, E.T.; Patnaik, A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018, 8, 1358–1365. [Google Scholar] [CrossRef]
- Comba, A.; Varela, M.L.; Faisal, S.M.; Abel, C.C., 2nd; Argento, A.E.; Al-Holou, W.N.; Hollon, T.C.; Perelman, J.D.; Dunn, P.J.; Motsch, S.; et al. Generation of 3D ex vivo mouse- and patient-derived glioma explant slice model for integration of confocal time-lapse imaging and spatial analysis. STAR Protoc. 2023, 4, 102174. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.L.; Balasubramaniyan, V.; Vaillant, B.; Ezhilarasan, R.; Hummelink, K.; Hollingsworth, F.; Wani, K.; Heathcock, L.; James, J.D.; Goodman, L.D.; et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 2013, 24, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42-56.e6. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef]
- Mann, B.; Artz, N.; Darawsheh, R.; Kram, D.E.; Hingtgen, S.; Satterlee, A.B. Opportunities and challenges for patient-derived models of brain tumors in functional precision medicine. npj Precis. Oncol. 2025, 9, 47. [Google Scholar] [CrossRef]
- Allen, T.M.; Brehm, M.A.; Bridges, S.; Ferguson, S.; Kumar, P.; Mirochnitchenko, O.; Palucka, K.; Pelanda, R.; Sanders-Beer, B.; Shultz, L.D.; et al. Humanized immune system mouse models: Progress, challenges and opportunities. Nat. Immunol. 2019, 20, 770–774. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef]
- Levin, V.A.; Phuphanich, S.; Yung, W.K.; Forsyth, P.A.; Maestro, R.D.; Perry, J.R.; Fuller, G.N.; Baillet, M. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J. Neurooncol. 2006, 78, 295–302. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023, 9, 112–121. [Google Scholar] [CrossRef]
- Heuer, S.; Burghaus, I.; Gose, M.; Kessler, T.; Sahm, F.; Vollmuth, P.; Venkataramani, V.; Hoffmann, D.; Schlesner, M.; Ratliff, M.; et al. PerSurge (NOA-30) phase II trial of perampanel treatment around surgery in patients with progressive glioblastoma. BMC Cancer 2024, 24, 135. [Google Scholar] [CrossRef]
- Raju R, R.; AlSawaftah, N.M.; Husseini, G.A. Modeling of brain tumors using in vitro, in vivo, and microfluidic models: A review of the current developments. Heliyon 2024, 10, e31402. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, R.; Song, Y.; Wei, W.; Baek, D.; Gillin, M.; Kurabayashi, K.; Chen, W. Cancer-on-a-chip for precision cancer medicine. Lab Chip 2025, 25, 3314–3347. [Google Scholar] [CrossRef]
- Hwangbo, H.; Chae, S.; Kim, W.; Jo, S.; Kim, G.H. Tumor-on-a-chip models combined with mini-tissues or organoids for engineering tumor tissues. Theranostics 2024, 14, 33–55. [Google Scholar] [CrossRef]
- Cai, X.; Briggs, R.G.; Homburg, H.B.; Young, I.M.; Davis, E.J.; Lin, Y.-H.; Battiste, J.D.; Sughrue, M.E. Application of microfluidic devices for glioblastoma study: Current status and future directions. Biomed. Microdevices 2020, 22, 60. [Google Scholar] [CrossRef]
- Skarne, N.; D’Souza, R.C.J.; Palethorpe, H.M.; Bradbrook, K.A.; Gomez, G.A.; Day, B.W. Personalising glioblastoma medicine: Explant organoid applications, challenges and future perspectives. Acta Neuropathol. Commun. 2025, 13, 6. [Google Scholar] [CrossRef]
- Liu, S.; Gong, Z.; Zhou, D.; Ayala-Nunez, V.; Xu, T.; Wick, P. Bridging the Gap: How Organ-on-a-Chip Technology Facilitates the Battle against Glioma. Small Sci. 2025, 2500154. [Google Scholar] [CrossRef]
- Pawlowski, K.D.; Duffy, J.T.; Babak, M.V.; Balyasnikova, I.V. Modeling glioblastoma complexity with organoids for personalized treatments. Trends Mol. Med. 2023, 29, 282–296. [Google Scholar] [CrossRef]
- Logun, M.; Zhao, W.; Mao, L.; Karumbaiah, L. Microfluidics in Malignant Glioma Research and Precision Medicine. Adv. Biosyst. 2018, 2, 1700221. [Google Scholar] [CrossRef]
- Wang, J.; Tao, X.; Zhu, J.; Dai, Z.; Du, Y.; Xie, Y.; Chu, X.; Fu, G.; Lei, Z. Tumor organoid-immune co-culture models: Exploring a new perspective of tumor immunity. Cell Death Discov. 2025, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, Y.; Wang, Y. Co-culture models for investigating cellular crosstalk in the glioma microenvironment. Cancer Pathog. Ther. 2024, 2, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Calar, K.; de la Puente, P. Mimicking tumor hypoxia and tumor-immune interactions employing three-dimensional in vitro models. J. Exp. Clin. Cancer Res. 2020, 39, 75. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, G.; Javed, B.; Singh, B.; Tian, F. Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics. Sensors 2024, 24, 2865. [Google Scholar] [CrossRef]
- Milde, T.; Fangusaro, J.; Fisher, M.J.; Hawkins, C.; Rodriguez, F.J.; Tabori, U.; Witt, O.; Zhu, Y.; Gutmann, D.H. Optimizing preclinical pediatric low-grade glioma models for meaningful clinical translation. Neuro Oncol. 2023, 25, 1920–1931. [Google Scholar] [CrossRef]
- Sundar, S.J.; Shakya, S.; Barnett, A.; Wallace, L.C.; Jeon, H.; Sloan, A.; Recinos, V.; Hubert, C.G. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Transl. Oncol. 2022, 15, 101251. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Abdoli, A.; Rahmanian, M.; Bardania, H.; Bayandori, M.; Moosavi Basri, S.M.; Kalbasi, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Microfluidic Brain-on-a-Chip: Perspectives for Mimicking Neural System Disorders. Mol. Neurobiol. 2019, 56, 8489–8512. [Google Scholar] [CrossRef]
- Rončević, A.; Koruga, N.; Soldo Koruga, A.; Rončević, R. Artificial Intelligence in Glioblastoma—Transforming Diagnosis and Treatment. Chin. Neurosurg. J. 2025, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Palavani, L.B.; Nogueira, B.V.; Mitre, L.P.; Chen, H.C.; Müller, G.C.; Vilardo, M.; Pereira, V.G.; Paleare, L.F.F.; Ribeiro, F.V.; Araujo, A.A.S.; et al. Artificial intelligence algorithms for differentiating pseudoprogression from true progression in high-grade gliomas: A systematic review and meta-analysis. Neurosurg. Rev. 2025, 48, 591. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, D.; Du, X.; Chen, W.; Lin, X.; Xu, B.; Xu, Y.; Ye, J.; Shen, Y. AI-driven Design of Drug Delivery Systems: Strategies and Challenges in Overcoming Biological Barriers. Acta Pharm. Sin. B 2025. [Google Scholar] [CrossRef]
| Model Category | Subtype | Key Advantages | Limitations | Reference |
|---|---|---|---|---|
| Spontaneous models | Natural occurrence | Mimics natural tumor initiation and progression | Requires a large cohort of animals | [31,32] |
| Chemically induced | Simple procedures, brief experiment times, and the ability to detect tumor progression from an early stage | [33] | ||
| Virus-induced | Mimicking the natural progression of human cancer | [34,35] | ||
| Genetically engineered models | Cre-loxP, traditional | High genetic accuracy and a stable model | Limited to spatial gene control; time-intensive | [36] |
| Cre-loxP, tamoxifen-inducible | Allows temporal gene control | [11,37] | ||
| Tet/dox-inducible | Provides temporal regulation of gene expression | [38] | ||
| Transposon-based: Sleeping Beauty (SB) and PiggyBac (PB) | Reduced generation time | [12] | ||
| CRISPR/Cas9 | Cost-effective, fast, and easy to implement | Risk of off-target effects | [39] | |
| Viral vector-based delivery | Rapid model establishment | Limited vector capacity (<2.5 Kb) | [40] | |
| Transplant models | Allograft (mouse-to-mouse) | Suitable for studying immune response and immunotherapy | Murine-specific immune interactions | [41] |
| Xenograft (human-to-mouse) | Recapitulates human tumor genetic and phenotypic traits | Lacks a functional human immune system | [14,42] | |
| Humanized mouse models | Hematopoietic stem cells (HSC)-engrafted | Supports a fully functional human immune system | Incomplete replication of human immune responses | [43] |
| Human microbiota-associated (HMA) | Reduces gut microbiota interference on immunity | [44] |
| Model Type | Key Features | Immune Compatibility | Strengths | Limitations |
|---|---|---|---|---|
| Cell line-derived xenograft (CDX) | High-passage, well-established cell lines (e.g., U-87 MG, LN-229, U-251 MG) have 80–100% engraftment rates. | Unable to mimic the complete human immune system. | Efficient tumor formation and rapid growth Useful for migration and invasion studies. Reproducible results. | Does not fully mimic patient tumor heterogeneity. Lacks proper invasion and microenvironment features. |
| Patient-derived xenograft (PDX) | Engraftment success rate is 60–80%. Retains patient tumor heterogeneity, but variable success depending on tumor subtype and sample viability. | Not ideal for studying immune responses due to the use of immunodeficient mice. | Better mimics human tumors. Higher clinical relevance. Retains patient tumor characteristics. | Requires immunodeficient animals; limited immune interactions; low engraftment for less aggressive tumors. |
| Allograft (Syngeneic) | Engraftment success rate is 90–100%. Uses murine glioma cell lines (e.g., GL261) and is highly efficient in immunocompetent mice. | The tumor cells and the host rodent are genetically identical, ensuring a higher immune compatibility. | Studies the immune response and tumor-immune interactions. Maintains original tumor genetics. | Limited to mouse tumor characteristics. May not fully replicate human glioblastoma behavior. |
| Genetically engineered models (GEMs) | Variable engraftment rate (often <50% without strong promoters or multiple mutations). Tumor development depends on the efficiency of genetic manipulation. Longer latency and lower penetrance unless multiple driver mutations are combined. | Fully competent immune system. | Precise genetic control; useful for studying tumor initiation, progression, and therapeutic targets. | Limited genetic diversity; unpredictable tumor onset. May not fully recapitulate human tumor heterogeneity. |
| Explant brain slice models | Ex vivo brain tissue slices preserve the brain microenvironment for real-time migration and invasion studies. | Depends on the retention of resident immune cells. | Preserves tissue architecture Real-time observation of tumor behavior. Useful for drug testing. | Limited to ex vivo studies. Lacks systemic physiological factors. Short experimental duration. |
| Category | Technique/Method | Purpose/Description | Application |
|---|---|---|---|
| Macroscopic imaging | MRI, PET, CT | Non-invasive imaging to monitor tumor growth and metastasis | Evaluating tumor burden and anatomical localization |
| Optical imaging | Fluorescence | Visualizing labeled cells or molecules in vivo | Tracking tumor progression and therapeutic response |
| Bioluminescence: luciferase-expressing tumor cells + substrate | Emits visible light for sensitive, high-throughput tumor/metastasis detection | ||
| Intravital microscopy | Two-photon, light-sheet fluorescence microscopy | High-resolution, real-time imaging of cell behavior in live tissues | Observing glioblastoma cell motility, vascular interaction, and invasion in brain tissues |
| Quantification methods | Cell tracking algorithms, morphometric analysis | Quantitative assessment of migration speed, directionality, and invasion depth | Analyzing dynamic cell movement and morphology |
| Transcriptomic profiling | Single-cell RNA sequencing (scRNA-seq) | Captures gene expression at single-cell resolution to reveal heterogeneity and invasive phenotypes | Identifying molecular programs linked to invasion, stemness, and therapy resistance |
| Dimension | Animal Models | Multicellular Spheroids/Organoids (3D) | Microfluidic/GBM-on-a-Chip |
| Tumor heterogeneity and architecture | PDX preserves patient heterogeneity and recapitulate white-matter and perivascular invasion; zebrafish enables rapid in vivo visualization; GEM maps genotype to phenotype invasion routes [11,84]. | Capture intra-spheroid gradients, cell-state heterogeneity, and chronic drug responses; scalable [141]. | Reconstructs tumor–vessel/BBB interfaces with human cells; supports engineered gradients and spatial niches [142]. |
| Microenvironmental complexity (ECM, vasculature, BBB, and neural activity) | Whole-brain ECM/white matter tracts, intact (or humanized) immunity, systemic physiology, and neuronal activity influencing invasion [12]. | ECM can be tuned but lacks vasculature/BBB and systemic cues [143]. | Adds perfused micro vessels/BBB, shear stress, and controllable stromal components; still partial compared to brain complexity [144]. |
| Immune context | Syngeneic/GEM: complete murine immunity; humanized mice: partial human immune function; zebrafish larvae: innate-biased [41,60]. | Largely immune-absent unless co-cultured [145]. | Immune co-cultures possible (e.g., macrophages/T cells) but typically simplified [146]. |
| Measurable invasion phenotypes | Long-range migration along white matter and perivascular tracks; live intravital or MRI/bioluminescence tracking; zebrafish: real-time perivascular guidance. | Collective and single-cell invasion into matrices; hypoxia-driven invasiveness [144,147]. | Perivascular invasion, pseudopalisading dynamics, vascular co-option and extravasation in controlled microchannels [148]. |
| Throughput, cost, and speed | Lower throughput, higher cost, weeks-months latency [149]. | High throughput, inexpensive, days-weeks [150]. | Moderate throughput: device fabrication and imaging expertise required [151]. |
| Experimental control and standardization | Biological realism high but experiment-to-experiment variability; species differences [149]. | Highly controllable; batch effects (size and matrix) need standardization [27]. | Highly controllable microenvironment and flow; device-to-device variability and PDMS/drug absorption issues [144]. |
| Ethical/regulatory | Heavier regulatory/ethical load. | Fewer ethical constraints. | Fewer ethical constraints. All three still require good experimental practice. |
| Best use cases | Validating human-cell findings; testing BBB penetration, PK/PD, neuro-immune interactions; mapping in vivo invasion routes. | Mechanism discovery, gene/drug screens, chronic treatment response under tumor-like gradients. | Dissecting transport/invasion at the BBB–tumor interface; perivascular co-option; patient-specific microenvironment engineering. |
| Key limitations | Species gaps; cost; lower throughput; immunodeficiency in many xenografts. | No systemic physiology; limited vasculature/BBB; matrix choice influences results. | Partial microenvironment; fabrication complexity; limited systemic metabolism. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hettiarachchi, P.; Park, T. Choice of Animal Models to Investigate Cell Migration and Invasion in Glioblastoma. Cancers 2025, 17, 2776. https://doi.org/10.3390/cancers17172776
Hettiarachchi P, Park T. Choice of Animal Models to Investigate Cell Migration and Invasion in Glioblastoma. Cancers. 2025; 17(17):2776. https://doi.org/10.3390/cancers17172776
Chicago/Turabian StyleHettiarachchi, Piyanka, and Taeju Park. 2025. "Choice of Animal Models to Investigate Cell Migration and Invasion in Glioblastoma" Cancers 17, no. 17: 2776. https://doi.org/10.3390/cancers17172776
APA StyleHettiarachchi, P., & Park, T. (2025). Choice of Animal Models to Investigate Cell Migration and Invasion in Glioblastoma. Cancers, 17(17), 2776. https://doi.org/10.3390/cancers17172776








