Microsurgical Reconstruction with Free Tissue Transfer in Skin Cancer Patients: A Systematic Review
Simple Summary
Abstract
1. Introduction
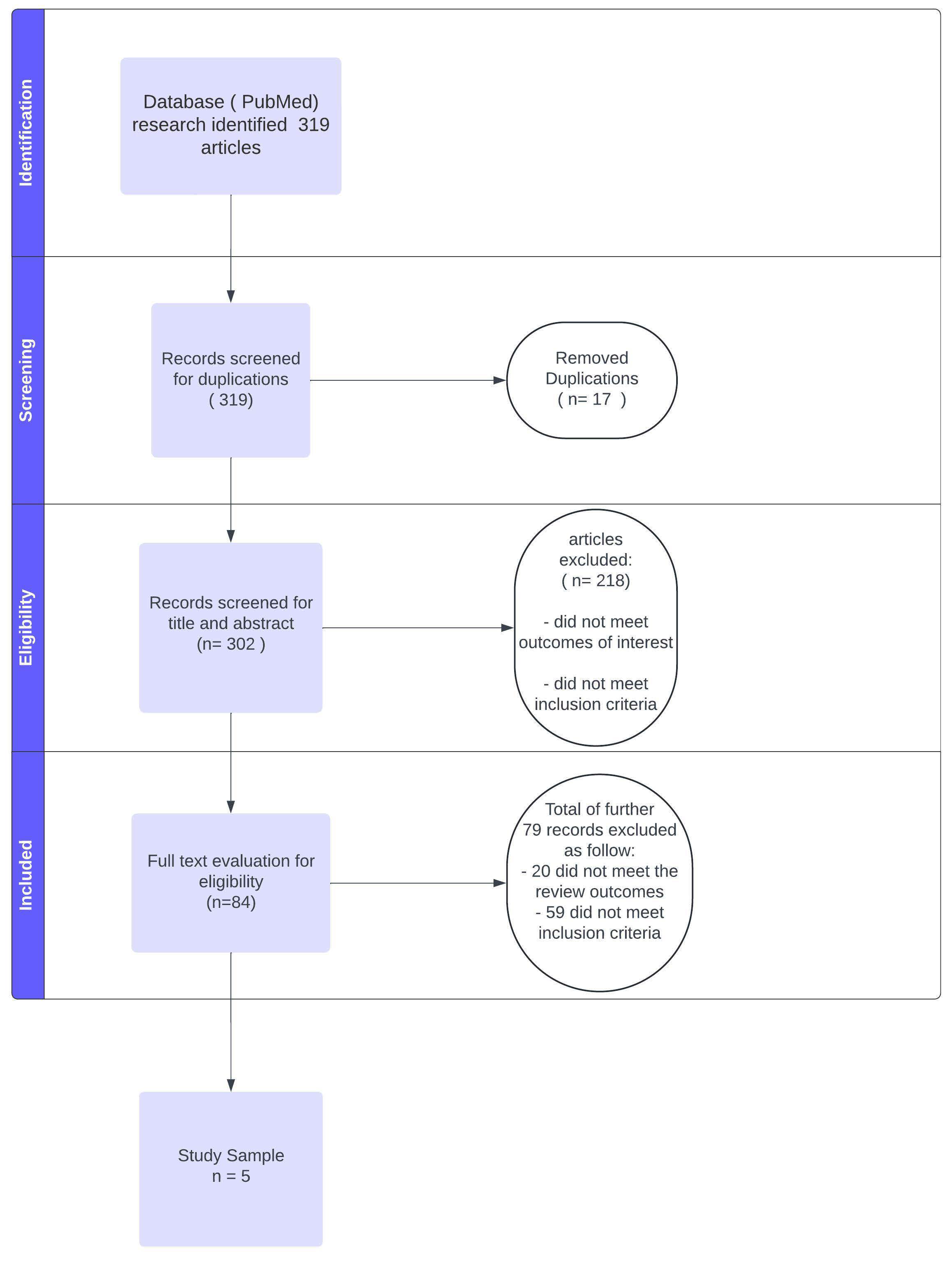
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction and Quality Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MSCs | Melanoma skin cancers |
| NMSCs | Non-melanoma skin cancers |
| BCC | Basocellular carcinoma |
| SCC | Squamocellular carcinoma |
References
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care Clin. Off. Pract. 2015, 42, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. Prevalence of a History of Skin Cancer in 2007. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, C.; Chen, D.; Zhang, G.; Wang, X. Non-Surgical Therapeutic Strategies for Non-Melanoma Skin Cancers. Curr. Treat. Options Oncol. 2023, 24, 1978–1993. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Fang, L.; Ni, R.; Zhang, H.; Pan, G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Leigh, I.M. Progress in skin cancer: The U.K. experience. Br. J. Dermatol. 2014, 171, 443–445. [Google Scholar] [CrossRef]
- Muzic, J.G.; Schmitt, A.R.; Wright, A.C.; Alniemi, D.T.; Zubair, A.S.; Lourido, J.M.O.; Seda, I.M.S.; Weaver, A.L.; Baum, C.L. Incidence and Trends of Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma. Mayo Clin. Proc. 2017, 92, 890–898. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; EGershenwald, J.; AScolyer, R. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bordeaux, J.; Chen, P.-L.; Contreras, C.M.; DiMaio, D.; et al. Basal Cell Skin Cancer, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1181–1203. [Google Scholar] [CrossRef]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Baumann, B.C.; Bordeaux, J.; Chen, P.-L.; Chin, R.; Contreras, C.M.; et al. NCCN Guidelines® Insights: Squamous Cell Skin Cancer, Version 1.2022. J. Natl. Compr. Cancer Netw. 2021, 19, 1382–1394. [Google Scholar] [CrossRef]
- Pathak, S.; Zito, P.M. Clinical Guidelines for the Staging, Diagnosis, and Management of Cutaneous Malignant Melanoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK572149/ (accessed on 22 July 2024).
- Han, D.; Zager, J.S.; Shyr, Y.; Chen, H.; Berry, L.D.; Iyengar, S.; Djulbegovic, M.; Weber, J.L.; Marzban, S.S.; Sondak, V.K.; et al. Clinicopathologic Predictors of Sentinel Lymph Node Metastasis in Thin Melanoma. J. Clin. Oncol. 2013, 31, 4387–4393. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Elashoff, R.; Essner, R.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; et al. Sentinel-Node Biopsy or Nodal Observation in Melanoma. N. Engl. J. Med. 2006, 355, 1307–1317. [Google Scholar] [CrossRef]
- Hallock, G.G. The Reconstructive Toolbox. Arch. Plast. Surg. 2023, 50, 331–334. [Google Scholar] [CrossRef]
- Simunovic, F.; Eisenhardt, S.U.; Penna, V.; Thiele, J.R.; Stark, G.B.; Bannasch, H. Microsurgical reconstruction of oncological scalp defects in the elderly. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Zeller, J.; Kiefer, J.; Braig, D.; Winninger, O.; Dovi-Akue, D.; Herget, G.W.; Stark, G.B.; Eisenhardt, S.U. Efficacy and Safety of Microsurgery in Interdisciplinary Treatment of Sarcoma Affecting the Bone. Front. Oncol. 2019, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Olivan, M.V.; Busnardo, F.F.; Faria, J.C.; Coltro, P.S.; Grillo, V.A.; Gemperli, R. Chimerical anterolateral thigh flap for plantar reconstruction. Microsurgery 2015, 35, 546–552. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.R.; Kwon, H.J.; Oh, D.Y.; Jun, Y.-J.; Rhie, J.W.; Moon, S.-H. Two-team-approached free flap reconstruction for plantar malignant melanoma: An observational (STROBE-compliant) trial. Medicine 2022, 101, e29442. [Google Scholar] [CrossRef]
- Suh, Y.C.; Suh, H.P.; Lee, J.S.; Chang, J.S.; Hong, J.P. Reconstruction using a perforator free flap after malignant melanoma resection of the ankle foot. J. Surg. Oncol. 2017, 116, 862–869. [Google Scholar] [CrossRef]
- Burch, M.B.; Chung, T.K.; Rosenthal, E.L.; Schmalbach, C.E. Multimodality Management of High-Risk Head and Neck Basal Cell Carcinoma Requiring Free-Flap Reconstruction. Otolaryngol. Neck Surg. 2015, 152, 868–873. [Google Scholar] [CrossRef]
- Pompucci, A.; Rea, G.; Farallo, E.; Salgarello, M.; Campanella, A.; Fernandez, E. Combined treatment of advanced stages of recurrent skin cancer of the head. J. Neurosurg. 2004, 100, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L., Jr.; Sober, A.J.; Kopf, A.W.; Lew, R.A.; Mihm, M.C., Jr.; Golomb, F.M.; Hennessey, P.; Harris, M.N.; Gumport, S.L.; Raker, J.W.; et al. A prognostic model for clinical stage I melanoma of the lower extremity. Location on foot as independent risk factor for recurrent disease. Surgery 1981, 89, 599–603. [Google Scholar] [PubMed]
- Walsh, S.M.; Fisher, S.G.; Sage, R.A. Survival of patients with primary pedal melanoma. J. Foot Ankle Surg. 2003, 42, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Zech, S.; Geerling, J.; Frink, M.; Knobloch, K.; Krettek, C. A new foot and ankle outcome score: Questionnaire based, subjective, Visual-Analogue-Scale, validated and computerized. Foot Ankle Surg. 2006, 12, 191–199. [Google Scholar] [CrossRef]
- Zhao, W.; Ukawa, S.; Tsushita, K.; Kawamura, T.; Wakai, K.; Ando, M.; Tamakoshi, A. Association of gait speed with mortality among the Japanese elderly in the New Integrated Suburban Seniority Investigation Project: A prospective cohort study. Age Ageing 2014, 44, 153–157. [Google Scholar] [CrossRef]
| First Author, Year | Population | Histology | Location | N+ | M+ | Margins Status | Adjuvant Radio-Therapy | Adj. Chemo or Immuno Therapy | Neo-Adjuvant Therapy | Mean Follow Up (mo.) | Survival | Local Recurrence | Metastasis c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim JH [20], 2022 | 21 | Melanoma | heel | 10 | 0 | R0 | 0 | 5 | 0 | 18 | 100% (overall surv.) | / | 23.8% |
| Suh YC [21], 2017 | 59 | Melanoma | foot and ankle | 4 | 0 | R0 | 8 | 7 | 0 | 31.5 | 73.2% (5-year overall surv.) 44% (progression-free surv.) 51.4 months (median progression-free surv. with 95% CI: 17.2–85.6 months) | 22.2% | 36% |
| M.B. Burch [22], 2015 | 38 | BCC | head and neck | 0 | 0 | / | 17 | 0 | 4 (10.5%) b | 19.9 | 80% (2-year overall surv.) 66% (5-year overall surv.) 69% (overall disease-specific surv.) 72% (2-year disease-free surv.) | 13% | 2.6% |
| Olivan [19], 2015 | 22 | Melanoma | plantar region | / | / | / | / | 0 | / | 24 | / | / | / |
| Pompucci [23], 2004 | 18 | SCC (8) BCC (10) | head | 4 | / | R0 (44.4%) R1 (33.3%) R2 (22.2%) | 10 a | / | 1 (5.5%) b | 25 | median survival time 32 months (the lower 95% confidence limit 23 months) | 11% | / |
| First Author, Year | Population | Type of Flap (no.) (%) | Mean Hospital Stay (Days) | Minor Complications a | Major Complications a | Flap Loss | Salvage Procedure (Success %) |
|---|---|---|---|---|---|---|---|
| Kim JH [20], 2022 | 21 | ALT (21) (100%) | 15.3 (±6.4) | lymphedema (1) marginal disruption (1) | venous congestion (3) hematoma (1) | 0 | 4 (100%) |
| Suh YC [21], 2017 | 59 | ALT (22) (36.6%) SCIP (30) (50%) PIAP (1) (1.6%) UMT (4) (6.6%) SGAP (3) (5%) | 17.1 | hematoma (3) seroma (1) wound infection (1) wound dehiscence (1) | arterial insufficiency (4) venous congestion (2) hematoma (3) | 2 (complete) 2 (partial) | 9 (77.7%) b |
| M.B. Burch [22], 2015 | 38 | RFFF (25) (65.7%) TRAM (6) (15.7%) Latissimus dorsi (4) (10.5%) ALT (3) (7.8%) | 5.2 (range 3–11) | exposed bone (4) hematoma/seroma (4) surgical site infection (3) osteoradionecrosis (2) donor site evisceration (2) | 0 | 0 | 0 |
| Olivan [19], 2015 | 22 | ALT (22) (100%) | / | 0 | flap necrosis (2) | 2 (partial) | 0 c |
| Pompucci [23], 2004 | 18 | Latissimus dorsi (15) (83.3%) TRAM (3) (16.7%) | / | / | radionecrosis (1) | 1 (partial) | 1 (100%) |
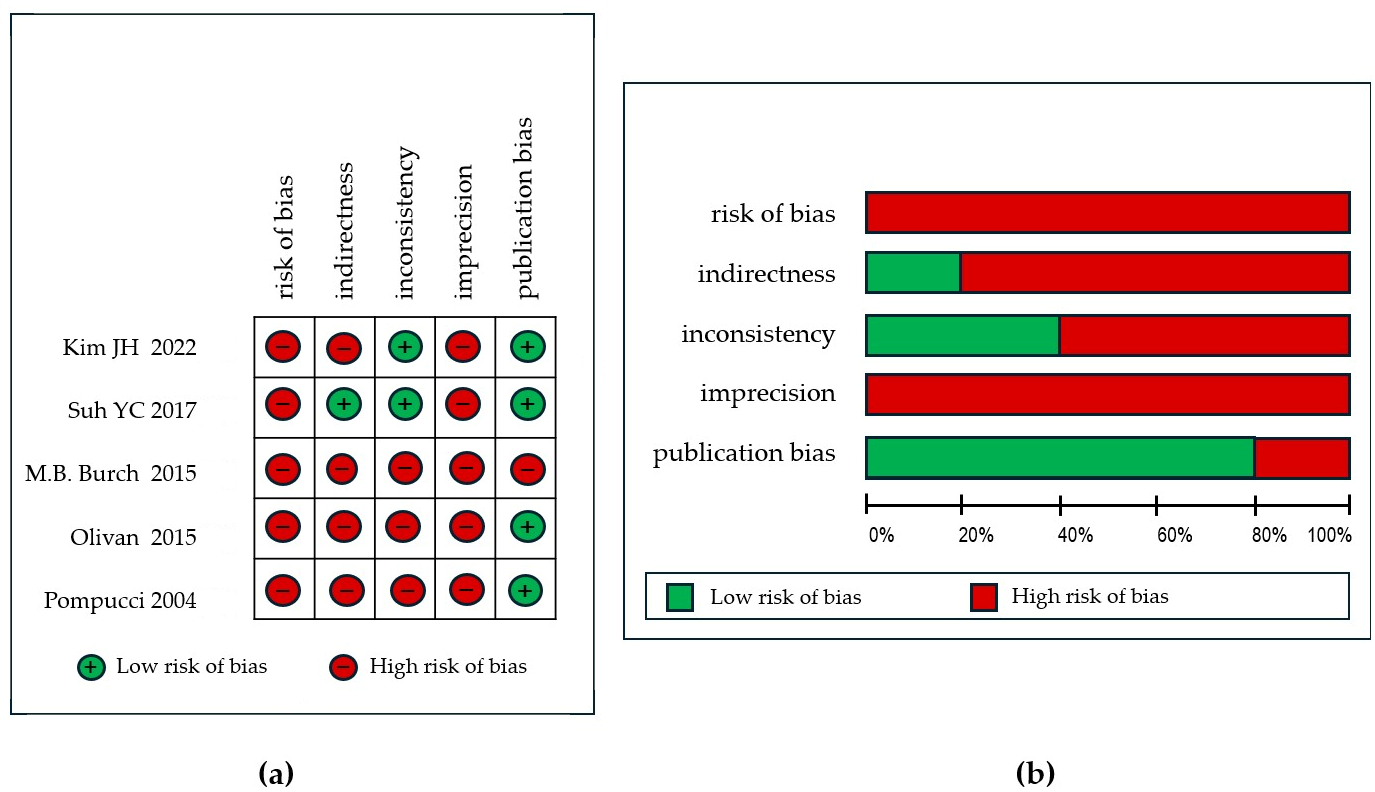
| First Author, Year | Population | Study Design | Risk of Bias | Indirectness | Inconsistency | Imprecision | Publication Bias | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|
| Kim JH [20], 2022 | 21 | retrospective | very serious a,b | serious c,d | not serious | very serious e | undetected | moderate |
| Suh YC [21], 2017 | 59 | retrospective | serious a | not serious | not serious | serious f | not serious | moderate |
| M.B. Burch [22], 2015 | 38 | retrospective | very serious a,b | serious g | serious h | serious f | Serious k | very low |
| Olivan [19], 2015 | 22 | retrospective | very serious a,b | very serious i | very serious j | serious f | not serious | very low |
| Pompucci [23], 2004 | 18 | retrospective | serious a | serious | very serious j | serious f | not serious | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brambullo, T.; L’Erario, S.; Marena, F.; Carpenito, R.; Costa, A.L.; Vindigni, V.; Bassetto, F. Microsurgical Reconstruction with Free Tissue Transfer in Skin Cancer Patients: A Systematic Review. Cancers 2025, 17, 2371. https://doi.org/10.3390/cancers17142371
Brambullo T, L’Erario S, Marena F, Carpenito R, Costa AL, Vindigni V, Bassetto F. Microsurgical Reconstruction with Free Tissue Transfer in Skin Cancer Patients: A Systematic Review. Cancers. 2025; 17(14):2371. https://doi.org/10.3390/cancers17142371
Chicago/Turabian StyleBrambullo, Tito, Stefano L’Erario, Francesco Marena, Roberta Carpenito, Alfio Luca Costa, Vincenzo Vindigni, and Franco Bassetto. 2025. "Microsurgical Reconstruction with Free Tissue Transfer in Skin Cancer Patients: A Systematic Review" Cancers 17, no. 14: 2371. https://doi.org/10.3390/cancers17142371
APA StyleBrambullo, T., L’Erario, S., Marena, F., Carpenito, R., Costa, A. L., Vindigni, V., & Bassetto, F. (2025). Microsurgical Reconstruction with Free Tissue Transfer in Skin Cancer Patients: A Systematic Review. Cancers, 17(14), 2371. https://doi.org/10.3390/cancers17142371









