Is Human Chorionic Gonadotropin a Reliable Marker for Testicular Germ Cell Tumor? New Perspectives for a More Accurate Diagnosis
Simple Summary
Abstract
1. Introduction
1.1. Testicular Germ Cell Tumor
1.2. Mutations
1.3. Metastatic Spread
1.4. Diagnosis and Disease Management
2. Cryptorchidism as a Crucial Risk Factor for TGCT
2.1. Cryptorchidism
2.2. Surgical Treatment
2.3. Testicular Alterations of Cryptorchid Testicles
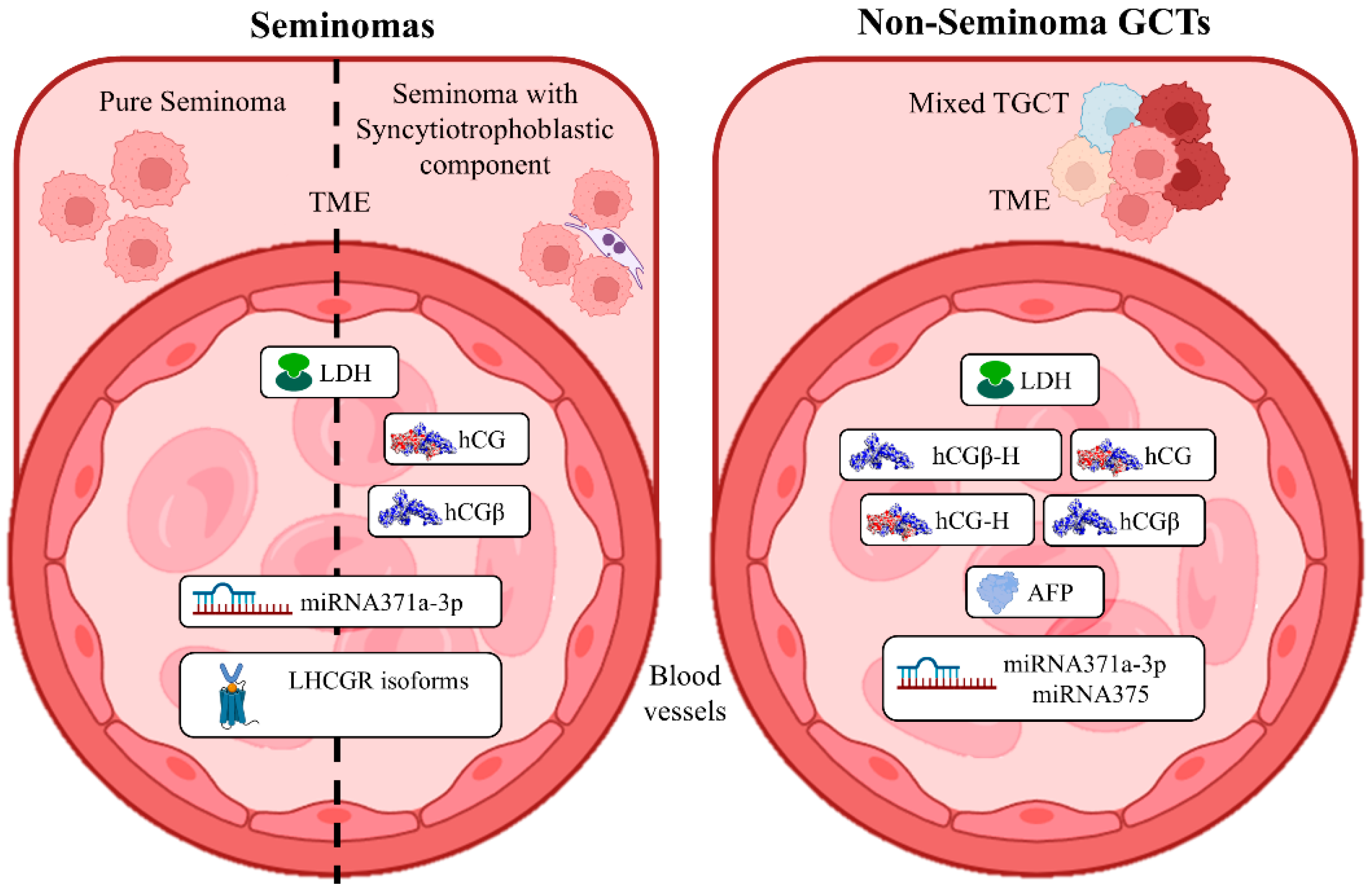
3. TGCT Serum Diagnostic Markers
3.1. Alpha-Fetoprotein
3.2. Lactate Dehydrogenase
3.3. Human Chorionic Gonadotropin
3.4. MicroRNAs as New Potential Diagnostic Markers
3.5. Other Potential Markers
4. hCG as a Critical Marker for TGCT Diagnosis
4.1. hCG Levels During TGCT Developmental Stages
4.2. hCG Expression and Epigenetic Regulations
4.3. Role of Hormones in hCG Expression
4.4. hCG Receptor: LHCGR and Its Variants
5. Improvement in an Effective Diagnosis of TGCT Based on hCG Expression and Signaling
| Germinoma Family of Tumors (~50%) | |||||||
| ICD-O-3.2 subtypes | Terminology | Incidence | Median Age | hCG, hCGβ marker positivity | References | ||
| 9061/3 | Seminoma | ~70% | 30–49 years | Negative | [35,78,142,143] | ||
| 9061/3 | Seminoma with syncytiotrophoblastic cells | ~30% | Positive | ||||
| Prognosis group | 5-year PFS | 5-year survival rate | Clinical stages | Treatment (Orchidectomy mandatory first step for all CSs) | References | ||
| Good | 89% | 95% | I | Surveillance; adjuvant carboplatin or radiotherapy | [5] | ||
| IIA/B | Chemotherapy (BEP × 3 or EP × 4) or radiotherapy (30–36 Gy) | ||||||
| IIC and III | Chemotherapy (BEP × 3 or EP × 4) | ||||||
| Intermediate | 79% | 88% | IIC and III | Chemotherapy (BEP × 4 or VIP × 4) | |||
| Poor | Nd | Nd | Not classified | Nd | |||
| Non-seminomatous germ cell tumors (~50%) | |||||||
| ICD-O-3.2 subtypes | Terminology | Incidence pure | Incidence mixed | Median Age | hCG, hCG-H, hCGβ, hCGβ-H marker positivity | References | |
| 9070/3 | Embryonal carcinoma | 16% | 80–90% | 30 years | Positive | [144,145,146,147,148] | |
| 9071/3 | Yolk sac tumor | <1% | 44% | 16–20 months | Rarely positive | ||
| 9100/3 | Choriocarcinoma | <1% | 8% | 25–30 years | Strongly positive | ||
| 9080/3 | Teratoma (post-pubertal) | 4–9% | 50% | 20–35 years | Slightly positive in mixed forms | ||
| Prognosis group | 5-year PFS | 5-year survival rate | Clinical stages | Treatment (Orchidectomy mandatory first step for all CSs) | References | ||
| Good | 90% | 96% | I | Surveillance, adjuvant chemotherapy (BEP × 1), and primary RPLND | [5] | ||
| IIA/B | Nerve sparing RPLND, adjuvant chemotherapy | ||||||
| IIC and III | Chemotherapy (BEP × 3 or EP × 4) | ||||||
| Intermediate | 78% | 89% | IIC and III | Chemotherapy (BEP × 3 or EP × 4) | |||
| IIA/B | Chemotherapy (BEP × 4 or VIP × 4) | ||||||
| Poor | 54% | 67% | IIC and III | Chemotherapy (BEP × 4 or VIP × 4) | |||
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 11 July 2025).
- Arranz Arija, J.A.; Del Muro, X.G.; Caro, R.L.; Méndez-Vidal, M.J.; Pérez-Valderrama, B.; Aparicio, J.; Climent Durán, M.Á.; Caballero Díaz, C.; Durán, I.; González-Billalabeitia, E. SEOM-GG Clinical Guidelines for the Management of Germ-Cell Testicular Cancer (2023). Clin. Transl. Oncol. 2024, 26, 2783–2799. [Google Scholar] [CrossRef]
- Brönimann, S.; Mun, D.-H.; Hackl, M.; Yang, L.; Shariat, S.F.; Waldhoer, T. Increase and Plateauing of Testicular Cancer Incidence in Austria-A Time Trend Analysis of the Past Four Decades. Eur. Urol. Open Sci. 2023, 49, 104–109. [Google Scholar] [CrossRef]
- Gurney, J.K.; Florio, A.A.; Znaor, A.; Ferlay, J.; Laversanne, M.; Sarfati, D.; Bray, F.; McGlynn, K.A. International Trends in the Incidence of Testicular Cancer: Lessons from 35 Years and 41 Countries. Eur. Urol. 2019, 76, 615–623. [Google Scholar] [CrossRef]
- Nicol, D.; Berney, D.M.; Boormanss, J.L.; Di Nardo, D.; Fankhauser, C.D.; Fischer, S.; Gremmels, H.; Cornes, R.; Heidenreich, A.; Leão, R.; et al. EAU Guidelines on Testicular Cancer—2024 Update. Presented at the EAU Annual Congress. European Association of Urology. Available online: https://uroweb.org/guidelines (accessed on 5 June 2025).
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA A Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, A.A.; Kelly, S.P.; Altekruse, S.F.; Rosenberg, P.S.; McGlynn, K.A. Future of Testicular Germ Cell Tumor Incidence in the United States: Forecast through 2026. Cancer 2017, 123, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Mhamane, S.; Bagal, S.; Shivshankar, S.; Kadam, P.; Prakash, G.; Budukh, A. Global Burden of Testicular Cancer and Its Risk Factors. Indian. J. Med. Paediatr. Oncol. 2024, 46, 142–149. [Google Scholar] [CrossRef]
- Znaor, A.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Laversanne, M.; Kuliš, T.; Gurney, J.; Sarfati, D.; McGlynn, K.A.; Bray, F. Testicular Cancer Incidence Predictions in Europe 2010–2035: A Rising Burden despite Population Ageing. Int. J. Cancer 2020, 147, 820–828. [Google Scholar] [CrossRef]
- Manku, G.; Culty, M. Mammalian Gonocyte and Spermatogonia Differentiation: Recent Advances and Remaining Challenges. Reproduction 2015, 149, R139-157. [Google Scholar] [CrossRef]
- Islam, R.; Heyer, J.; Figura, M.; Wang, X.; Nie, X.; Nathaniel, B.; Indumathy, S.; Hartmann, K.; Pleuger, C.; Fijak, M.; et al. T Cells in Testicular Germ Cell Tumors: New Evidence of Fundamental Contributions by Rare Subsets. Br. J. Cancer 2024, 130, 1893–1903. [Google Scholar] [CrossRef]
- Shen, H.; Shih, J.; Hollern, D.P.; Wang, L.; Bowlby, R.; Tickoo, S.K.; Thorsson, V.; Mungall, A.J.; Newton, Y.; Hegde, A.M.; et al. Integrated Molecular Characterization of Testicular Germ Cell Tumors. Cell Rep. 2018, 23, 3392–3406. [Google Scholar] [CrossRef]
- Satomi, K.; Takami, H.; Fukushima, S.; Yamashita, S.; Matsushita, Y.; Nakazato, Y.; Suzuki, T.; Tanaka, S.; Mukasa, A.; Saito, N.; et al. 12p Gain Is Predominantly Observed in Non-Germinomatous Germ Cell Tumors and Identifies an Unfavorable Subgroup of Central Nervous System Germ Cell Tumors. Neuro Oncol. 2022, 24, 834–846. [Google Scholar] [CrossRef]
- Ozgun, G.; Nichols, C.; Kollmannsberger, C.; Nappi, L. Genomic Features of Mediastinal Germ Cell Tumors: A Narrative Review. Mediastinum 2022, 6, 34. [Google Scholar] [CrossRef]
- Hemminki, K.; Vaittinen, P.; Dong, C.; Easton, D. Sibling Risks in Cancer: Clues to Recessive or X-Linked Genes? Br. J. Cancer 2001, 84, 388–391. [Google Scholar] [CrossRef]
- Tateo, V.; Thompson, Z.J.; Gilbert, S.M.; Cortessis, V.K.; Daneshmand, S.; Masterson, T.A.; Feldman, D.R.; Pierorazio, P.M.; Prakash, G.; Heidenreich, A.; et al. Epidemiology and Risk Factors for Testicular Cancer: A Systematic Review. Eur. Urol. 2025, 87, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; McGlynn, K.A.; Rajpert-De Meyts, E.; Bishop, D.T.; Chung, C.C.; Dalgaard, M.D.; Greene, M.H.; Gupta, R.; Grotmol, T.; Haugen, T.B.; et al. Meta-Analysis of Five Genome-Wide Association Studies Identifies Multiple New Loci Associated with Testicular Germ Cell Tumor. Nat. Genet. 2017, 49, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Cabral, E.R.M.; Pacanhella, M.F.; Lengert, A.V.H.; Dos Reis, M.B.; Leal, L.F.; de Lima, M.A.; da Silva, A.L.V.; Pinto, I.A.; Reis, R.M.; Pinto, M.T.; et al. Somatic Mutation Detection and KRAS Amplification in Testicular Germ Cell Tumors. Front. Oncol. 2023, 13, 1133363. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Summersgill, B.; Yost, S.; Sultana, R.; Labreche, K.; Dudakia, D.; Renwick, A.; Seal, S.; Al-Saadi, R.; Broderick, P.; et al. Whole-Exome Sequencing Reveals the Mutational Spectrum of Testicular Germ Cell Tumours. Nat. Commun. 2015, 6, 5973. [Google Scholar] [CrossRef]
- Nakhaei-Rad, S.; Soleimani, Z.; Vahedi, S.; Gorjinia, Z. Testicular Germ Cell Tumors: Genomic Alternations and RAS-Dependent Signaling. Crit. Rev. Oncol. Hematol. 2023, 183, 103928. [Google Scholar] [CrossRef]
- Coffey, J.; Linger, R.; Pugh, J.; Dudakia, D.; Sokal, M.; Easton, D.F.; Timothy Bishop, D.; Stratton, M.; Huddart, R.; Rapley, E.A. Somatic KIT Mutations Occur Predominantly in Seminoma Germ Cell Tumors and Are Not Predictive of Bilateral Disease: Report of 220 Tumors and Review of Literature. Genes Chromosomes Cancer 2008, 47, 34–42. [Google Scholar] [CrossRef]
- Kemmer, K.; Corless, C.L.; Fletcher, J.A.; McGreevey, L.; Haley, A.; Griffith, D.; Cummings, O.W.; Wait, C.; Town, A.; Heinrich, M.C. KIT Mutations Are Common in Testicular Seminomas. Am. J. Pathol. 2004, 164, 305–313. [Google Scholar] [CrossRef]
- Galvez-Carvajal, L.; Sanchez-Muñoz, A.; Ribelles, N.; Saez, M.; Baena, J.; Ruiz, S.; Ithurbisquy, C.; Alba, E. Targeted Treatment Approaches in Refractory Germ Cell Tumors. Crit. Rev. Oncol. Hematol. 2019, 143, 130–138. [Google Scholar] [CrossRef]
- Onorato, A.; Guida, E.; Colopi, A.; Dolci, S.; Grimaldi, P. RAS/Mitogen-Activated Protein Kinase Signaling Pathway in Testicular Germ Cell Tumors. Life 2024, 14, 327. [Google Scholar] [CrossRef]
- Ottaviano, M.; Giunta, E.F.; Rescigno, P.; Pereira Mestre, R.; Marandino, L.; Tortora, M.; Riccio, V.; Parola, S.; Casula, M.; Paliogiannis, P.; et al. The Enigmatic Role of TP53 in Germ Cell Tumours: Are We Missing Something? Int. J. Mol. Sci. 2021, 22, 7160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dou, Z.; Sammons, M.A.; Levine, A.J.; Berger, S.L. Lysine Methylation Represses P53 Activity in Teratocarcinoma Cancer Cells. Proc. Natl. Acad. Sci. USA 2016, 113, 9822–9827. [Google Scholar] [CrossRef] [PubMed]
- Widschwendter, M.; Jones, A.; Evans, I.; Reisel, D.; Dillner, J.; Sundström, K.; Steyerberg, E.W.; Vergouwe, Y.; Wegwarth, O.; Rebitschek, F.G.; et al. Epigenome-based cancer risk prediction: Rationale, opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.H.; Nielsen, J.E.; Daugaard, G.; Hansen, T.v.O.; Rajpert-De Meyts, E.; Almstrup, K. Differences in Global DNA Methylation of Testicular Seminoma Are Not Associated with Changes in Histone Modifications, Clinical Prognosis, BRAF Mutations or Gene Expression. Cancer Genet. 2016, 209, 506–514. [Google Scholar] [CrossRef]
- Jostes, S.; Nettersheim, D.; Schorle, H. Epigenetic Drugs and Their Molecular Targets in Testicular Germ Cell Tumours. Nat. Rev. Urol. 2019, 16, 245–259. [Google Scholar] [CrossRef]
- Nestler, T.; Dalvi, P.; Haidl, F.; Wittersheim, M.; von Brandenstein, M.; Paffenholz, P.; Wagener-Ryczek, S.; Pfister, D.; Koitzsch, U.; Hellmich, M.; et al. Transcriptome Analysis Reveals Upregulation of Immune Response Pathways at the Invasive Tumour Front of Metastatic Seminoma Germ Cell Tumours. Br. J. Cancer 2022, 126, 937–947. [Google Scholar] [CrossRef]
- Patel, H.D.; Singla, N.; Ghandour, R.A.; Freifeld, Y.; Cheaib, J.G.; Woldu, S.L.; Pierorazio, P.M.; Bagrodia, A. Site of Extranodal Metastasis Impacts Survival in Patients with Testicular Germ Cell Tumors. Cancer 2019, 125, 3947–3952. [Google Scholar] [CrossRef]
- McHugh, D.J.; Gleeson, J.P.; Feldman, D.R. Testicular Cancer in 2023: Current Status and Recent Progress. CA Cancer J. Clin. 2024, 74, 167–186. [Google Scholar] [CrossRef]
- Gupta, V.; Shah, D.; Gatiya, K.; Shetty, S. Evaluation of Testicular Nonseminomatous Germ Cell Tumor Using Contrast-Enhanced Ultrasound. Case Rep. Radiol. 2025, 2025, 6614645. [Google Scholar] [CrossRef] [PubMed]
- Conduit, C.; Koh, T.T.; Hofman, M.S.; Toner, G.C.; Goad, J.; Lawrentschuk, N.; Tai, K.-H.; Lewin, J.H.; Tran, B. Two Decades of FDG-PET/CT in Seminoma: Exploring Its Role in Diagnosis, Surveillance and Follow-Up. Cancer Imaging 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.-P.; Simonsen-Richter, H.; Kulejewski, M.; Anheuser, P.; Zecha, H.; Isbarn, H.; Pichlmeier, U. Serum Tumour Markers in Testicular Germ Cell Tumours: Frequencies of Elevated Levels and Extents of Marker Elevation Are Significantly Associated with Clinical Parameters and with Response to Treatment. Biomed. Res. Int. 2019, 2019, 5030349. [Google Scholar] [CrossRef] [PubMed]
- Hoei-Hansen, C.E.; Rajpert-De Meyts, E.; Daugaard, G.; Skakkebaek, N.E. Carcinoma in Situ Testis, the Progenitor of Testicular Germ Cell Tumours: A Clinical Review. Ann. Oncol. 2005, 16, 863–868. [Google Scholar] [CrossRef]
- Kilic, I.; Acosta, A.M.; Idrees, M.T. Evolution of Testicular Germ Cell Tumors in the Molecular Era With Histogenetic Implications. Adv. Anat. Pathol. 2024, 31, 206–214. [Google Scholar] [CrossRef]
- Ruf, C.G.; Schmidt, S.; Kliesch, S.; Oing, C.; Pfister, D.; Busch, J.; Heinzelbecker, J.; Winter, C.; Zengerling, F.; Albers, P.; et al. Testicular Germ Cell Tumours’ Clinical Stage I: Comparison of Surveillance with Adjuvant Treatment Strategies Regarding Recurrence Rates and Overall Survival-a Systematic Review. World J. Urol. 2022, 40, 2889–2900. [Google Scholar] [CrossRef]
- Gurney, J.K.; McGlynn, K.A.; Stanley, J.; Merriman, T.; Signal, V.; Shaw, C.; Edwards, R.; Richiardi, L.; Hutson, J.; Sarfati, D. Risk Factors for Cryptorchidism. Nat. Rev. Urol. 2017, 14, 534–548. [Google Scholar] [CrossRef]
- Shin, J.; Jeon, G.W. Comparison of Diagnostic and Treatment Guidelines for Undescended Testis. Clin. Exp. Pediatr. 2020, 63, 415–421. [Google Scholar] [CrossRef]
- Banks, K.; Tuazon, E.; Berhane, K.; Koh, C.J.; De Filippo, R.E.; Chang, A.; Kim, S.S.; Daneshmand, S.; Davis-Dao, C.; Lewinger, J.P.; et al. Cryptorchidism and Testicular Germ Cell Tumors: Comprehensive Meta-Analysis Reveals That Association between These Conditions Diminished over Time and Is Modified by Clinical Characteristics. Front. Endocrinol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Ergül, R.B.; Bayramoğlu, Z.; Keçeli, A.M.; Dönmez, M.İ. Risk for Testicular Germ Cell Tumors and Spermatogenesis Failure in Post-Pubertal Undescended Testes. Int. Urol. Nephrol. 2024, 56, 2269–2274. [Google Scholar] [CrossRef]
- Ferguson, L.; Agoulnik, A.I. Testicular Cancer and Cryptorchidism. Front. Endocrinol. 2013, 4, 32. [Google Scholar] [CrossRef]
- Rajfer, J.; Handelsman, D.J.; Swerdloff, R.S.; Hurwitz, R.; Kaplan, H.; Vandergast, T.; Ehrlich, R.M. Hormonal Therapy of Cryptorchidism. A Randomized, Double-Blind Study Comparing Human Chorionic Gonadotropin and Gonadotropin-Releasing Hormone. N. Engl. J. Med. 1986, 314, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.A.; Abdul-Karim, F.W.; Elder, J.S. Effect of Preoperative Human Chorionic Gonadotropin on Intra-Abdominal Rat Testes Undergoing Standard and Fowler-Stephens Orchiopexy. J. Urol. 1997, 157, 2315–2317. [Google Scholar] [CrossRef] [PubMed]
- Rodprasert, W.; Virtanen, H.E.; Mäkelä, J.-A.; Toppari, J. Hypogonadism and Cryptorchidism. Front. Endocrinol. 2020, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, N.; Caridha, D.; Patanè, S.; Bianchi, F.; Vestri, E.; Bruno, C.; Camoglio, F. Elastosonographic Evaluation of the Post-Operative Morpho-Volumetric Recovery of the Gonad in the Cryptorchid Patient. Am. J. Clin. Exp. Urol. 2019, 7, 182–187. [Google Scholar]
- Nicola, Z.; Virginia, M.; Francesco Saverio, C. Post-Operative Use of Human Chorionic Gonadotrophin (u-hCG) Inpatients Treated for Intrabdominal Unilateral Undescended Testes. Am. J. Clin. Exp. Urol. 2018, 6, 133–137. [Google Scholar]
- Errico, A.; Ambrosini, G.; Vinco, S.; Bottani, E.; Dalla Pozza, E.; Marroncelli, N.; Brandi, J.; Cecconi, D.; Decimo, I.; Migliorini, F.; et al. In Vitro Effect of hCG on Cryptorchid Patients’ Gubernacular Cells: A Predictive Model for Adjuvant Personalized Therapy. Cell Commun. Signal 2025, 23, 19. [Google Scholar] [CrossRef]
- Cobellis, G.; Noviello, C.; Nino, F.; Romano, M.; Mariscoli, F.; Martino, A.; Parmeggiani, P.; Papparella, A. Spermatogenesis and Cryptorchidism. Front. Endocrinol. 2014, 5, 63. [Google Scholar] [CrossRef]
- Gupta, V.; Giridhar, A.; Sharma, R.; Ahmed, S.M.; Raju, K.V.V.N.; Rao, T.S. Malignancy in an Undescended Intra-Abdominal Testis: A Single Institution Experience. Indian. J. Surg. Oncol. 2021, 12, 133–138. [Google Scholar] [CrossRef]
- Wong, D.G.; Singla, N.; Bagrodia, A. Massive Intra-Abdominal Germ Cell Tumors: A Case Series and Review of Literature. Rev. Urol. 2019, 21, 136–140. [Google Scholar]
- Ferragut Cardoso, A.P.; Gomide, L.M.M.; Souza, N.P.; de Jesus, C.M.N.; Arnold, L.L.; Cohen, S.M.; de Camargo, J.L.V.; Nascimento, E.; Pontes, M.G. Time Response of Rat Testicular Alterations Induced by Cryptorchidism and Orchiopexy. Int. J. Exp. Pathol. 2021, 102, 57–69. [Google Scholar] [CrossRef]
- O’Donnell, L.; Smith, L.B.; Rebourcet, D. Sertoli Cells as Key Drivers of Testis Function. Semin. Cell Dev. Biol. 2022, 121, 2–9. [Google Scholar] [CrossRef]
- Zivkovic, D.; Bica, D.T.G.; Hadziselimovic, F. Relationship between Adult Dark Spermatogonia and Secretory Capacity of Leydig Cells in Cryptorchidism. BJU Int. 2007, 100, 1147–1149; discussion 1149. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Tramontano, L.; Adel, M.; Fleming, S. Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. J. Pers. Med. 2024, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- E Virtanen, H.; Bjerknes, R.; Cortes, D.; Jørgensen, N.; Meyts, E.R.; Thorsson, A.V.; Thorup, J.; Main, K.M. Cryptorchidism: Classification, Prevalence and Long-Term Consequences. Acta Paediatr. 2007, 96, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.-P.; Belge, G. Testicular Germ Cell Tumours—Features and prospects of the novel tumour marker microRNA-371a-3p (M371 test): A narrative review. Aktuelle Urol. 2024, 55, 510–519. [Google Scholar] [CrossRef]
- Aykut, D.; Halil, B. Can Serum Tumor Marker Densities According to Tumor Volume and Testicle Size Be Used to Predict Progression in Patients with Testicular Cancer? Curr. Urol. 2024, 18, 218–224. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Jones, N.R.; Protheroe, A.; Joseph, J.; Roberts, N.W.; Van den Bruel, A.; Fanshawe, T.R. The Diagnostic Performance of Current Tumour Markers in Surveillance for Recurrent Testicular Cancer: A Diagnostic Test Accuracy Systematic Review. Cancer Epidemiol. 2019, 59, 15–21. [Google Scholar] [CrossRef]
- Raos, D.; Krasic, J.; Masic, S.; Abramovic, I.; Coric, M.; Kruslin, B.; Katusic Bojanac, A.; Bulic-Jakus, F.; Jezek, D.; Ulamec, M.; et al. In Search of TGCT Biomarkers: A Comprehensive In Silico and Histopathological Analysis. Dis. Markers 2020, 2020, 8841880. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Parraga-Alava, J. Meta-Analysis of Gene Expressions in Testicular Germ Cell Tumor Histologies. Int. J. Mol. Sci. 2020, 21, 4487. [Google Scholar] [CrossRef]
- Pluta, J.; Pyle, L.C.; Nead, K.T.; Wilf, R.; Li, M.; Mitra, N.; Weathers, B.; D’Andrea, K.; Almstrup, K.; Anson-Cartwright, L.; et al. Identification of 22 Susceptibility Loci Associated with Testicular Germ Cell Tumors. Nat. Commun. 2021, 12, 4487. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Li, J.; Yang, Z.; Fan, S.; Shao, Z.; Xia, Y.; Wang, Z.; Wang, X.; Sun, N.; et al. Half-Life of Serum Alpha-Fetoprotein in Prepubertal Testicular Yolk Sac Tumors: An Index Significantly Associated with Prognosis. World J. Urol. 2024, 42, 429. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T.-Y. Update on the Applications and Limitations of Alpha-Fetoprotein for Hepatocellular Carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef]
- Langner, J.L.; Millard, F.; Vavinskaya, V.; Zhang, H.; Yodkhunnatham, N.; Bagrodia, A. Evaluation and Management of Testicular Cancer After Late Relapse. Oncology 2024, 38, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chen, X.; Yang, J.; Ding, J. Lactate Dehydrogenase D Is a General Dehydrogenase for D-2-Hydroxyacids and Is Associated with D-Lactic Acidosis. Nat. Commun. 2023, 14, 6638. [Google Scholar] [CrossRef] [PubMed]
- Milose, J.C.; Filson, C.P.; Weizer, A.Z.; Hafez, K.S.; Montgomery, J.S. Role of Biochemical Markers in Testicular Cancer: Diagnosis, Staging, and Surveillance. Open Access J. Urol. 2011, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The Usefulness of Lactate Dehydrogenase Measurements in Current Oncological Practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Lei, Z.M.; Li, X.; Greenwold, N.; Nakajima, S.T.; Jauniaux, E.; Rao, C.V. Human Fetal Nongonadal Tissues Contain Human Chorionic Gonadotropin/Luteinizing Hormone Receptors. J. Clin. Endocrinol. Metab. 2004, 89, 952–956. [Google Scholar] [CrossRef]
- Cole, L.A. New Discoveries on the Biology and Detection of Human Chorionic Gonadotropin. Reprod. Biol. Endocrinol. 2009, 7, 8. [Google Scholar] [CrossRef]
- Cole, L.A.; Butler, S. Hyperglycosylated hCG, hCGβ and Hyperglycosylated hCGβ: Interchangeable Cancer Promoters. Mol. Cell. Endocrinol. 2012, 349, 232–238. [Google Scholar] [CrossRef]
- Cole, L.A. Biological Functions of hCG and hCG-Related Molecules. Reprod. Biol. Endocrinol. 2010, 8, 102. [Google Scholar] [CrossRef]
- Cole, L.A. hCG, Five Independent Molecules. Clin. Chim. Acta 2012, 413, 48–65. [Google Scholar] [CrossRef]
- Berndt, S.; Blacher, S.; Munaut, C.; Detilleux, J.; d’Hauterive, S.P.; Huhtaniemi, I.; Evain-Brion, D.; Noël, A.; Fournier, T.; Foidart, J.-M. Hyperglycosylated Human Chorionic Gonadotropin Stimulates Angiogenesis through TGF-β Receptor Activation. FASEB J. 2013, 27, 1309–1321. [Google Scholar] [CrossRef]
- Herghelegiu, C.G.; Veduta, A.; Stefan, M.F.; Magda, S.L.; Ionascu, I.; Radoi, V.E.; Oprescu, D.N.; Calin, A.M. Hyperglycosylated-hCG: Its Role in Trophoblast Invasion and Intrauterine Growth Restriction. Cells 2023, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Lempiäinen, A.; Sankila, A.; Hotakainen, K.; Haglund, C.; Blomqvist, C.; Stenman, U.-H. Expression of Human Chorionic Gonadotropin in Testicular Germ Cell Tumors. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Daugaard, G.; Nestler, T.; Tryakin, A.; Fedyanin, M.; Fankhauser, C.; Hermanns, T.; Aparicio, J.; Heinzelbecker, J.; Paffenholz, P.; et al. Human Chorionic Gonadotropin–Positive Seminoma Patients: A Registry Compiled by the Global Germ Cell Tumor Collaborative Group (G3). Eur. J. Cancer 2020, 132, 127–135. [Google Scholar] [CrossRef]
- Patrikidou, A.; Cazzaniga, W.; Berney, D.; Boormans, J.; de Angst, I.; Di Nardo, D.; Fankhauser, C.; Fischer, S.; Gravina, C.; Gremmels, H.; et al. European Association of Urology Guidelines on Testicular Cancer: 2023 Update. Eur. Urol. 2023, 84, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Rothermundt, C.; Stalder, O.; Terbuch, A.; Hermanns, T.; Zihler, D.; Müller, B.; Fankhauser, C.D.; Hirschi-Blickenstorfer, A.; Seifert, B.; et al. The Value of Tumour Markers in the Detection of Relapse—Lessons Learned from the Swiss Austrian German Testicular Cancer Cohort Study. Eur. Urol. Open Sci. 2023, 50, 57–60. [Google Scholar] [CrossRef]
- Knight, A.K.; Bingemann, T.; Cole, L.; Cunningham-Rundles, C. Frequent False Positive Beta Human Chorionic Gonadotropin Tests in Immunoglobulin A Deficiency. Clin. Exp. Immunol. 2005, 141, 333–337. [Google Scholar] [CrossRef]
- Arrieta, O.; Michel Ortega, R.M.; Angeles-Sánchez, J.; Villarreal-Garza, C.; Avilés-Salas, A.; Chanona-Vilchis, J.G.; Aréchaga-Ocampo, E.; Luévano-González, A.; Jiménez, M.A.; Aguilar, J.L. Serum Human Chorionic Gonadotropin Is Associated with Angiogenesis in Germ Cell Testicular Tumors. J. Exp. Clin. Cancer Res. 2009, 28, 120. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Yodkhunnatham, N.; Pandit, K.; Puri, D.; Yuen, K.L.; Bagrodia, A. MicroRNAs in Testicular Germ Cell Tumors: The Teratoma Challenge. Int. J. Mol. Sci. 2024, 25, 2156. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Haugen, Ø.P.; Haugen, T.B. Diverse Roles and Targets of miRNA in the Pathogenesis of Testicular Germ Cell Tumour. Cancers 2022, 14, 1190. [Google Scholar] [CrossRef] [PubMed]
- Chavarriaga, J.; Hamilton, R.J. miRNAs for Testicular Germ Cell Tumours: Contemporary Indications for Diagnosis, Surveillance and Follow-Up. Andrology 2023, 11, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lafin, J.T.; Kenigsberg, A.P.; Meng, X.; Abe, D.; Savelyeva, A.; Singla, N.; Woldu, S.L.; Lotan, Y.; Mauck, R.J.; Lewis, C.M.; et al. Serum Small RNA Sequencing and miR-375 Assay Do Not Identify the Presence of Pure Teratoma at Postchemotherapy Retroperitoneal Lymph Node Dissection. Eur. Urol. Open Sci. 2021, 26, 83–87. [Google Scholar] [CrossRef]
- Belge, G.; Grobelny, F.; Matthies, C.; Radtke, A.; Dieckmann, K.-P. Serum Level of microRNA-375-3p Is Νot a Reliable Biomarker of Teratoma. Vivo 2020, 34, 163–168. [Google Scholar] [CrossRef]
- Saliyeva, S.; Boranbayeva, R.; Bulegenova, M.; Beloussov, V. Application of microRNAs in the Diagnosis and Monitoring of Pediatric Germ Cell Tumors: Kazakh Experience. Pediatr. Hematol. Oncol. 2024, 41, 121–134. [Google Scholar] [CrossRef]
- Ujfaludi, Z.; Fazekas, F.; Biró, K.; Oláh-Németh, O.; Buzogany, I.; Sükösd, F.; Beöthe, T.; Pankotai, T. miR-21, miR-29a, and miR-106b: Serum and Tissue Biomarkers with Diagnostic Potential in Metastatic Testicular Cancer. Sci. Rep. 2024, 14, 20151. [Google Scholar] [CrossRef]
- SWOG Cancer Research Network. A Prospective Observational Cohort Study to Assess MiRNA 371 for Outcome Prediction in Patients with Newly Diagnosed Germ Cell Tumors; clinicaltrials.gov; SWOG Cancer Research Network: Portland, OR, USA, 2025.
- Children’s Oncology Group. A Phase 3 Study of Active Surveillance for Low Risk and a Randomized Trial of Carboplatin vs. Cisplatin for Standard Risk Pediatric and Adult Patients With Germ Cell Tumors; clinicaltrials.gov; Children’s Oncology Group: Monrovia, CA, USA, 2025.
- Ellinger, J.; Wittkamp, V.; Albers, P.; Perabo, F.G.E.; Mueller, S.C.; von Ruecker, A.; Bastian, P.J. Cell-Free Circulating DNA: Diagnostic Value in Patients With Testicular Germ Cell Cancer. J. Urol. 2009, 181, 363–371. [Google Scholar] [CrossRef]
- Kawakami, T.; Okamoto, K.; Ogawa, O.; Okada, Y. XISTunmethylated DNA Fragments in Male-Derived Plasma as a Tumour Marker for Testicular Cancer. Lancet 2004, 363, 40–42. [Google Scholar] [CrossRef]
- Ellinger, J.; Albers, P.; Perabo, F.G.; Müller, S.C.; von Ruecker, A.; Bastian, P.J. CpG Island Hypermethylation of Cell-Free Circulating Serum DNA in Patients With Testicular Cancer. J. Urol. 2009, 182, 324–329. [Google Scholar] [CrossRef]
- Lobo, J.; van Zogchel, L.M.J.; Nuru, M.G.; Gillis, A.J.M.; van der Schoot, C.E.; Tytgat, G.A.M.; Looijenga, L.H.J. Combining Hypermethylated RASSF1A Detection Using ddPCR with miR-371a-3p Testing: An Improved Panel of Liquid Biopsy Biomarkers for Testicular Germ Cell Tumor Patients. Cancers 2021, 13, 5228. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, J.; Albers, P.; Müller, S.C.; Von Ruecker, A.; Bastian, P.J. Circulating Mitochondrial DNA in the Serum of Patients with Testicular Germ Cell Cancer as a Novel Noninvasive Diagnostic Biomarker. BJU Int. 2009, 104, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Krasic, J.; Skara, L.; Bojanac, A.K.; Ulamec, M.; Jezek, D.; Kulis, T.; Sincic, N. The Utility of cfDNA in TGCT Patient Management: A Systematic Review. Ther. Adv. Med. Oncol. 2022, 14, 17588359221090365. [Google Scholar] [CrossRef] [PubMed]
- Nastały, P.; Honecker, F.; Pantel, K.; Riethdorf, S. Detection of Circulating Tumor Cells (CTCs) in Patients with Testicular Germ Cell Tumors. In Testicular Germ Cell Tumors: Methods and Protocols; Bagrodia, A., Amatruda, J.F., Eds.; Springer: New York, NY, USA, 2021; pp. 245–261. ISBN 978-1-0716-0860-9. [Google Scholar]
- Sykes, J.; Kaldany, A.; Jang, T.L. Current and Evolving Biomarkers in the Diagnosis and Management of Testicular Germ Cell Tumors. J. Clin. Med. 2024, 13, 7448. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, Z.; Zhou, Q.; Fan, J.; Zhou, D.; Xing, L.; Bo, H.; Tang, L.; Fan, L. Identification of mRNA Prognostic Markers for TGCT by Integration of Co-Expression and CeRNA Network. Front. Endocrinol. 2021, 12, 743155. [Google Scholar] [CrossRef]
- Murez, T.; Fléchon, A.; Branger, N.; Savoie, P.-H.; Rocher, L.; Camparo, P.; Neuville, P.; Ferretti, L.; Van Hove, A.; Roupret, M. French AFU Cancer Committee Guidelines—Update 2022-2024: Testicular Germ Cell Cancer. Prog. Urol. 2022, 32, 1066–1101. [Google Scholar] [CrossRef]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P.; Nicolai, N.; Oldenburg, J. Guidelines on Testicular Cancer: 2015 Update. Eur. Urol. 2015, 68, 1054–1068. [Google Scholar] [CrossRef]
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leão, R.; et al. Testicular Seminoma and Non-Seminoma: ESMO-EURACAN Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2022, 33, 362–375. [Google Scholar] [CrossRef]
- Krege, S.; Albers, P.; Heidenreich, A. The role of tumour markers in diagnosis and management of testicular germ cell tumours. Urol. A 2011, 50, 313–321. [Google Scholar] [CrossRef]
- Knöfler, M.; Saleh, L.; Bauer, S.; Galos, B.; Rotheneder, H.; Husslein, P.; Helmer, H. Transcriptional Regulation of the Human Chorionic Gonadotropin Beta Gene during Villous Trophoblast Differentiation. Endocrinology 2004, 145, 1685–1694. [Google Scholar] [CrossRef]
- Hallast, P.; Rull, K.; Laan, M. The Evolution and Genomic Landscape of CGB1 and CGB2 Genes. Mol. Cell Endocrinol. 2007, 260–262, 2–11. [Google Scholar] [CrossRef]
- Białas, P.; Śliwa, A.; Szczerba, A.; Jankowska, A. The Study of the Expression of CGB1 and CGB2 in Human Cancer Tissues. Genes 2020, 11, 1082. [Google Scholar] [CrossRef]
- Śliwa, A.; Kubiczak, M.; Szczerba, A.; Walkowiak, G.; Nowak-Markwitz, E.; Burczyńska, B.; Butler, S.; Iles, R.; Białas, P.; Jankowska, A. Regulation of Human Chorionic Gonadotropin Beta Subunit Expression in Ovarian Cancer. BMC Cancer 2019, 19, 746. [Google Scholar] [CrossRef]
- Hotakainen, K.; Lintula, S.; Jarvinen, R.; Paju, A.; Stenman, J.; Rintala, E.; Stenman, U.-H. Overexpression of Human Chorionic Gonadotropin Beta Genes 3, 5 and 8 in Tumor Tissue and Urinary Cells of Bladder Cancer Patients. Tumour Biol. 2007, 28, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Corona, D.; Contreras-Sanzón, E.; Puente-Rivera, J.; Arreola, R.; Camacho-Nuez, M.; Cruz Santiago, J.; Estrella-Parra, E.A.; Torres-Romero, J.C.; López-Camarillo, C.; Alvarez-Sánchez, M.E. Epigenetic Factors and ncRNAs in Testicular Cancer. Int. J. Mol. Sci. 2023, 24, 12194. [Google Scholar] [CrossRef] [PubMed]
- Głodek, A.; Kubiczak, M.J.; Walkowiak, G.P.; Nowak-Markwitz, E.; Jankowska, A. Methylation Status of Human Chorionic Gonadotropin Beta Subunit Promoter and TFAP2A Expression as Factors Regulating CGB Gene Expression in Placenta. Fertil. Steril. 2014, 102, 1175–1182.e8. [Google Scholar] [CrossRef] [PubMed]
- Biskup, K.; Blanchard, V.; Castillo-Binder, P.; Alexander, H.; Engeland, K.; Schug, S. N- and O-Glycosylation Patterns and Functional Testing of CGB7 versus CGB3/5/8 Variants of the Human Chorionic Gonadotropin (hCG) Beta Subunit. Glycoconj. J. 2020, 37, 599–610. [Google Scholar] [CrossRef]
- Liu, L.; Roberts, R.M. Silencing of the Gene for the β Subunit of Human Chorionic Gonadotropin by the Embryonic Transcription Factor Oct-3/4*. J. Biol. Chem. 1996, 271, 16683–16689. [Google Scholar] [CrossRef]
- Fournier, T.; Guibourdenche, J.; Handschuh, K.; Tsatsaris, V.; Rauwel, B.; Davrinche, C.; Evain-Brion, D. PPARγ and Human Trophoblast Differentiation. J. Reprod. Immunol. 2011, 90, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Handschuh, K.; Guibourdenche, J.; Cocquebert, M.; Tsatsaris, V.; Vidaud, M.; Evain-Brion, D.; Fournier, T. Expression and Regulation by PPARγ of hCG α- and β-Subunits: Comparison between Villous and Invasive Extravillous Trophoblastic Cells. Placenta 2009, 30, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Giovangrandi, Y.; Parfait, B.; Asheuer, M.; Olivi, M.; Lidereau, R.; Vidaud, M.; Bièche, I. Analysis of the Human CGB/LHB Gene Cluster in Breast Tumors by Real-Time Quantitative RT-PCR Assays. Cancer Lett. 2001, 168, 93–100. [Google Scholar] [CrossRef]
- Nwabuobi, C.; Arlier, S.; Schatz, F.; Guzeloglu-Kayisli, O.; Lockwood, C.J.; Kayisli, U.A. hCG: Biological Functions and Clinical Applications. Int. J. Mol. Sci. 2017, 18, 2037. [Google Scholar] [CrossRef]
- Stenman, U.-H.; Alfthan, H.; Hotakainen, K. Human Chorionic Gonadotropin in Cancer. Clin. Biochem. 2004, 37, 549–561. [Google Scholar] [CrossRef]
- Ramakrishnappa, N.; Rajamahendran, R.; Lin, Y.-M.; Leung, P.C.K. GnRH in Non-Hypothalamic Reproductive Tissues. Anim. Reprod. Sci. 2005, 88, 95–113. [Google Scholar] [CrossRef]
- Lin, Y.M.; Poon, S.L.; Choi, J.H.; Lin, J.S.N.; Leung, P.C.K.; Huang, B.M. Transcripts of Testicular Gonadotropin-Releasing Hormone, Steroidogenic Enzymes, and Intratesticular Testosterone Levels in Infertile Men. Fertil. Steril. 2008, 90, 1761–1768. [Google Scholar] [CrossRef]
- Ciereszko, R.; Opałka, M.; Kamińska, B.; Kamiński, T.; Dusza, L. Prolactin Involvement in the Regulation of the Hypothalamic-Pituitary-Ovarian Axis during the Early Luteal Phase of the Porcine Estrous Cycle. Anim. Reprod. Sci. 2002, 69, 99–115. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.; Cugati, G.; Singh, A.K. Hyperprolactinemia: An Often Missed Cause of Male Infertility. J. Hum. Reprod. Sci. 2011, 4, 102–103. [Google Scholar] [CrossRef]
- Würfel, W.; Beckmann, M.W.; Austin, R.; Herzog, U.; Albert, P.J. Effects of Prolactin on Secretion and Synthesis of Human Chorionic Gonadotropin in Human Term Placentas in Vitro: Short-Term Increase in Secretion, Followed by Medium-Term Suppression of Synthesis and Secretion. Gynecol. Obstet. Investig. 2010, 33, 129–133. [Google Scholar] [CrossRef]
- Wilson, E.A.; Jawad, M.J.; Dickson, L.R. Suppression of Human Chorionic Gonadotropin by Progestational Steroids. Am. J. Obstet. Gynecol. 1980, 138, 708–713. [Google Scholar] [CrossRef]
- Errico, A.; Vinco, S.; Ambrosini, G.; Dalla Pozza, E.; Marroncelli, N.; Zampieri, N.; Dando, I. Mitochondrial Dynamics as Potential Modulators of Hormonal Therapy Effectiveness in Males. Biology 2023, 12, 547. [Google Scholar] [CrossRef]
- Gromoll, J.; Wistuba, J.; Terwort, N.; Godmann, M.; Müller, T.; Simoni, M. A New Subclass of the Luteinizing Hormone/Chorionic Gonadotropin Receptor Lacking Exon 10 Messenger RNA in the New World Monkey (Platyrrhini) Lineage. Biol. Reprod. 2003, 69, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.T.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. Human Gene Mutation Database (HGMD): 2003 Update. Hum. Mutat. 2003, 21, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Kooij, C.D.; Mavinkurve-Groothuis, A.M.C.; Kremer Hovinga, I.C.L.; Looijenga, L.H.J.; Rinne, T.; Giltay, J.C.; de Kort, L.M.O.; Klijn, A.J.; de Krijger, R.R.; Verrijn Stuart, A.A. Familial Male-Limited Precocious Puberty (FMPP) and Testicular Germ Cell Tumors. J. Clin. Endocrinol. Metab. 2022, 107, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Flippo, C.; Kolli, V.; Andrew, M.; Berger, S.; Bhatti, T.; Boyce, A.M.; Casella, D.; Collins, M.T.; Délot, E.; Devaney, J.; et al. Precocious Puberty in a Boy With Bilateral Leydig Cell Tumors Due to a Somatic Gain-of-Function LHCGR Variant. J. Endocr. Soc. 2022, 6, bvac127. [Google Scholar] [CrossRef]
- Stavrou, S.S.; Zhu, Y.S.; Cai, L.Q.; Katz, M.D.; Herrera, C.; Defillo-Ricart, M.; Imperato-McGinley, J. A Novel Mutation of the Human Luteinizing Hormone Receptor in 46XY and 46XX Sisters. J. Clin. Endocrinol. Metab. 1998, 83, 2091–2098. [Google Scholar] [CrossRef]
- Latronico, A.C.; Chai, Y.; Arnhold, I.J.; Liu, X.; Mendonca, B.B.; Segaloff, D.L. A Homozygous Microdeletion in Helix 7 of the Luteinizing Hormone Receptor Associated with Familial Testicular and Ovarian Resistance Is Due to Both Decreased Cell Surface Expression and Impaired Effector Activation by the Cell Surface Receptor. Mol. Endocrinol. 1998, 12, 442–450. [Google Scholar] [CrossRef][Green Version]
- Yariz, K.O.; Walsh, T.; Uzak, A.; Spiliopoulos, M.; Duman, D.; Onalan, G.; King, M.-C.; Tekin, M. Inherited Mutation of the Luteinizing Hormone/Choriogonadotropin Receptor (LHCGR) in Empty Follicle Syndrome. Fertil. Steril. 2011, 96, e125–e130. [Google Scholar] [CrossRef]
- Thomas, S.M.; Veerabathiran, R. Evaluating the Impact of LHCGR Gene Polymorphism on Polycystic Ovary Syndrome: A Comprehensive Meta-Analysis and Power Assessment. J. Turk. Ger. Gynecol. Assoc. 2024, 25, 207–218. [Google Scholar] [CrossRef]
- Piersma, D.; Berns, E.M.J.J.; Verhoef-Post, M.; Uitterlinden, A.G.; Braakman, I.; Pols, H.A.P.; Themmen, A.P.N. A Common Polymorphism Renders the Luteinizing Hormone Receptor Protein More Active by Improving Signal Peptide Function and Predicts Adverse Outcome in Breast Cancer Patients. J. Clin. Endocrinol. Metab. 2006, 91, 1470–1476. [Google Scholar] [CrossRef]
- Kossack, N.; Troppmann, B.; Richter-Unruh, A.; Kleinau, G.; Gromoll, J. Aberrant Transcription of the LHCGR Gene Caused by a Mutation in Exon 6A Leads to Leydig Cell Hypoplasia Type II. Mol. Cell. Endocrinol. 2013, 366, 59–67. [Google Scholar] [CrossRef]
- Troppmann, B.; Kleinau, G.; Krause, G.; Gromoll, J. Structural and Functional Plasticity of the Luteinizing Hormone/Choriogonadotrophin Receptor. Hum. Reprod. Update 2013, 19, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Kossack, N.; Simoni, M.; Richter-Unruh, A.; Themmen, A.P.N.; Gromoll, J. Mutations in a Novel, Cryptic Exon of the Luteinizing Hormone/Chorionic Gonadotropin Receptor Gene Cause Male Pseudohermaphroditism. PLoS Med. 2008, 5, e88. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, M.; Nielsen, J.E.; Andreassen, C.H.; Juul, A.; Toft, B.G.; Rajpert-De Meyts, E.; Daugaard, G.; Blomberg Jensen, M. Luteinizing Hormone Receptor Is Expressed in Testicular Germ Cell Tumors: Possible Implications for Tumor Growth and Prognosis. Cancers 2020, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, Y.; Beck, S.D.W.; Foster, R.S.; Bihrle, R.; Einhorn, L.H. Serum Tumor Markers in Testicular Cancer. Urol. Oncol. 2013, 31, 17–23. [Google Scholar] [CrossRef]
- Lempiäinen, A.; Stenman, U.-H.; Blomqvist, C.; Hotakainen, K. Free β-Subunit of Human Chorionic Gonadotropin in Serum Is a Diagnostically Sensitive Marker of Seminomatous Testicular Cancer. Clin. Chem. 2008, 54, 1840–1843. [Google Scholar] [CrossRef]
- Kao, C.-S.; Ulbright, T.M.; Young, R.H.; Idrees, M.T. Testicular Embryonal Carcinoma: A Morphologic Study of 180 Cases Highlighting Unusual and Unemphasized Aspects. Am. J. Surg. Pathol. 2014, 38, 689–697. [Google Scholar] [CrossRef]
- Lendel, A.; Zynger, D.L. Embryonal Carcinoma. Available online: https://www.pathologyoutlines.com/topic/testisembryonal.html (accessed on 10 June 2025).
- Lei, N.; Lei, L.-L.; Wang, C.-H.; Mei, C.-R. Pure Testicular Choriocarcinoma, a Rare and Highly Malignant Subtype with Challenging Treatment: A Case Report and Review of the Literature. Mol. Clin. Oncol. 2024, 20, 1. [Google Scholar] [CrossRef]
- Al-Khayal, A.; Noureldin, Y.; Alghafees, M.; Shafqat, A.; Sabbah, B.N.; Elhossiny, A.H.; Bakir, M.; Omar, M.A.; Arabi, T.Z.; Abdul Rab, S.; et al. A Decade in Focus: Mixed Germ Cell Tumors with Choriocarcinoma Components. Ann. Med. Surg. 2023, 85, 5355–5358. [Google Scholar] [CrossRef]
- Chavarriaga, J.; Clark, R.; Atenafu, E.G.; Anson-Cartwright, L.; Warde, P.; Chung, P.; Bedard, P.L.; Jiang, D.M.; O’Malley, M.; Prendeville, S.; et al. Long-Term Relapse and Survival in Clinical Stage I Testicular Teratoma. Eur. Urol. Focus. 2024. [Google Scholar] [CrossRef]
- Aydin, A.M.; Zemp, L.; Cheriyan, S.K.; Sexton, W.J.; Johnstone, P.A.S. Contemporary Management of Early Stage Testicular Seminoma. Transl. Androl. Urol. 2020, 9, S36–S44. [Google Scholar] [CrossRef]

| Chromosomal | TGCT Type | Type of Alteration |
| 12p | SE and NSTs | Gain of chromosome 12p, i (12p) |
| 11q | SE | Less frequent copies |
| 2q, 8q, 8p, 10q, 15, 19q, 19p, 22 | NSTs | Less frequent copies |
| Somatic mutations/copy number gains | TGCT type | Type of alteration |
| KIT | SE > NSTs | Activating mutations |
| KRAS | SE and NSTs | Activating mutations, copy number gain |
| NRAS | SE and NSTs | Activating mutations |
| TP53 | SE and NSTs | Inactivating mutations |
| MDM2 | SE and NSTs | Copy number gain |
| DNA methylation status | TGCT type | |
| Hypomethylation | SE | |
| Hypermethylation | NSTs |
| TNM Classification | |||
|---|---|---|---|
| pT—Primary Tumor | |||
| pTX | Primary tumor cannot be assessed | ||
| pT0 | No evidence of primary tumor | ||
| pTis | Germ cell neoplasia in situ | ||
| pT1 | Tumor limited to testis (including rete testis) and epididymis without vascular/lymphatic invasion and without invasion of the epididymis | ||
| pT2 | Tumor limited to testis with vascular/lymphatic invasion, invading hilar soft tissue or the epididymis, or tumor extending through tunica albuginea with involvement of visceral tunica vaginalis | ||
| pT3 | Tumor invades spermatic cord with or without vascular/lymphatic invasion | ||
| pT4 | Tumor invades scrotum with or without vascular or lymphatic invasion | ||
| N—Regional Lymph Nodes—Clinical | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis with a lymph node mass 2 cm or less in greatest dimensions or multiple lymph nodes, with no more than 2 cm in greatest dimension | ||
| N2 | Metastasis with a lymph node mass of more than 2 cm but no more than 5 cm in greatest dimension; more than 5 nodes positive, with no more than 5 cm; or evidence of extragonadal extension of tumor | ||
| N3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | ||
| Pn—Regional Lymph Nodes—Pathological | |||
| pNx | Regional lymph nodes cannot be assessed | ||
| pNo | No regional lymph node metastasis | ||
| pN1 | Metastasis with a lymph node mass of 2 cm or less in greatest dimension and 5 or fewer positive nodes, with no more than 2 cm in greatest dimension | ||
| pN2 | Metastasis with a lymph node mass of more than 2 cm but no more than 5 cm in greatest dimension; or more than 5 nodes positive, with no more than 5 cm; or evidence of extranodal extension of tumour | ||
| pN3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | ||
| M—Distant metastasis | |||
| MX | Distant metastasis cannot be assessed | ||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| M1a | Non-regional lymph node(s) or lung metastasis | ||
| M1b | Distant metastasis other than non-regional lymph nodes and lung | ||
| S—Serum Tumor Markers (Pre-chemotherapy) | |||
| SX | Serum marker studies not available or not performed | ||
| S0 | Serum marker study levels within normal limits | ||
| S1 S2 S3 | LDH (U/L) <1.5 × ULN and 1.5–10 × ULN or >10 × ULN or | hCG (mIU/mL) <5000 and 5000–50,000 or >50,000 or | AFP (ng/mL) <1000 1000–10,000 >10,000 |
| Good Prognosis Group | |
| Non-seminomas 5-year PFS 90% 5-year OS 96% | All the following criteria:
|
| Seminoma 5-year PFS 90% 5-year OS 96% | All the following criteria:
|
| Intermediate prognosis group | |
| Non-seminomas 5-year PFS 78% 5-year OS 89% | Any of the following criteria:
|
| Seminoma 5-year PFS 79% 5-year OS 88% | All the following criteria:
|
| Poor prognosis group | |
| Non-seminomas 5-year PFS 78% 5-year OS 89% | Any of the following criteria:
|
| Seminoma | No patients classified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marroncelli, N.; Ambrosini, G.; Errico, A.; Vinco, S.; Dalla Pozza, E.; Cogo, G.; Cristanini, I.; Migliorini, F.; Zampieri, N.; Dando, I. Is Human Chorionic Gonadotropin a Reliable Marker for Testicular Germ Cell Tumor? New Perspectives for a More Accurate Diagnosis. Cancers 2025, 17, 2409. https://doi.org/10.3390/cancers17142409
Marroncelli N, Ambrosini G, Errico A, Vinco S, Dalla Pozza E, Cogo G, Cristanini I, Migliorini F, Zampieri N, Dando I. Is Human Chorionic Gonadotropin a Reliable Marker for Testicular Germ Cell Tumor? New Perspectives for a More Accurate Diagnosis. Cancers. 2025; 17(14):2409. https://doi.org/10.3390/cancers17142409
Chicago/Turabian StyleMarroncelli, Nunzio, Giulia Ambrosini, Andrea Errico, Sara Vinco, Elisa Dalla Pozza, Giulia Cogo, Ilaria Cristanini, Filippo Migliorini, Nicola Zampieri, and Ilaria Dando. 2025. "Is Human Chorionic Gonadotropin a Reliable Marker for Testicular Germ Cell Tumor? New Perspectives for a More Accurate Diagnosis" Cancers 17, no. 14: 2409. https://doi.org/10.3390/cancers17142409
APA StyleMarroncelli, N., Ambrosini, G., Errico, A., Vinco, S., Dalla Pozza, E., Cogo, G., Cristanini, I., Migliorini, F., Zampieri, N., & Dando, I. (2025). Is Human Chorionic Gonadotropin a Reliable Marker for Testicular Germ Cell Tumor? New Perspectives for a More Accurate Diagnosis. Cancers, 17(14), 2409. https://doi.org/10.3390/cancers17142409









