Trends in Cancer Incidence and Associated Risk Factors in People Living with and Without HIV in Botswana: A Population-Based Cancer Registry Data Analysis from 1990 to 2021
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design, Setting and Population
2.2. Data Source and Collection
2.2.1. Botswana National Cancer Registry
2.2.2. The National Data Warehouse
2.2.3. Antiretroviral Program in Botswana
2.3. Statistical Analysis
3. Results
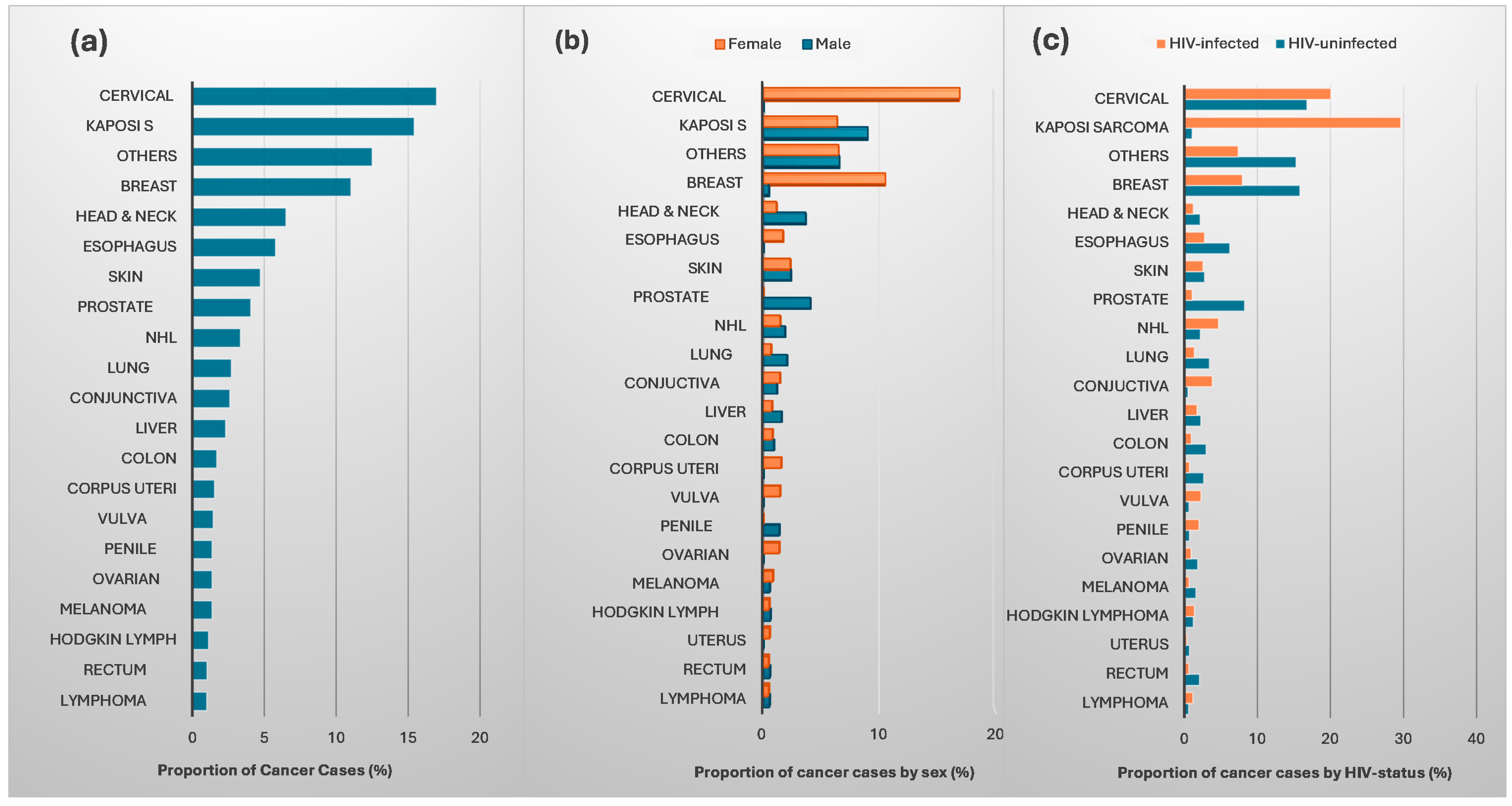
3.1. Overall
3.2. Cancer Incidence
3.2.1. Incidences of AIDS-Defining Cancers
3.2.2. Incidences of Non-AIDS-Defining Cancers
3.2.3. SIRs for Males and Females Living with HIV
3.3. Risk Factors Associated with AIDS and Non-AIDS Defining Cancers
4. Discussion
4.1. Strength
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Health Topics: HIV/AIDS. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 12 November 2024).
- Statistics Botswana. Fifth Botswana AIDS Impact Survey (BAIS V) Summary. 2022. Available online: https://www.statsbots.org.bw/sites/default/files/BAIS%20V%20Preliminary%20Report.pdf (accessed on 11 May 2024).
- Shiels, M.S.; Engels, E.A. Evolving epidemiology of HIV-associated malignancies. Curr. Opin. HIV AIDS 2017, 12, 6–11. [Google Scholar] [CrossRef]
- Dubrow, R.; Silverberg, M.J.; Park, L.S.; Crothers, K.; Justice, A.C. HIV infection, aging, and immune function: Implications for cancer risk and prevention. Curr. Opin. Oncol. 2012, 24, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Engels, E.A. Increased risk of histologically defined cancer subtypes in human immunodeficiency virus-infected individuals: Clues for possible immunosuppression-related or infectious etiology. Cancer 2012, 118, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, P.G.; Aboulafia, D.M.; Zloza, A. Malignancies in HIV/AIDS: From epidemiology to therapeutic challenges. AIDS 2014, 28, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Ministry of Health and Wellness. Annual Report to African Cancer Registry Network: Update on Botswana National Cancer Registry; Ministry of Health and Wellness: Singapore, 2017. [Google Scholar]
- Mitsuyasu, R.T. Non-AIDS-defining cancers. Top. Antivir. Med. 2014, 22, 660–665. [Google Scholar]
- Hernandez-Ramirez, R.U.; Shiels, M.S.; Dubrow, R.; Engels, E.A. Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. Lancet HIV 2017, 4, e495–e504. [Google Scholar] [CrossRef]
- Bohlius, J.; Valeri, F.; Maskew, M.; Prozesky, H.; Garone, D.; Sengayi, M.; Fox, M.P.; Davies, M.A.; Egger, M. Kaposi’s Sarcoma in HIV-infected patients in South Africa: Multicohort study in the antiretroviral therapy era. Int. J. Cancer 2014, 135, 2644–2652. [Google Scholar] [CrossRef]
- Dryden-Peterson, S.; Medhin, H.; Kebabonye-Pusoentsi, M.; Seage, G.R., 3rd; Suneja, G.; Kayembe, M.K.; Mmalane, M.; Rebbeck, T.; Rider, J.R.; Essex, M.; et al. Cancer Incidence following Expansion of HIV Treatment in Botswana. PLoS ONE 2015, 10, e0135602. [Google Scholar] [CrossRef]
- Elmore, S.N.; Bigger, E.S.; Kayembe, M.K.A.; Musimar, Z.; Suneja, G.; Efstathiou, J.A.; Dryden-Peterson, S. Demographic Characteristics and Preliminary Outcomes in a Cohort of HIV-Positive Patients With Kaposi’s Sarcoma in a High ART Coverage Setting: A Report from Botswana. J. Glob. Oncol. 2016, 2, 70s–71s. [Google Scholar] [CrossRef]
- Milligan, M.G.; Bigger, E.; Abramson, J.S.; Sohani, A.R.; Zola, M.; Kayembe, M.K.A.; Medhin, H.; Suneja, G.; Lockman, S.; Chabner, B.A.; et al. Impact of HIV Infection on the Clinical Presentation and Survival of Non-Hodgkin Lymphoma: A Prospective Observational Study From Botswana. J. Glob. Oncol. 2018, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dryden-Peterson, S.; Bvochora-Nsingo, M.; Suneja, G.; Efstathiou, J.A.; Grover, S.; Chiyapo, S.; Ramogola-Masire, D.; Kebabonye-Pusoentsi, M.; Clayman, R.; Mapes, A.C.; et al. HIV Infection and Survival Among Women With Cervical Cancer. J. Clin. Oncol. 2016, 34, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Rogena, E.A.; Simbiri, K.O.; De Falco, G.; Leoncini, L.; Ayers, L.; Nyagol, J. A review of the pattern of AIDS defining, HIV associated neoplasms and premalignant lesions diagnosed from 2000–2011 at Kenyatta National Hospital, Kenya. Infect. Agent. Cancer 2015, 10, 28. [Google Scholar] [CrossRef]
- Bender Ignacio, R.; Ghadrshenas, M.; Low, D.; Orem, J.; Casper, C.; Phipps, W. HIV Status and Associated Clinical Characteristics Among Adult Patients With Cancer at the Uganda Cancer Institute. J. Glob. Oncol. 2018, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.; Althoff, K.N.; Mugglin, C.; Wools-Kaloustian, K.; Koller, M.; Dabis, F.; Nash, D.; Gsponer, T.; Sungkanuparph, S.; McGowan, C.; et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J. Acquir. Immune Defic. Syndr. 2014, 65, e8–e16. [Google Scholar] [CrossRef]
- Goncalves, P.H.; Montezuma-Rusca, J.M.; Yarchoan, R.; Uldrick, T.S. Cancer prevention in HIV-infected populations. Semin. Oncol. 2016, 43, 173–188. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Gail, M.H.; Hall, H.I.; Li, J.; Chaturvedi, A.K.; Bhatia, K.; Uldrick, T.S.; Yarchoan, R.; Goedert, J.J.; et al. Cancer burden in the HIV-infected population in the United States. J. Natl. Cancer Inst. 2011, 103, 753–762. [Google Scholar] [CrossRef]
- Silverberg, M.J.; Leyden, W.; Warton, E.M.; Quesenberry, C.P., Jr.; Engels, E.A.; Asgari, M.M. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J. Natl. Cancer Inst. 2013, 105, 350–360. [Google Scholar] [CrossRef]
- Global Cancer Observatory-International Agency for Research on Cancer: Globocan 2022. 72-Botswana Cancer-Fact-Sheets. 2022. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/72-botswana-fact-sheet.pdf (accessed on 23 April 2024).
- Casper, C. The increasing burden of HIV-associated malignancies in resource-limited regions. Annu. Rev. Med. 2011, 62, 157–170. [Google Scholar] [CrossRef]
- Coghill, A.E.; Newcomb, P.A.; Madeleine, M.M.; Richardson, B.A.; Mutyaba, I.; Okuku, F.; Phipps, W.; Wabinga, H.; Orem, J.; Casper, C. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS 2013, 27, 2933–2942. [Google Scholar] [CrossRef]
- African Cancer Registry Network. Botswana National Cancer Registry. 2012. Available online: https://afcrn.org/membership/members/118-bncr (accessed on 11 August 2024).
- Botswana-Rutgers Partnership for Health. A Quantitative and Qualitative Assessment of Cancer Services and Needs at the Four Public Oncology Centers within the Botswana Health System. 2022. Available online: https://globalhealth.rutgers.edu/wp-content/uploads/Botswana-Cancer-Care-and-Prevention-Needs-Assessment-Report.pdf (accessed on 25 August 2024).
- I-TECH. National Data Warehouse and Dashboards in Botswana. 2019. Available online: https://www.go2itech.org/2019/08/national-data-warehouse-and-dashboards-in-botswana/ (accessed on 25 August 2024).
- World Health Organization. Botswana Launches Treat All Strategy. 2016. Available online: https://www.afro.who.int/countries/botswana/news/botswana-launches-treat-all-strategy (accessed on 5 February 2024).
- Kirkwood, B.R.; Sterne, J.A.C. Essential Medical Statistics; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lorenzoni, C.F.; Ferro, J.; Carrilho, C.; Colombet, M.; Parkin, D.M. Cancer in Mozambique: Results from two population-based cancer registries. Int. J. Cancer 2020, 147, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Horner, M.J.; Haas, C.B.; McGee-Avila, J.K.; Pfeiffer, R.M.; Engels, E.A.; Pawlish, K.; Monterosso, A.; Riedel, D.J.; Wu, X.C.; et al. Differences in Trends in Cancer Incidence Rates Among People with HIV during 2001-2019 By Race and Ethnicity and By Risk Group in the United States. Clin. Infect. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Abd Elhafeez, S.; D’Arrigo, G.; Leonardis, D.; Fusaro, M.; Tripepi, G.; Roumeliotis, S. Methods to Analyze Time-to-Event Data: The Cox Regression Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 1302811. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef]
- Pusoentsi, M.; Shatera, B.P.; Motlogi, S.; Monagen, T.; Tapela, N.; Masupe, T.; Medhin, H.G. Quality of Cancer Registry Data: Botswana Experience, Demonstrating Improvements Over Time. J. Glob. Oncol. 2016, 2, 43s. [Google Scholar] [CrossRef]
- Mutyaba, I.; Phipps, W.; Krantz, E.M.; Goldman, J.D.; Nambooze, S.; Orem, J.; Wabinga, H.R.; Casper, C. A Population-Level Evaluation of the Effect of Antiretroviral Therapy on Cancer Incidence in Kyadondo County, Uganda, 1999–2008. J. Acquir. Immune Defic. Syndr. 2015, 69, 481–486. [Google Scholar] [CrossRef][Green Version]
- Wong, I.K.J.; Grulich, A.E.; Poynten, I.M.; Polizzotto, M.N.; van Leeuwen, M.T.; Amin, J.; McGregor, S.; Law, M.; Templeton, D.J.; Vajdic, C.M.; et al. Time trends in cancer incidence in Australian people living with HIV between 1982 and 2012. HIV Med. 2022, 23, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ramogola-Masire, D. HPV Vaccine for Cervical Cancer Prevention in Botswana. Commonw. Health Partnersh. 2014, 81–84. Available online: https://www.commonwealthhealth.org/wp-content/uploads/2014/05/7-HPV-botswana-Masire.pdf (accessed on 18 May 2025).
- Mathoma, A.; Sartorius, B.; Mahomed, S. The Trends and Risk Factors of AIDS-Defining Cancers and Non-AIDS-Defining Cancers in Adults Living with and without HIV: A Narrative Review. J. Cancer Epidemiol. 2024, 2024, 7588928. [Google Scholar] [CrossRef]
- Sengayi-Muchengeti, M.; Singh, E.; Chen, W.C.; Bradshaw, D.; de Villiers, C.B.; Newton, R.; Waterboer, T.; Mathew, C.G.; Sitas, F. Thirteen cancers associated with HIV infection in a Black South African cancer patient population (1995–2016). Int. J. Cancer 2023, 152, 183–194. [Google Scholar] [CrossRef]
- Wabinga, H.R.; Nambooze, S.; Amulen, P.M.; Okello, C.; Mbus, L.; Parkin, D.M. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int. J. Cancer 2014, 135, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Msyamboza, K.P.; Dzamalala, C.; Mdokwe, C.; Kamiza, S.; Lemerani, M.; Dzowela, T.; Kathyola, D. Burden of cancer in Malawi; common types, incidence and trends: National population-based cancer registry. BMC Res. Notes 2012, 5, 149. [Google Scholar] [CrossRef] [PubMed]
- Gotti, D.; Raffetti, E.; Albini, L.; Sighinolfi, L.; Maggiolo, F.; Di Filippo, E.; Ladisa, N.; Angarano, G.; Lapadula, G.; Pan, A.; et al. Survival in HIV-infected patients after a cancer diagnosis in the cART Era: Results of an italian multicenter study. PLoS ONE 2014, 9, e94768. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.G.; Smith, D.; Salters, K.A.; Zhang, W.; Kanters, S.; Milan, D.; Montaner, J.S.G.; Coldman, A.; Hogg, R.S.; Wiseman, S.M. Overview of cancer incidence and mortality among people living with HIV/AIDS in British Columbia, Canada: Implications for HAART use and NADM development. BMC Cancer 2017, 17, 270. [Google Scholar] [CrossRef]
- Hleyhel, M.; Hleyhel, M.; Bouvier, A.M.; Belot, A.; Tattevin, P.; Pacanowski, J.; Genet, P.; De Castro, N.; Berger, J.L.; Dupont, C.; et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: Results from a French cohort. AIDS 2014, 28, 2109–2118. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. 2024. Available online: https://gco.iarc.who.int/today (accessed on 13 March 2025).
- Schneider, J.W.; Dittmer, D.P. Diagnosis and Treatment of Kaposi Sarcoma. Am. J. Clin. Dermatol. 2017, 18, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Ballon, G.; Akar, G.; Cesarman, E. Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling In Vivo. PLoS Pathog. 2015, 11, e1004581. [Google Scholar] [CrossRef]
- Calabresi, A.; Ferraresi, A.; Festa, A.; Scarcella, C.; Donato, F.; Vassallo, F.; Limina, R.; Castelli, F.; Quiros-Roldan, E.; Brescia, H.I.V.C.S.G. Incidence of AIDS-defining cancers and virus-related and non-virus-related non-AIDS-defining cancers among HIV-infected patients compared with the general population in a large health district of Northern Italy, 1999–2009. HIV Med. 2013, 14, 481–490. [Google Scholar] [CrossRef]
- Jaquet, A.; Odutola, M.; Ekouevi, D.K.; Tanon, A.; Oga, E.; Akakpo, J.; Charurat, M.; Zannou, M.D.; Eholie, S.P.; Sasco, A.J.; et al. Cancer and HIV infection in referral hospitals from four West African countries. Cancer Epidemiol. 2015, 39, 1060–1065. [Google Scholar] [CrossRef]
- Kauma, G.; Ddungu, H.; Ssewanyana, I.; Nyesiga, S.; Bogere, N.; Namulema-Diiro, T.; Byakika-Kibwika, P.; Namukwaya, E.; Kizza, H.M. Virologic Nonsuppression Among Patients With HIV Newly Diagnosed With Cancer at Uganda Cancer Institute: A Cross-Sectional Study. JCO Glob. Oncol. 2023, 9, e2200262. [Google Scholar] [CrossRef]
- Muturi, D.; Mwanzi, S.N.; Riunga, F.M.; Shah, J.; Shah, R. HIV Prevalence and Characteristics Among Patients with AIDS-Defining and Non-AIDS-Defining Cancers in a Tertiary Hospital in Kenya. JCO Glob. Oncol. 2023, 9, e2200360. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.T.; Chiao, E.Y. Malignancies in women with HIV infection. Curr. Opin. HIV AIDS 2017, 12, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Brickman, C.; Palefsky, J.M. Human papillomavirus in the HIV-infected host: Epidemiology and pathogenesis in the antiretroviral era. Curr. HIV/AIDS Rep. 2015, 12, 6–15. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Fisher, J.L.; Paskett, E.D. Marital status and stage at diagnosis of cutaneous melanoma: Results from the Surveillance Epidemiology and End Results (SEER) program, 1973–2006. Cancer 2011, 117, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Thuret, R.; Sun, M.; Budaus, L.; Abdollah, F.; Liberman, D.; Shariat, S.F.; Iborra, F.; Guiter, J.; Patard, J.J.; Perrotte, P.; et al. A population-based analysis of the effect of marital status on overall and cancer-specific mortality in patients with squamous cell carcinoma of the penis. Cancer Causes Control 2013, 24, 71–79. [Google Scholar] [CrossRef]
- Mihor, A.; Tomsic, S.; Zagar, T.; Lokar, K.; Zadnik, V. Socioeconomic inequalities in cancer incidence in Europe: A comprehensive review of population-based epidemiological studies. Radiol. Oncol. 2020, 54, 1–13. [Google Scholar] [CrossRef]
- Wang, C.C.; Silverberg, M.J.; Abrams, D.I. Non-AIDS-Defining Malignancies in the HIV-Infected Population. Curr. Infect. Dis. Rep. 2014, 16, 406. [Google Scholar] [CrossRef]
- Park, L.S.; Tate, J.P.; Sigel, K.; Brown, S.T.; Crothers, K.; Gibert, C.; Goetz, M.B.; Rimland, D.; Rodriguez-Barradas, M.C.; Bedimo, R.J.; et al. Association of Viral Suppression With Lower AIDS-Defining and Non-AIDS-Defining Cancer Incidence in HIV-Infected Veterans: A Prospective Cohort Study. Ann. Intern. Med. 2018, 169, 87–96. [Google Scholar] [CrossRef]
| All Patients N = 27,726 | Patients Without HIV N = 3505 | Patients with HIV N = 13,737 | p Value * | |
|---|---|---|---|---|
| n (%) | ||||
| Cancer diagnosis age, median (IQR) | 52 (39–66) | 61 (50–72) | 42 (34–52) | <0.001 † |
| Cancer diagnosis age (years) | ||||
| 18–29 | 2277 (8.2%) | 207 (5.9%) | 1578 (11.5%) | <0.001 |
| 30–39 | 5063 (18.3%) | 244 (7.0%) | 4092 (29.8%) | |
| 40–49 | 5408 (19.5%) | 419 (12.0%) | 3841 (28.0%) | |
| 50–59 | 5058 (18.2%) | 729 (20.8%) | 2394 (17.4%) | |
| ≥60 | 9920 (35.8%) | 1906 (54.4%) | 1832 (13.3%) | |
| Sex | ||||
| Female | 16,021 (57.8%) | 2075 (59.2%) | 8172 (59.5%) | 0.757 |
| Male | 11,705 (42.2%) | 1430 (40.8%) | 5565 (40.5%) | |
| Marital Status | ||||
| Married | 8341 (30.1%) | 1572 (44.8%) | 2828 (20.6%) | <0.001 |
| Single | 14,815 (53.4%) | 1523 (43.5%) | 9388 (68.3%) | |
| Previously married | 288 (1.0%) | 66 (1.9%) | 104 (0.8%) | |
| Other (separated and cohabitation) | 1367 (4.9%) | 283 (8.1%) | 352 (2.6%) | |
| Missing | 2915 (10.5%) | 61 (1.7%) | 1065 (7.8%) | |
| Employed | ||||
| Yes | 5186 (18.7%) | 677 (19.3%) | 2665 (19.4%) | <0.001 |
| No | 10,270 (37.0%) | 821 (23.4%) | 6313 (46.0%) | |
| Other | 5432 (19.6%) | 984 (28.1%) | 1013 (7.4%) | |
| Missing | 6838 (24.7%) | 1023 (29.2%) | 3746 (27.3%) | |
| Alcohol intake | ||||
| Current | 814 (2.9%) | 123 (3.5%) | 359 (2.6%) | <0.001 |
| Past | 1639 (5.9%) | 225 (6.4%) | 850 (6.2%) | |
| Never | 3583 (12.9%) | 754 (21.5%) | 1685 (12.3%) | |
| Missing | 21,690 (78.2%) | 2403 (68.6%) | 10,843 (78.9%) | |
| Smoking | ||||
| Current | 859 (3.1%) | 124 (3.5%) | 359 (2.6%) | 0.059 |
| Past | 1042 (3.8%) | 197 (5.6%) | 497 (3.6%) | |
| Never | 4169 (11.4%) | 789 (22.5%) | 2049 (14.9%) | |
| Missing | 21,656 (81.7%) | 2395 (68.3%) | 10,832 (78.9%) | |
| Cancer category | ||||
| ADCs | 9904 (35.7%) | 704 (20.1%) | 7465 (54.3%) | <0.001 |
| NADCs | 17,822 (64.3%) | 2801 (79.9%) | 6272 (45.7%) | |
| ART period | ||||
| <2002 | 2510 (9.1%) | 96 (2.7%) | 1057 (7.7%) | <0.001 |
| 2002–2007 | 7937 (28.6%) | 607 (17.3%) | 3531 (25.7%) | |
| 2008–2012 | 7124 (25.7%) | 943 (26.9%) | 3559 (25.9%) | |
| 2013–2015 | 3798 (13.7%) | 817 (23.3%) | 2042 (14.9%) | |
| 2016–2021 | 6357 (22.9%) | 1042 (29.7%) | 3548 (25.8%) | |
| Cancer staging | ||||
| Early staging (I and II) | 1710 (6.2%) | 372 (10.6%) | 964 (7.0%) | 0.004 |
| Late staging (III and IV) | 3076 (11.1%) | 708 (20.2%) | 1423 (10.4%) | |
| Missing | 22,940 (82.7%) | 2425 (69.2%) | 11,350 (82.6%) | |
| Outcome status | ||||
| Alive | 17,574 (63.4%) | 2266 (64.7%) | 9004 (65.5%) | 0.412 |
| Dead | 9609 (34.7%) | 1183 (33.8%) | 4548 (33.1%) | |
| Missing | 543 (2.0%) | 56 (1.6%) | 185 (1.3%) | |
| Initial CD4 count, median (IQR) | 89 (167–262) | |||
| Initial CD4 count(cells/mm3) | ||||
| <200 | 1426 (10.4%) | |||
| 200–349 | 587 (4.3%) | |||
| 350–500 | 161 (1.2%) | |||
| >500 | 150 (1.1%) | |||
| Missing | 11,413 (83.2%) | |||
| Recent CD4 count, median (IQR) | 459 (277–672) | |||
| Recent CD4 count (cells/mm3) | ||||
| <200 | 802 (5.8%) | |||
| 200–349 | 1111 (8.1%) | |||
| 350–500 | 1126 (8.2%) | |||
| >500 | 2437 (17.7%) | |||
| Missing | 8261 (60.1%) | |||
| Initial viral load (copies/mL) | ||||
| <400 (undetectable) | 2288 (16.6%) | |||
| ≥400 | 2932 (21.3%) | |||
| Missing | 8517 (62.0%) | |||
| Recent viral load (copies/mL) | ||||
| <400 (undetectable) | 4534 (33.0%) | |||
| ≥400 | 665 (4.8%) | |||
| Missing | 137 (66.5%) | |||
| Cancer | Living with HIV | Living Without HIV | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | 1990–2001 | 2002–2007 | 2008–2012 | 2013–2015 | 2016–2021 | p-Value † | Age Group | 1990–2001 | 2002–2007 | 2008–2012 | 2013–2015 | 2016–2021 | p-Value † | |
| ALL | 18–29 | 31.61 | 516.44 | 88.78 | 108.65 | 114.52 | 0.010 | 18–29 | 0.59 | 3.12 | 2.93 | 4.87 | 2.39 | 0.244 |
| 30–39 | 36.26 | 1304.52 | 199.21 | 358.78 | 380.48 | 0.309 | 30–39 | 1.59 | 4.82 | 5.31 | 7.63 | 6.20 | 0.220 | |
| 40–49 | 34.92 | 1947.31 | 288.00 | 714.04 | 1146.75 | <0.001 | 40–49 | 1.72 | 15.44 | 19.19 | 23.20 | 10.75 | 0.021 | |
| 50–59 | 43.38 | 3024.62 | 449.37 | 1316.85 | 2179.68 | <0.001 | 50–59 | 3.00 | 32.75 | 45.81 | 53.06 | 25.69 | <0.001 | |
| ≥60 | 44.19 | 3294.69 | 713.66 | 2760.66 | 4825.81 | <0.001 | ≥60 | 1.46 | 51.29 | 109.96 | 139.29 | 76.39 | <0.001 | |
| Overall | 34.74 | 1227.05 | 233.59 | 539.63 | 1047.59 | <0.001 | Overall | 1.38 | 15.27 | 23.06 | 31.10 | 27.22 | <0.001 | |
| ADCs | 18–29 | 21.31 | 383.81 | 61.72 | 75.39 | 75.61 | 0.006 | 18–29 | 0.08 | 0.20 | 0.18 | 0.70 | 0.10 | 0.718 |
| 30–39 | 23.35 | 974.33 | 128.32 | 204.92 | 228.40 | 0.058 | 30–39 | 1.06 | 1.18 | 1.77 | 1.33 | 0.95 | 0.962 | |
| 40–49 | 19.53 | 1241.70 | 164.31 | 378.02 | 585.24 | <0.001 | 40–49 | 0.86 | 4.60 | 4.63 | 7.37 | 2.19 | 0.263 | |
| 50–59 | 19.61 | 1488.11 | 193.40 | 445.41 | 820.52 | <0.001 | 50–59 | 2.35 | 7.97 | 10.78 | 8.58 | 4.12 | 0.364 | |
| ≥60 | 17.67 | 1086.03 | 213.94 | 704.17 | 1328.75 | <0.001 | ≥60 | 1.66 | 12.82 | 24.48 | 26.97 | 10.77 | 0.002 | |
| Overall | 21.49 | 799.83 | 128.18 | 250.52 | 475.67 | <0.001 | Overall | 0.67 | 3.70 | 5.18 | 6.09 | 4.00 | 0.051 | |
| CERVICAL | 18–29 | 7.37 | 32.33 | 7.08 | 12.20 | 15.57 | 0.904 | 18–29 | 0.04 | 0.07 | 0.00 | 0.20 | 0.00 | 0.943 |
| 30–39 | 5.12 | 148.33 | 38.18 | 86.82 | 121.78 | <0.001 | 30–39 | 0.33 | 1.06 | 1.33 | 0.83 | 0.73 | 0.954 | |
| 40–49 | 6.90 | 252.00 | 63.55 | 207.74 | 404.47 | <0.001 | 40–49 | 0.26 | 4.01 | 4.28 | 6.00 | 1.87 | 0.281 | |
| 50–59 | 5.94 | 423.45 | 93.15 | 316.30 | 566.55 | <0.001 | 50–59 | 1.17 | 6.64 | 9.21 | 8.58 | 3.57 | 0.368 | |
| ≥60 | 3.31 | 463.70 | 95.79 | 448.11 | 951.94 | <0.001 | ≥60 | 0.62 | 11.29 | 21.78 | 22.23 | 8.81 | 0.005 | |
| Overall | 6.34 | 140.20 | 42.93 | 127.90 | 301.46 | <0.001 | Overall | 0.34 | 15.27 | 4.48 | 4.99 | 3.27 | <0.001 | |
| KS | 18–29 | 13.15 | 327.44 | 47.55 | 53.22 | 48.92 | <0.001 | 18–29 | 0.04 | 0.00 | 0.06 | 0.10 | 0.00 | 0.971 |
| 30–39 | 17.64 | 761.99 | 78.52 | 94.48 | 88.11 | <0.001 | 30–39 | 0.26 | 0.12 | 0.33 | 0.50 | 0.07 | 0.996 | |
| 40–49 | 12.43 | 882.02 | 79.98 | 137.36 | 126.91 | <0.001 | 40–49 | 0.17 | 0.00 | 0.00 | 0.27 | 0.11 | 0.951 | |
| 50–59 | 13.07 | 955.78 | 76.08 | 87.14 | 159.08 | <0.001 | 50–59 | 0.00 | 0.22 | 0.22 | 0.00 | 0.27 | 0.750 | |
| ≥60 | 14.36 | 573.52 | 103.78 | 192.05 | 218.15 | <0.001 | ≥60 | 0.21 | 0.57 | 0.41 | 1.19 | 0.98 | 0.425 | |
| Overall | 14.56 | 603.61 | 71.61 | 97.25 | 126.96 | 0.004 | Overall | 0.10 | 0.13 | 0.17 | 0.34 | 0.31 | 0.105 | |
| NHL | 18–29 | 0.79 | 24.04 | 7.08 | 9.98 | 11.12 | 0.228 | 18–29 | 0.00 | 0.14 | 0.12 | 0.40 | 0.10 | 0.691 |
| 30–39 | 0.59 | 64.01 | 11.62 | 23.62 | 18.52 | 0.428 | 30–39 | 0.13 | 0.00 | 0.11 | 0.00 | 0.15 | 0.984 | |
| 40–49 | 0.20 | 107.67 | 20.77 | 32.92 | 53.87 | 0.007 | 40–49 | 0.17 | 0.59 | 0.34 | 1.09 | 0.22 | 0.742 | |
| 50–59 | 0.59 | 108.89 | 24.18 | 41.96 | 94.89 | <0.001 | 50–59 | 0.00 | 1.11 | 1.35 | 0.00 | 0.22 | 0.979 | |
| ≥60 | 0.00 | 48.81 | 14.37 | 64.02 | 158.66 | <0.001 | ≥60 | 0.21 | 0.96 | 2.28 | 3.56 | 0.98 | 0.254 | |
| Overall | 0.59 | 56.01 | 13.65 | 25.37 | 47.24 | <0.001 | Overall | 0.06 | 0.40 | 0.54 | 0.76 | 0.42 | 0.633 | |
| NADCs | 18–29 | 10.30 | 135.95 | 27.06 | 33.26 | 38.91 | 0.603 | 18–29 | 0.51 | 2.91 | 2.75 | 4.18 | 2.29 | 0.268 |
| 30–39 | 12.91 | 330.19 | 70.88 | 153.85 | 152.08 | <0.001 | 30–39 | 2.12 | 3.65 | 3.54 | 6.30 | 5.25 | 0.172 | |
| 40–49 | 15.39 | 705.61 | 123.70 | 336.02 | 561.51 | <0.001 | 40–49 | 2.57 | 10.84 | 14.57 | 15.83 | 8.56 | 0.043 | |
| 50–59 | 23.77 | 1536.51 | 255.97 | 871.45 | 1359.16 | <0.001 | 50–59 | 3.65 | 24.79 | 35.03 | 44.48 | 21.57 | <0.001 | |
| ≥60 | 26.51 | 2208.66 | 499.72 | 2056.49 | 3497.06 | <0.001 | ≥60 | 1.25 | 38.46 | 85.48 | 112.32 | 65.62 | <0.001 | |
| Overall | 13.24 | 428.62 | 105.41 | 289.11 | 571.92 | <0.001 | Overall | 1.34 | 11.58 | 17.88 | 25.01 | 23.22 | <0.001 | |
| BREAST | 18–29 | 2.85 | 15.75 | 2.78 | 3.33 | 2.22 | 0.184 | 18–29 | 0.20 | 0.20 | 0.24 | 0.20 | 0.25 | 0.943 |
| 30–39 | 3.65 | 37.59 | 11.29 | 23.62 | 32.55 | 0.001 | 30–39 | 0.93 | 1.53 | 1.44 | 1.82 | 1.60 | 0.642 | |
| 40–49 | 3.95 | 132.88 | 20.15 | 70.38 | 144.26 | <0.001 | 40–49 | 0.00 | 4.16 | 6.34 | 4.37 | 3.29 | 0.148 | |
| 50–59 | 4.75 | 217.77 | 35.55 | 154.92 | 265.13 | <0.001 | 50–59 | 0.78 | 5.09 | 7.63 | 13.34 | 5.22 | 0.021 | |
| ≥60 | 3.31 | 231.85 | 51.09 | 248.06 | 680.90 | <0.001 | ≥60 | 0.00 | 4.02 | 12.86 | 14.82 | 10.41 | <0.001 | |
| Overall | 3.42 | 58.80 | 14.83 | 47.83 | 122.83 | <0.001 | Overall | 0.31 | 2.21 | 3.67 | 4.61 | 4.70 | 0.781 | |
| HEAD and NECK | 18–29 | 0.48 | 8.29 | 1.52 | 0.00 | 4.45 | 0.809 | 18–29 | 0.00 | 0.41 | 0.48 | 0.40 | 0.30 | 0.674 |
| 30–39 | 0.89 | 20.32 | 3.49 | 4.47 | 8.42 | 0.665 | 30–39 | 0.13 | 0.12 | 0.33 | 1.00 | 0.36 | 0.516 | |
| 40–49 | 2.17 | 54.98 | 11.47 | 23.84 | 40.17 | 0.001 | 40–49 | 0.00 | 1.93 | 1.71 | 1.36 | 0.33 | 0.76 | |
| 50–59 | 1.19 | 187.53 | 37.68 | 103.28 | 161.87 | <0.001 | 50–59 | 0.00 | 4.43 | 4.04 | 7.31 | 2.20 | 0.141 | |
| ≥60 | 4.42 | 256.25 | 71.84 | 320.08 | 350.37 | <0.001 | ≥60 | 0.21 | 4.78 | 7.68 | 17.49 | 6.37 | 0.003 | |
| Overall | 1.05 | 36.88 | 10.63 | 26.43 | 51.38 | <0.001 | Overall | 0.04 | 1.64 | 1.86 | 3.69 | 2.14 | 0.035 | |
| SKIN * | 18–29 | 1.03 | 13.26 | 3.29 | 3.33 | 10.01 | 0.202 | 18–29 | 0.04 | 0.27 | 0.12 | 0.29 | 0.20 | 0.819 |
| 30–39 | 1.18 | 32.51 | 6.97 | 19.79 | 21.32 | 0.010 | 30–39 | 0.00 | 0.12 | 0.22 | 0.33 | 0.29 | 0.575 | |
| 40–49 | 1.18 | 64.15 | 11.16 | 40.87 | 62.09 | <0.001 | 40–49 | 0.34 | 0.30 | 0.69 | 1.09 | 0.33 | 0.725 | |
| 50–59 | 1.19 | 96.79 | 24.89 | 77.46 | 156.29 | <0.001 | 50–59 | 0.26 | 2.43 | 2.25 | 2.22 | 1.51 | 0.457 | |
| ≥60 | 2.21 | 195.24 | 46.30 | 240.06 | 442.92 | <0.001 | ≥60 | 0.00 | 2.87 | 7.88 | 8.89 | 3.80 | 0.028 | |
| Overall | 1.15 | 37.57 | 10.17 | 32.77 | 70.27 | <0.001 | Overall | 0.08 | 0.83 | 1.37 | 1.71 | 1.38 | 0.628 | |
| ESOPHAGUS | 18–29 | 0.24 | 0.83 | 0.25 | 0.00 | 0.00 | 0.525 | 18–29 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.964 |
| 30–39 | 0.49 | 4.06 | 1.16 | 1.92 | 0.56 | 0.834 | 30–39 | 0.13 | 0.00 | 0.00 | 0.17 | 0.00 | 0.854 | |
| 40–49 | 0.79 | 27.49 | 6.20 | 15.89 | 18.26 | 0.017 | 40–49 | 0.00 | 0.45 | 0.34 | 0.27 | 0.22 | 0.789 | |
| 50–59 | 1.19 | 139.13 | 27.73 | 112.97 | 97.68 | <0.001 | 50–59 | 0.00 | 1.11 | 3.82 | 3.50 | 0.96 | 0.226 | |
| ≥60 | 2.21 | 231.85 | 47.90 | 336.08 | 396.64 | <0.001 | ≥60 | 0.42 | 4.02 | 10.17 | 16.30 | 4.90 | 0.007 | |
| Overall | 0.53 | 20.53 | 6.37 | 24.84 | 34.25 | <0.001 | Overall | 0.06 | 0.73 | 1.69 | 2.59 | 1.28 | 0.273 | |
| PROSTATE | 18–29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.000 | 18–29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.000 |
| 30–39 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 1.000 | 30–39 | 0.26 | 0.00 | 0.00 | 0.00 | 0.00 | 1.000 | |
| 40–49 | 0.59 | 2.29 | 0.00 | 0.00 | 4.57 | 0.199 | 40–49 | 0.69 | 0.00 | 0.34 | 0.00 | 0.22 | 0.504 | |
| 50–59 | 3.57 | 36.30 | 2.13 | 6.46 | 44.65 | <0.001 | 50–59 | 1.04 | 0.66 | 1.80 | 2.54 | 1.10 | 0.593 | |
| ≥60 | 2.21 | 134.23 | 36,72 | 176.04 | 323.92 | <0.001 | ≥60 | 0.00 | 4.40 | 11.41 | 12.45 | 15.67 | <0.001 | |
| Overall | 0.39 | 6.26 | 1.71 | 6.34 | 20.67 | 0.108 | Overall | 0.21 | 0.65 | 1.59 | 1.90 | 3.61 | 0.137 | |
| CONJUCTIVA | 18–29 | 0.16 | 24.87 | 3.04 | 5.54 | 0.00 | 0.153 | 18–29 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.964 |
| 30–39 | 0.39 | 98.55 | 13.61 | 20.43 | 6.73 | 0.035 | 30–39 | 0.00 | 0.12 | 0.00 | 0.00 | 0.00 | 0.867 | |
| 40–49 | 0.00 | 107.67 | 16.43 | 23.84 | 21.00 | 0.531 | 40–49 | 0.00 | 0.30 | 0.34 | 0.00 | 0.00 | 0.916 | |
| 50–59 | 0.00 | 139.13 | 14.93 | 32.28 | 44.65 | 0.458 | 50–59 | 0.00 | 0.44 | 0.00 | 0.32 | 0.00 | 0.997 | |
| ≥60 | 0.00 | 85.42 | 25.54 | 32.01 | 59.50 | <0.001 | ≥60 | 0.00 | 0.38 | 1.24 | 0.00 | 0.12 | 0.883 | |
| Overall | 0.20 | 70.97 | 12.08 | 19.03 | 17.72 | 0.877 | Overall | 0.00 | 0.18 | 0.22 | 0.04 | 0.03 | 0.070 | |
| LUNG | 18–29 | 0.24 | 0.83 | 0.00 | 1.11 | 1.11 | 0.464 | 18–29 | 0.08 | 0.07 | 0.12 | 0.00 | 0.00 | 0.793 |
| 30–39 | 0.39 | 0.00 | 0.66 | 1.92 | 1.68 | 0.200 | 30–39 | 0.00 | 0.00 | 0.11 | 0.00 | 0.07 | 0.803 | |
| 40–49 | 0.39 | 16.04 | 1.55 | 6.81 | 10.04 | 0.134 | 40–49 | 0.17 | 0.30 | 0.69 | 0.55 | 0.11 | 0.874 | |
| 50–59 | 1.19 | 108.89 | 12.80 | 48.41 | 61.40 | <0.001 | 50–59 | 0.00 | 0.66 | 3.82 | 0.64 | 0.96 | 0.416 | |
| ≥60 | 2.21 | 146.43 | 39.91 | 56.01 | 132.21 | <0.001 | ≥60 | 0.00 | 2.49 | 4.77 | 5.04 | 2.69 | 0.101 | |
| Overall | 0.43 | 13.22 | 3.41 | 8.46 | 16.83 | 0.002 | Overall | 0.06 | 0.48 | 1.15 | 0.80 | 0.81 | 0.417 | |
| LIVER * | 18–29 | 0.32 | 1.66 | 0.25 | 0.00 | 0.00 | 0.400 | 18–29 | 0.00 | 0.07 | 0.06 | 0.20 | 0.00 | 0.844 |
| 30–39 | 0.30 | 9.14 | 1.99 | 5.11 | 2.24 | 0,705 | 30–39 | 0.13 | 0.00 | 0.00 | 0.00 | 0.07 | 0.792 | |
| 40–49 | 0.99 | 48.11 | 5.89 | 14.76 | 8.22 | 0.638 | 40–49 | 0.00 | 0.15 | 0.00 | 0.27 | 0.22 | 0.644 | |
| 50–59 | 0.00 | 90.74 | 15.64 | 48.41 | 13.95 | 0.522 | 50–59 | 0.00 | 1.55 | 0.67 | 1.27 | 0.41 | 0.708 | |
| ≥60 | 3.31 | 256.25 | 43.11 | 72.02 | 72.72 | 0.477 | ≥60 | 0.00 | 2.11 | 2.90 | 4.74 | 1.35 | 0.19 | |
| Overall | 0.49 | 23.66 | 5.32 | 11.89 | 8.56 | 0.304 | Overall | 0.02 | 0.50 | 0.44 | 0.88 | 0.44 | 0.571 | |
| Cancer Type | SIR (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ART | Post-ART | |||||||||
| 1990–2001 | 2002–2007 | 2008–2012 | 2013–2015 | 2016–2021 | ||||||
| All | 27.40 | (25.77–29.10) | 23.64 | (22.87–24.43) | 14.91 | (14.42–15.40) | 9.64 | (9.22–10.07) | 12.93 | (12.51–13.36) |
| ADCs | 30.36 | (28.07–32.77) | 66.29 | (63.61–69.93) | 34.39 | (32.88–35.95) | 20.76 | (19.46–22.12) | 36.84 | (35.06–38.68) |
| Cervical | 25.07 | (21.66–28.87) | 13.72 | (12.41–15.12) | 13.51 | (12.49–14.59) | 13.22 | (12.07–14.45) | 28.57 | (26.85–30.38) |
| Kaposi Sarcoma | 104.50 | (95.00–114.70) | 1449.82 | (1382.39–1519.69) | 388.01 | (365.32–411.74) | 97.54 | (87.83–108.04) | 144.29 | (130.97–158.60) |
| NHL | 7.49 | (4.44–11.84) | 39.23 | (33.40–45.78) | 37.35 | (32.45–42.79) | 18.13 | (14.69–22.14) | 31.88 | (27.13–37.22) |
| NADCs | 8.36 | (7.56–9.21) | 10.76 | (10.17–11.38) | 8.82 | (8.40–9.27) | 6.58 | (6.20–6.98) | 8.40 | (8.03–8.78) |
| Breast | 7.88 | (6.44–9.55) | 5.95 | (5.08–6.91) | 4.63 | (4.04–5.27) | 4.69 | (4.03–5.42) | 6.84 | (6.20–7.53) |
| Head and Neck | 20.88 | (14.28–29.48) | 6.87 | (5.62–8.31) | 8.14 | (6.93–9.49) | 4.40 | (3.58–5.36) | 9.57 | (8.20–11.11) |
| Skin * | 13.11 | (9.13–18.23) | 14.88 | (12.20–17.96) | 12.80 | (10.86–14.98) | 11.94 | (9.93–14.24) | 18.24 | (15.99–20.71) |
| Esophagus | 9.30 | (5.31–15.10) | 13.91 | (10.59–17.95) | 7.42 | (6.01–9.05) | 8.66 | (6.99–10.59) | 14.58 | (12.05–17.49) |
| Prostate | 1.51 | (0.78–2.65) | 6.38 | (3.78–10.08) | 2.41 | (1.58–3.53) | 3.41 | (2.19–5.08) | 3.88 | (3.03–4.91) |
| Conjunctiva | NA | NA | 97.15 | (84.27–111.43) | 86.74 | (74.66–100.22) | 243.79 | (190.74–307.02) | 540 | (412.05–695.11) |
| Lung | 7.01 | (3.73–11.99) | 12.06 | (8.54–16.56) | 4.44 | (3.32–5.82) | 8.16 | (5.58–11.52) | 15.49 | (11.73–20.07) |
| Liver * | 11.16 | (6.24–18.40) | 19.71 | (15.30–24.98) | 26.98 | (21.42–33.53) | 10.67 | (7.78–14.27) | 6.70 | (4.48–9.62) |
| SIR (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Type | Pre-ART | Post-ART | Cancer Type | Pre-ART | Post-ART | ||||||
| 1990–2001 | 2002–2007 | 2008–2012 | 2013–2015 | 2016–2021 | 1990–2001 | 2002–2007 | 2008–2012 | 2013–2015 | 2016–2021 | ||
| Males | Females | ||||||||||
| ALL | 87.5 (79.6–95.9) | 37.2 (35.4–39.0) | 22.7 (21.6–23.9) | 12.2 (11.4–13.1) | 14.7 (13.9–15.5) | ALL | 18.2 (17.3–19.0) | 14.1 (13.5–14.7) | 9.8 (9.3–10.3) | 12.0 (11.6–12.6) | 38.3 (35.3–41.5) |
| ADCs | 344.4 (305.1–387.4) | 475.0 (447.1–504.1) | 224.3 (208.5–241.0) | 75.6 (67.0–85.0) | 126.5 (113.5–140.5) | ADCs | 32.6 (30.8–34.5) | 24.2 (22.9–25.6) | 17.5 (16.2–18.8) | 28.1 (26.5–29.7) | 56.4 (50.9–62.4) |
| KS | 553.4 (489.1–623.7) | 2630.9 (2470.2–2799.3) | 1213.8 (1121.1–1312.1) | 303.6 (265.1–346.0) | 461.1 (406.0–521.6) | KS | 886.7 (823.8–953.0) | 441.4 (401.8–483.8) | 105.2 (88.7–123.8) | 60.2 (51.7–69.8) | 354.4 (303.9–411.0) |
| NHL | 31.0 (14.9–57.0) | 43.8 (34.9–54.3) | 38.8 (31.8–46.8) | 18.6 (14.0–24.3) | 42.8 (34.6–52.4) | NHL | 46.9 (37.1–58.6) | 64.4 (52.4–78.3) | 37.4 (26.9–50.8) | 50.5 (39.1–64.3) | 32.4 (14.0–63.9) |
| NADCs | 39.9 (34.2–46.3) | 13.8 (12.7–14.9) | 12.0 (11.1–12.9) | 8.3 (7.6–9.1) | 10.8 (10.0–11.5) | NADCs | 9.9 (9.2–10.7) | 8.9 (8.3–9.5) | 6.6 (6.0–7.1) | 7.3 (6.9–7.7) | 25.1 (21.9–28.5) |
| Breast | NA | 52.7 (25.3–97.0) | 6.7 (2.7–13.8) | 6.8 (2.2–15.8) | 17.3 (10.4–27.0) | Breast | 6.6 (5.6–7.7) | 5.9 (5.1–6.7) | 5.5 (4.7–6.4) | 7.0 (6.3–7.7) | 25.9 (21.0–31.7) |
| Head and Neck | 71.2 (45.2–106.9) | 7.5 (6.0–9.4) | 12.1 (10.1–14.5) | 5.2 (4.1–6.5) | 12.9 (10.8–15.3) | Head and Neck | 10.4 (6.8–15.3) | 8.4 (5.9–11.6) | 5.2 (3.2–8.1) | 4.7 (3.3–6.5) | NA |
| Skin * | 21.7 (8.7–44.7) | 15.8 (11.1–21.9) | 15.9 (12.2–20.5) | 12.1 (8.6–16.4) | 18.5 (14.4–23.3) | Skin * | 8.8 (6.1–12.3) | 6.5 (4.8–8.8) | 8.9 (6.5–12.0) | 14.6 (11.8–17.8) | NA |
| Esophagus | 24.8 (12.8–43.3) | 9.9 (7.3–13.2) | 8.4 (6.4–10.7) | 9.7 (7.5–12.3) | 14.4 (11.6–17.8) | Esophagus | 10.8 (5.6–18.9) | 5.1 (3.5–7.2) | 3.3 (2.2–4.8) | 5.6 (3.7–8.0) | NA |
| Prostate | 7.4 (3.8–13.0) | 3.65 (2.16–5.77) | 2.3 (1.5–3.4) | 2.6 (1.7–3.9) | 2.8 (2.2–3.5) | Cervical | 11.5 (10.4–12.7) | 13.8 (12.8–14.9) | 13.5 (12.3–14.8) | 25.0 (23.5–26.6) | 32.6 (28.1–37.5) |
| Conjunctiva | NA | 90.7 (72.5–112.0) | 95.3 (75.9–118.2) | NA | NA | Conjunctiva | 213.0 (176.3–255.0) | 97.6 (79.5–118.5) | 157.1 (113.7–211.5) | 91.9 (62.0–131.2) | NA |
| Lung | 49.6 (21.3–97.6) | 13.2 (8.9–18.8) | 7.8 (5.7–10.3) | 7.1 (4.4–10.9) | 13.9 (9.8–19.0) | Lung | 4.1 (1.8–8.1) | 1.8 (0.7–3.9) | 8.0 (4.0–14.4) | 3.6 (2.2–5.7) | 10.1 (3.3–23.7) |
| Liver * | NA | 2.6 (0.3–9.5) | 51.7 (23.6–98.1) | 19.9 (9.9–35.6) | 4.1 (0.9–12.0) | Liver * | 3.6 (1.2–8.4) | 9.7 (4.6–17.8) | 2.4 (0.7–6.2) | 6.1 (2.3–13.3) | NA |
| Factor | AIDS Defining Cancers | Non-AIDS Defining Cancers | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | Crude HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |
| Age (years) | ||||||||
| 18–29 | Reference | - | Reference | - | Reference | - | Reference | - |
| 30–39 | 1.58 (1.47–1.69) | <0.001 | - | - | 1.29 (1.19–1.39) | <0.001 | 1.53 (1.39–1.69) | <0.001 |
| 40–49 | 1.58 (1.47–1.69) | <0.001 | - | - | 2.05 (1.91–2.20) | <0.001 | 2.67 (2.43–2.94) | <0.001 |
| 50–59 | 0.99 (0.92–1.07) | 0.834 | - | - | 2.78 (2.59–2.98) | <0.001 | 3.86 (3.51–4.25) | <0.001 |
| ≥60 | 0.57 (0.53–0.61) | <0.001 | - | - | 3.22 (3.02–3.44) | <0.001 | 5.23 (4.73–5.78) | <0.001 |
| Sex | ||||||||
| Male | Reference | - | Reference | - | Reference | - | Reference | - |
| Female | 1.73 (1.66–1.81) | <0.001 | 1.35 (1.27–1.42) | <0.001 | 0.80 (0.78–0.82) | <0.001 | 0.89 (0.86–0.93) | <0.001 |
| Marital status | ||||||||
| Married | Reference | - | Reference | - | Reference | - | Reference | - |
| Single | 1.82 (1.73–1.92) | <0.001 | 1.11 (1.03–1.19) | 0.004 | 0.77 (0.74–0.79) | <0.001 | 1.16 (1.11–1.22) | <0.001 |
| Prev. married | 0.79 (0.61–1.01) | 0.065 | 0.77 (0.56–1.06) | 0.105 | 0.91 (0.79–1.04) | 0.154 | 0.92 (0.77–1.11) | 0.399 |
| Employed | ||||||||
| Yes | Reference | - | Reference | - | Reference | - | Reference | - |
| No | 1.12 (1.06–1.18) | <0.001 | 1.02 (0.96–1.09) | 0.501 | 0.98 (0.94–1.03) | 0.423 | - | - |
| Living with HIV | ||||||||
| No | Reference | - | Reference | - | Reference | - | Reference | - |
| Yes | 1.89 (1.74–2.04) | <0.001 | 1.73 (1.58–1.90) | <0.001 | 0.43 (0.41–0.45) | <0.001 | 0.71 (0.67–0.75) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathoma, A.; Tshisimogo, G.; Sartorius, B.; Mahomed, S. Trends in Cancer Incidence and Associated Risk Factors in People Living with and Without HIV in Botswana: A Population-Based Cancer Registry Data Analysis from 1990 to 2021. Cancers 2025, 17, 2374. https://doi.org/10.3390/cancers17142374
Mathoma A, Tshisimogo G, Sartorius B, Mahomed S. Trends in Cancer Incidence and Associated Risk Factors in People Living with and Without HIV in Botswana: A Population-Based Cancer Registry Data Analysis from 1990 to 2021. Cancers. 2025; 17(14):2374. https://doi.org/10.3390/cancers17142374
Chicago/Turabian StyleMathoma, Anikie, Gontse Tshisimogo, Benn Sartorius, and Saajida Mahomed. 2025. "Trends in Cancer Incidence and Associated Risk Factors in People Living with and Without HIV in Botswana: A Population-Based Cancer Registry Data Analysis from 1990 to 2021" Cancers 17, no. 14: 2374. https://doi.org/10.3390/cancers17142374
APA StyleMathoma, A., Tshisimogo, G., Sartorius, B., & Mahomed, S. (2025). Trends in Cancer Incidence and Associated Risk Factors in People Living with and Without HIV in Botswana: A Population-Based Cancer Registry Data Analysis from 1990 to 2021. Cancers, 17(14), 2374. https://doi.org/10.3390/cancers17142374







