Plasma WFDC2 (HE4) as a Predictive Biomarker for Clinical Outcomes in Cancer Patients Receiving Anti-PD-1 Therapy: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information and Plasma Sample Collection
2.2. Quantification of Plasma WFDC2 by ELISA
2.3. Statistical Analysis
3. Results
3.1. Association Between Tumor WFDC2 mRNA Expression and Survival Outcomes in Patients Undergoing Anti-PD-1 Therapy
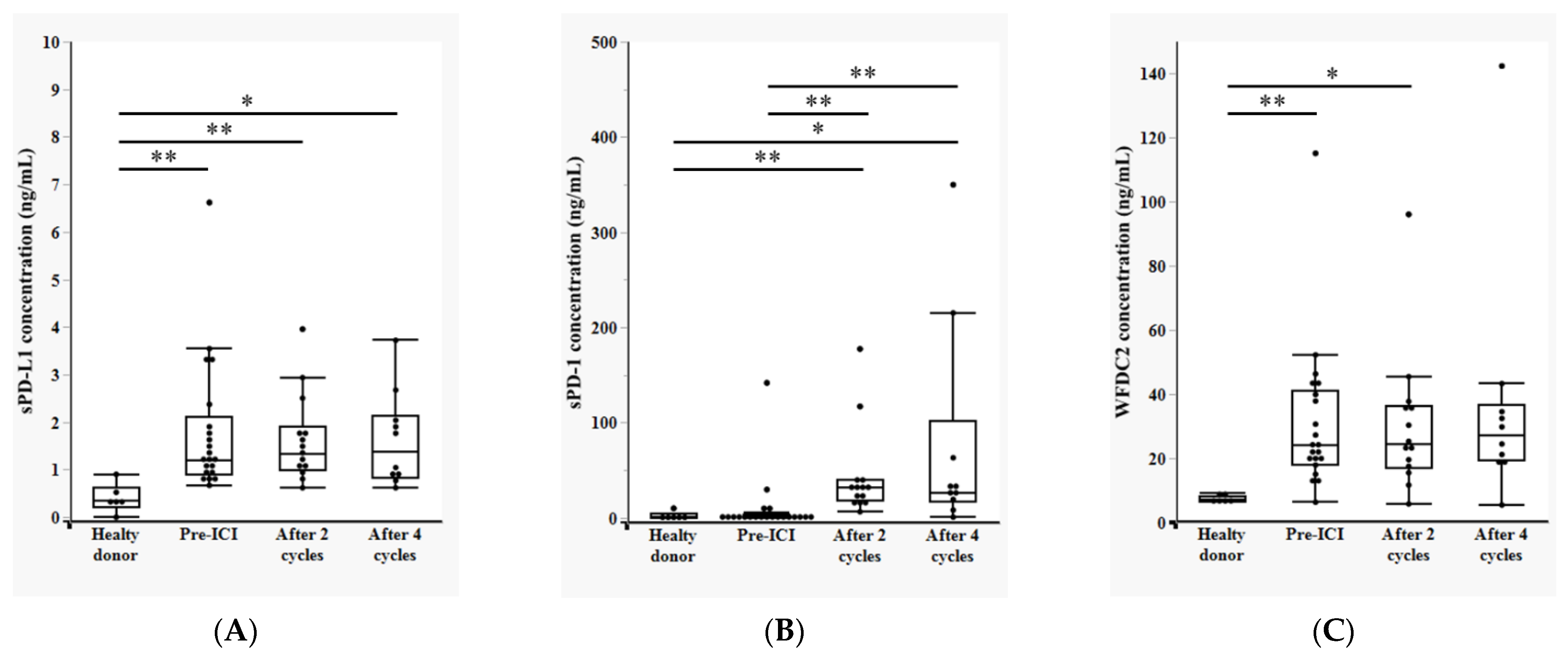
3.2. Temporal Changes in Plasma sPD-L1, sPD-1, and WFDC2 Levels During Anti-PD-1 Therapy
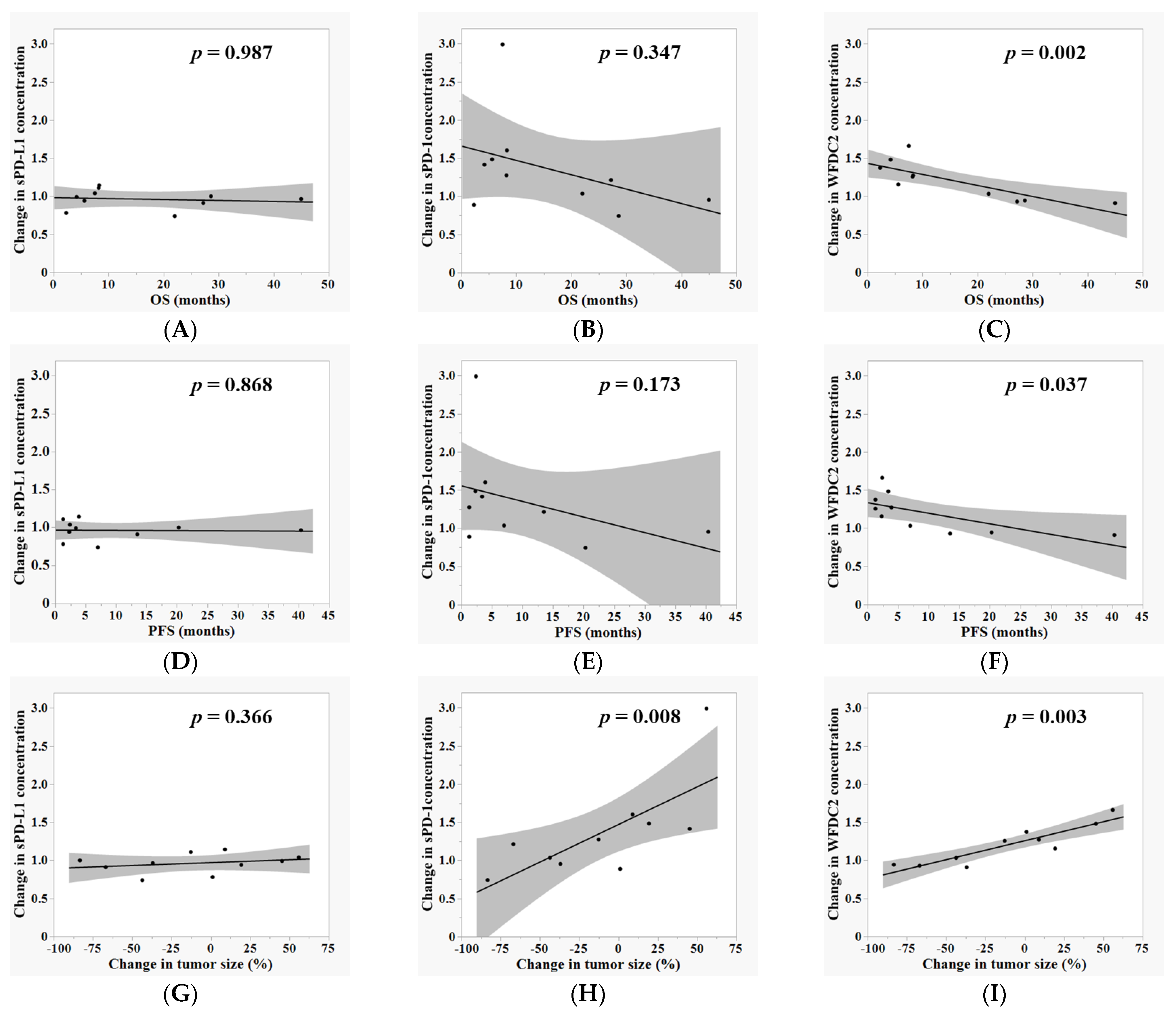
3.3. Correlation Between Plasma Biomarker Dynamics and Clinical Outcomes During ICI Therapy
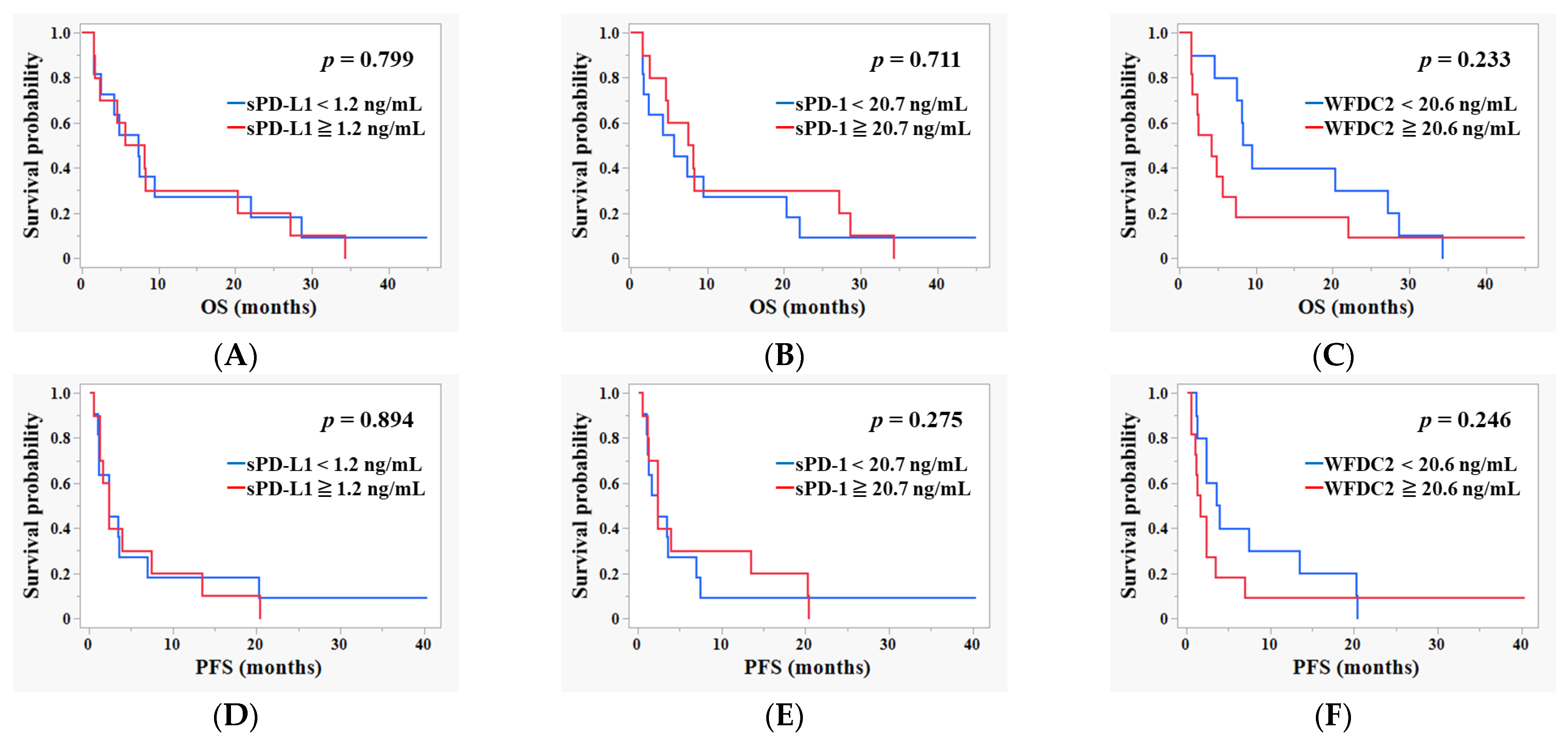
3.4. Survival Analysis Based on Baseline Pre-ICI Plasma Levels of sPD-L1, sPD-1, and WFDC2
3.5. Evaluation of the Diagnostic Performance of Baseline Plasma Biomarkers for Predicting BOR to ICI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICI | Immune checkpoint inhibitor |
| WFDC2 | Whey acidic protein four-disulfide core domain 2 |
| HE4 | Human Epididymis Protein 4 |
| OS | Overall survival |
| PFS | Progression-free survival |
| ROC | Receiver operating characteristic |
| PD-L1 | Programmed death-ligand 1 |
| PD-1 | Programmed cell death protein 1 |
| sPD-L1 | Soluble programmed death-ligand 1 |
| sPD-1 | Soluble programmed cell death protein 1 |
| NSCLC | Non-small cell lung cancer |
| ELISA | Enzyme-linked immunosorbent assay |
| BOR | Best overall response |
| CR | Complete response |
| PR | Partial response |
| SD | Stable disease |
| PD | Progressive disease |
| AUC | Area under the curve |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| HR | Hazard ratio |
| CI | Confidence interval |
| OR | Odds ratio |
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.-P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Rother, C.; John, T.; Wong, A. Biomarkers for immunotherapy resistance in non-small cell lung cancer. Front. Oncol. 2024, 14, 1489977. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Risch, E.; Halaban, R.; Zhen, P.; Bacchiocchi, A.; Risch, H.A. Dynamic changes of circulating soluble PD-1/PD-L1 and its association with patient survival in immune checkpoint blockade-treated melanoma. Int. Immunopharmacol. 2023, 118, 110092. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.; Han, G.; Schalper, K.A.; Carvajal-Hausdorf, D.; Pelekanou, V.; Rehman, J.; Velcheti, V.; Herbst, R.; Lorusso, P.; Rimm, D.L. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016, 2, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [PubMed]
- Duchemann, B.; Remon, J.; Naigeon, M.; Mezquita, L.; Ferrara, R.; Cassard, L.; Jouniaux, J.M.; Boselli, L.; Grivel, J.; Auclin, E.; et al. Integrating Circulating Biomarkers in the Immune Checkpoint Inhibitor Treatment in Lung Cancer. Cancers 2020, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a Soluble Form of B7-H1 That Retains Immunosuppressive Activity and Is Associated with Aggressive Renal Cell Carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dang, F.; Ren, J.; Wei, W. Biochemical Aspects of PD-L1 Regulation in Cancer Immunotherapy. Trends Biochem. Sci. 2018, 43, 1014–1032. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, R.; Ieguchi, K.; Watanabe, M.; Takayanagi, D.; Goshima, T.; Onoue, R.; Hamada, K.; Kubota, Y.; Horiike, A.; Ishiguro, T.; et al. Increased Plasma Soluble PD-1 Concentration Correlates with Disease Progression in Patients with Cancer Treated with Anti-PD-1 Antibodies. Biomedicines 2021, 9, 1929. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Hamada, K.; Watanabe, M.; Ohkuma, R.; Shida, M.; Onoue, R.; Kubota, Y.; Matsui, H.; Ishiguro, T.; Hirasawa, Y.; et al. Plasma Levels of Soluble PD-L1 Correlate with Tumor Regression in Patients with Lung and Gastric Cancer Treated With Immune Checkpoint Inhibitors. Anticancer. Res. 2019, 39, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Inoue, E.; Ohkuma, R.; Kobayashi, S.; Tsunoda, T.; Wada, S. Soluble PD-L1 changes in advanced non-small cell lung cancer patients treated with PD-1 inhibitors: An individual patient data meta-analysis. Front. Immunol. 2023, 14, 1308381. [Google Scholar] [CrossRef]
- Hamed, E.; Ahmed, H.; Sedeek, O.B.; Mohammed, A.M.; A Abd-Alla, A.; Ghaffar, H.M.A. Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn. Pathol. 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Kicman, A.; Gacuta, E.; Kulesza, M.; Będkowska, E.G.; Marecki, R.; Klank-Sokołowska, E.; Knapp, P.; Niczyporuk, M.; Ławicki, S. Diagnostic Utility of Selected Matrix Metalloproteinases (MMP-2, MMP-3, MMP-11, MMP-26), HE4, CA125 and ROMA Algorithm in Diagnosis of Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 6265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chu, L.; Tan, W.; Ye, C.; Dong, H. Human epididymis protein 4, a novel potential biomarker for diagnostic and prognosis monitoring of lung cancer. Clin. Respir. J. 2024, 18, e13774. [Google Scholar] [CrossRef] [PubMed]
- Lamy, P.-J.; Plassot, C.; Pujol, J.-L. Serum HE4: An Independent Prognostic Factor in Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0128836. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Wang, Y. WFDC2 is a potential prognostic and immunotherapy biomarker in lung adenocarcinoma. J. Int. Med Res. 2024, 52, 3000605241258893. [Google Scholar] [CrossRef] [PubMed]
- Kovács, S.A.; Fekete, J.T.; Győrffy, B. Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors. Acta Pharmacol. Sin. 2023, 44, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Hellström, I.; Raycraft, J.; Hayden-Ledbetter, M.; A Ledbetter, J.; Schummer, M.; McIntosh, M.; Drescher, C.; Urban, N.; Hellström, K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 2003, 63, 3695–3700. [Google Scholar] [PubMed]
- Zhang, Y.; Yang, W.; Han, X.; Qiao, Y.; Wang, H.; Chen, T.; Li, T.; Ou, W.-B. Knockdown of HE4 suppresses tumor growth and invasiveness in lung adenocarcinoma through regulation of EGFR signaling. Oncol. Res. 2024, 32, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- O’NEal, R.L.; Nam, K.T.; LaFleur, B.J.; Barlow, B.; Nozaki, K.; Lee, H.-J.; Kim, W.H.; Yang, H.-K.; Shi, C.; Maitra, A.; et al. Human epididymis protein 4 is up-regulated in gastric and pancreatic adenocarcinomas. Hum. Pathol. 2013, 44, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Hill, E.K.; Horan, T.; Yano, N.; Kim, K.; MacLaughlan, S.; Lambert-Messerlian, G.; Tseng, Y.D.; Padbury, J.F.; Miller, M.C.; et al. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci. Rep. 2014, 4, 3574. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Cantillo, E.; Oliver, M.T.; Rowswell-Turner, R.B.; Ribeiro, J.R.; Kim, K.; O Chichester, C.; A DiSilvestro, P.; Moore, R.G.; Singh, R.K.; et al. HE4 suppresses the expression of osteopontin in mononuclear cells and compromises their cytotoxicity against ovarian cancer cells. Clin. Exp. Immunol. 2018, 193, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhuang, H.; Wang, H.; Tan, M.; Schwab, C.L.; Deng, L.; Gao, J.; Hao, Y.; Li, X.; Gao, S.; et al. Overexpression of HE4 (human epididymis protein 4) enhances proliferation, invasion and metastasis of ovarian cancer. Oncotarget 2015, 7, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Rowswell-Turner, R.B.; Singh, R.K.; Urh, A.; Yano, N.; Kim, K.K.; Khazan, N.; Pandita, R.; Sivagnanalingam, U.; Hovanesian, V.; E James, N.; et al. HE4 Overexpression by Ovarian Cancer Promotes a Suppressive Tumor Immune Microenvironment and Enhanced Tumor and Macrophage PD-L1 Expression. J. Immunol. 2021, 206, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liang, J.; Lao, X.; Xia, X.; Li, S.; Liu, S. Single-Cell Sequencing Reveals PD-L1-Mediated Immune Escape Signaling in Lung Adenocarcinoma. J. Cancer 2025, 16, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Emerson, J.B.; Borgstadt, A.D.; Beffa, L.; Oliver, M.T.; Hovanesian, V.; Urh, A.; Singh, R.K.; Rowswell-Turner, R.; DiSilvestro, P.A.; et al. The biomarker HE4 (WFDC2) promotes a pro-angiogenic and immunosuppressive tumor microenvironment via regulation of STAT3 target genes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jin, C.; Tian, X.; Wang, Y.; Li, H. Knockdown of HE4 suppresses aggressive cell growth and malignant progression of ovarian cancer by inhibiting the JAK/STAT3 pathway. Biol. Open 2019, 8, bio043570. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Chen, J.; Wu, J.; Gu, Y.; Huang, Q.; Deng, Z.; Chen, S.; Wu, X.; Lv, Y.; Zeng, Z.; et al. Human epididymis protein 4 aggravates airway inflammation and remodeling in chronic obstructive pulmonary disease. Respir. Res. 2022, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Xu, Z.; Liu, Q.; Jin, G.; Wang, C. Tumor marker elevations in chronic kidney disease: A retrospective study. PeerJ 2025, 13, e19240. [Google Scholar] [CrossRef] [PubMed]
- Lycke, M.; Ulfenborg, B.; Lauesgaard, J.M.; Kristjansdottir, B.; Sundfeldt, K. Consideration should be given to smoking, endometriosis, renal function (eGFR) and age when interpreting CA125 and HE4 in ovarian tumor diagnostics. Clin. Chem. Lab. Med. 2021, 59, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, P.; Swisher, E.; Yip, Y.Y.; Tse, J.H.; Agnew, K.; Hellström, K.E.; Hellström, I. Silencing of the TGF-β1 Gene Increases the Immunogenicity of Cells from Human Ovarian Carcinoma. J. Immunother. 2012, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Ieguchi, K.; Shimizu, T.; Ohkuma, R.; Suzuki, R.; Mura, E.; Iriguchi, N.; Ishiguro, T.; Hirasawa, Y.; Ikeda, G.; et al. Plasma WFDC2 (HE4) as a Predictive Biomarker for Clinical Outcomes in Cancer Patients Receiving Anti-PD-1 Therapy: A Pilot Study. Cancers 2025, 17, 2384. https://doi.org/10.3390/cancers17142384
Watanabe M, Ieguchi K, Shimizu T, Ohkuma R, Suzuki R, Mura E, Iriguchi N, Ishiguro T, Hirasawa Y, Ikeda G, et al. Plasma WFDC2 (HE4) as a Predictive Biomarker for Clinical Outcomes in Cancer Patients Receiving Anti-PD-1 Therapy: A Pilot Study. Cancers. 2025; 17(14):2384. https://doi.org/10.3390/cancers17142384
Chicago/Turabian StyleWatanabe, Makoto, Katsuaki Ieguchi, Takashi Shimizu, Ryotaro Ohkuma, Risako Suzuki, Emiko Mura, Nana Iriguchi, Tomoyuki Ishiguro, Yuya Hirasawa, Go Ikeda, and et al. 2025. "Plasma WFDC2 (HE4) as a Predictive Biomarker for Clinical Outcomes in Cancer Patients Receiving Anti-PD-1 Therapy: A Pilot Study" Cancers 17, no. 14: 2384. https://doi.org/10.3390/cancers17142384
APA StyleWatanabe, M., Ieguchi, K., Shimizu, T., Ohkuma, R., Suzuki, R., Mura, E., Iriguchi, N., Ishiguro, T., Hirasawa, Y., Ikeda, G., Shimokawa, M., Ariizumi, H., Yoshimura, K., Horiike, A., Tsunoda, T., Tsuji, M., Kobayashi, S., Oguchi, T., Kiuchi, Y., & Wada, S. (2025). Plasma WFDC2 (HE4) as a Predictive Biomarker for Clinical Outcomes in Cancer Patients Receiving Anti-PD-1 Therapy: A Pilot Study. Cancers, 17(14), 2384. https://doi.org/10.3390/cancers17142384






