Epidemiological Insights into Colorectal Cancer Survival in Kazakhstan (2014–2023): A Retrospective Analysis Using the National Electronic Registry of Oncological Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Selection Criteria
2.2. Exposure and Covariates
2.3. Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Baseline Characterisitcs of the Study Cohort
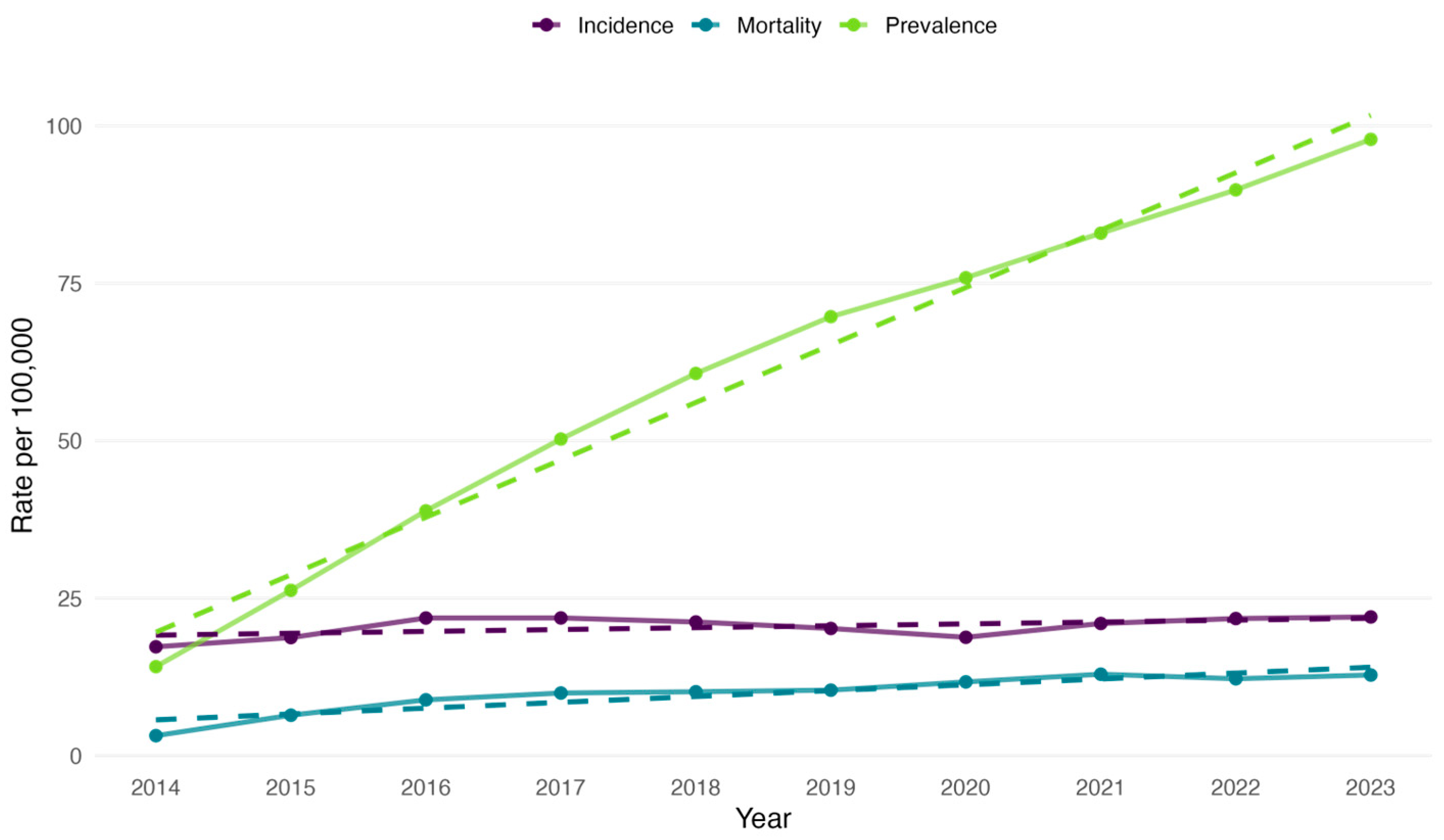
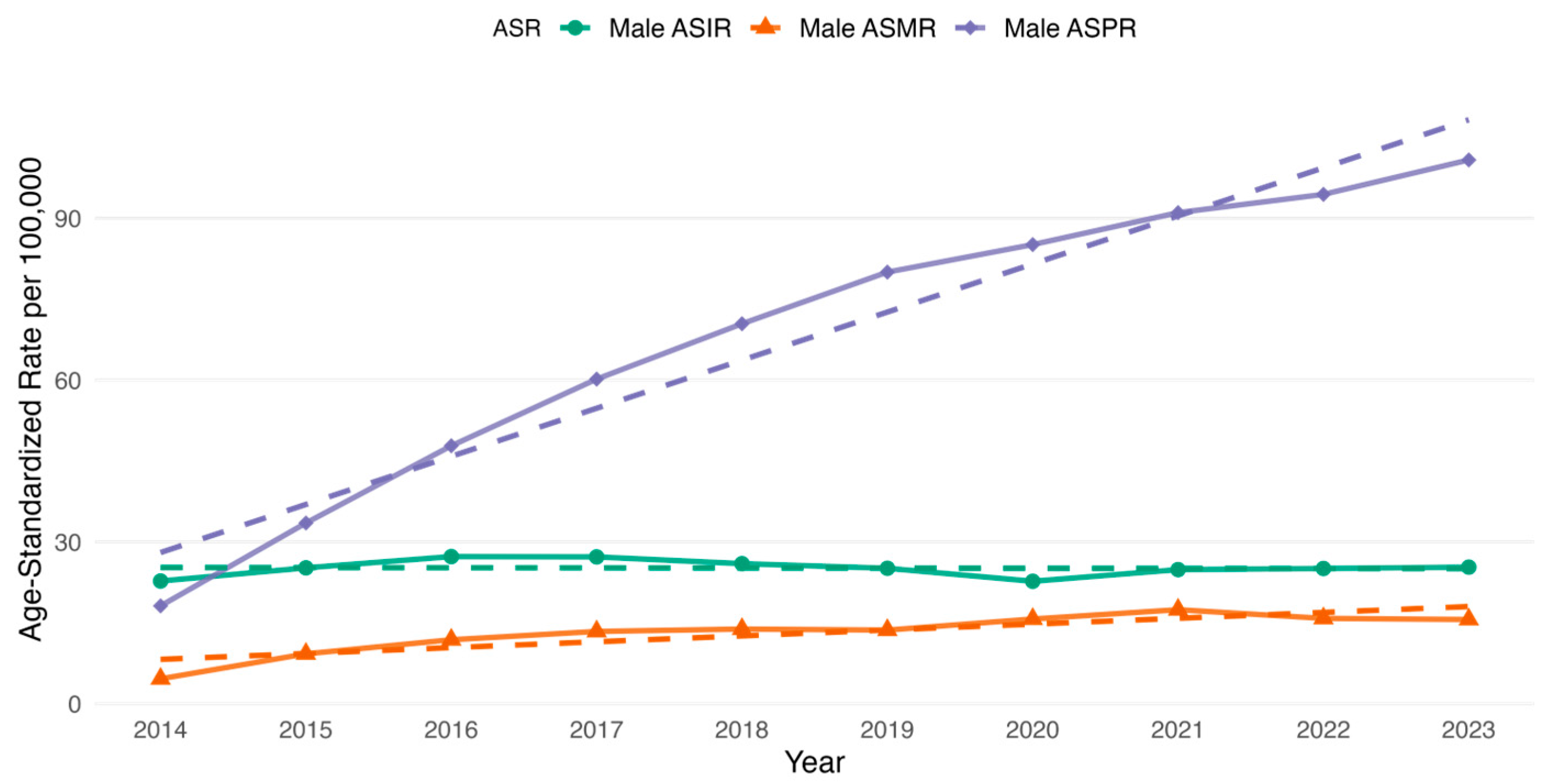
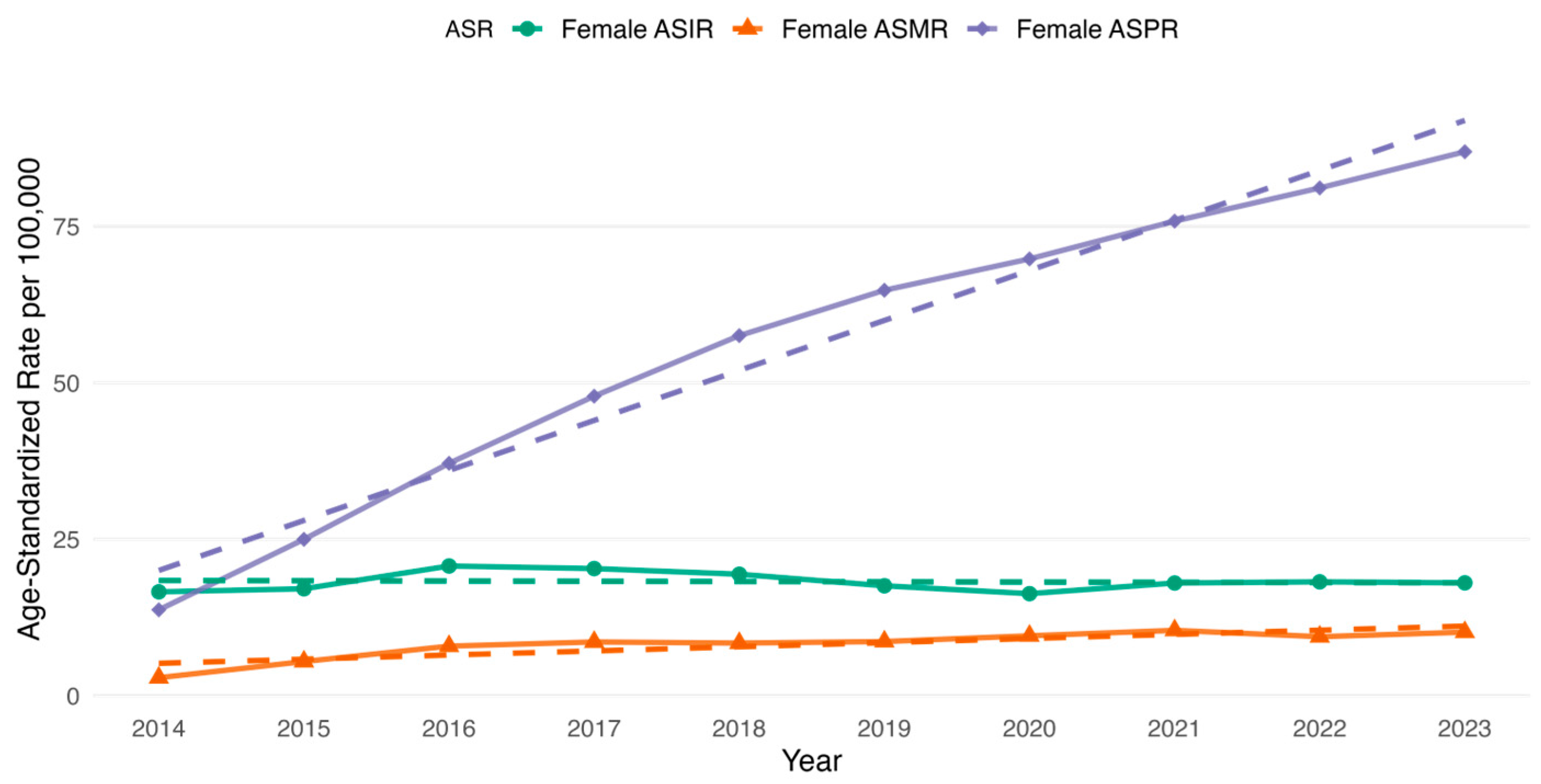
3.2. CRC Incidence, Prevalence and All-Cause Mortality Rate Among the Study Cohort
3.3. Survival Analysis
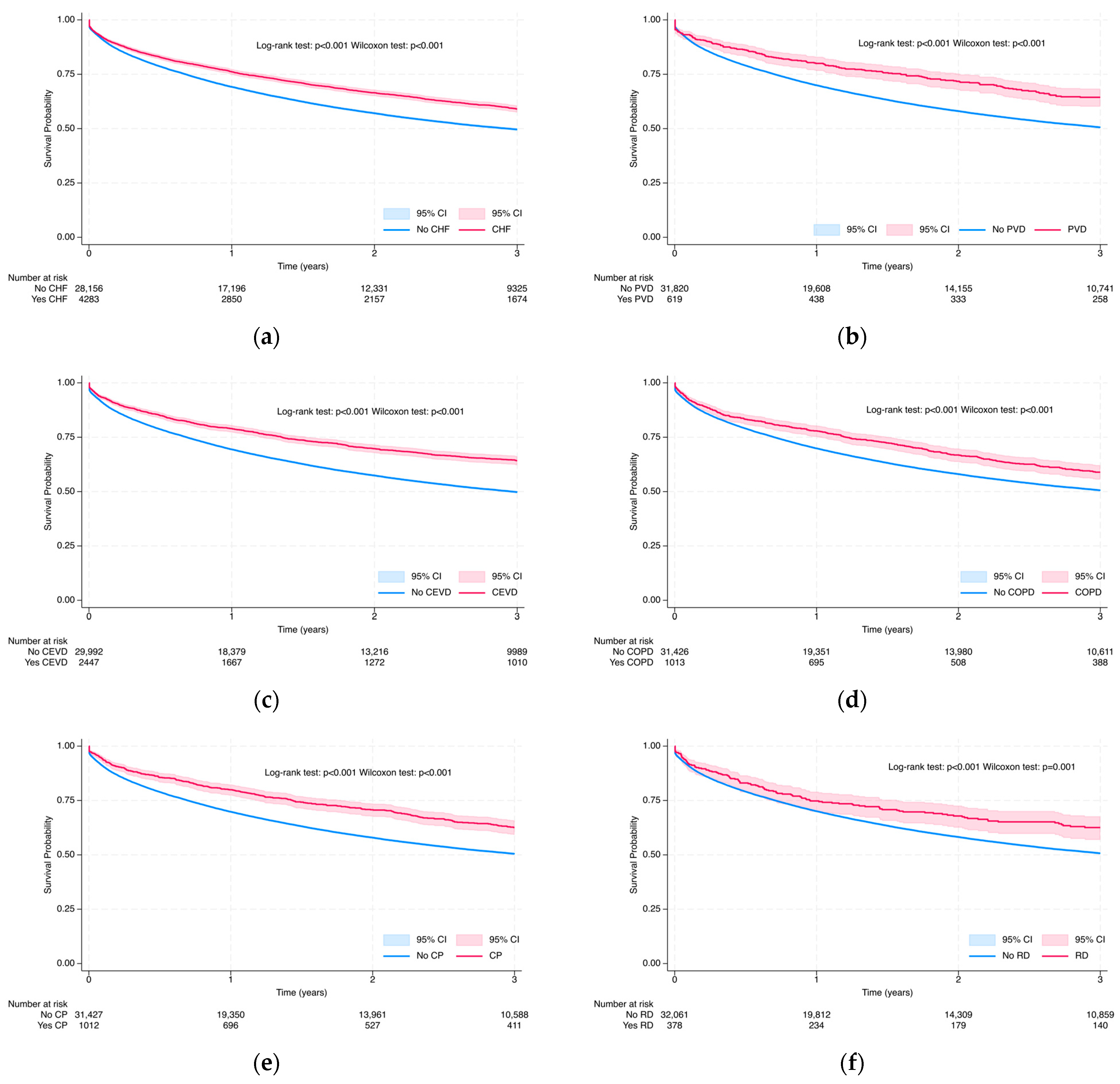
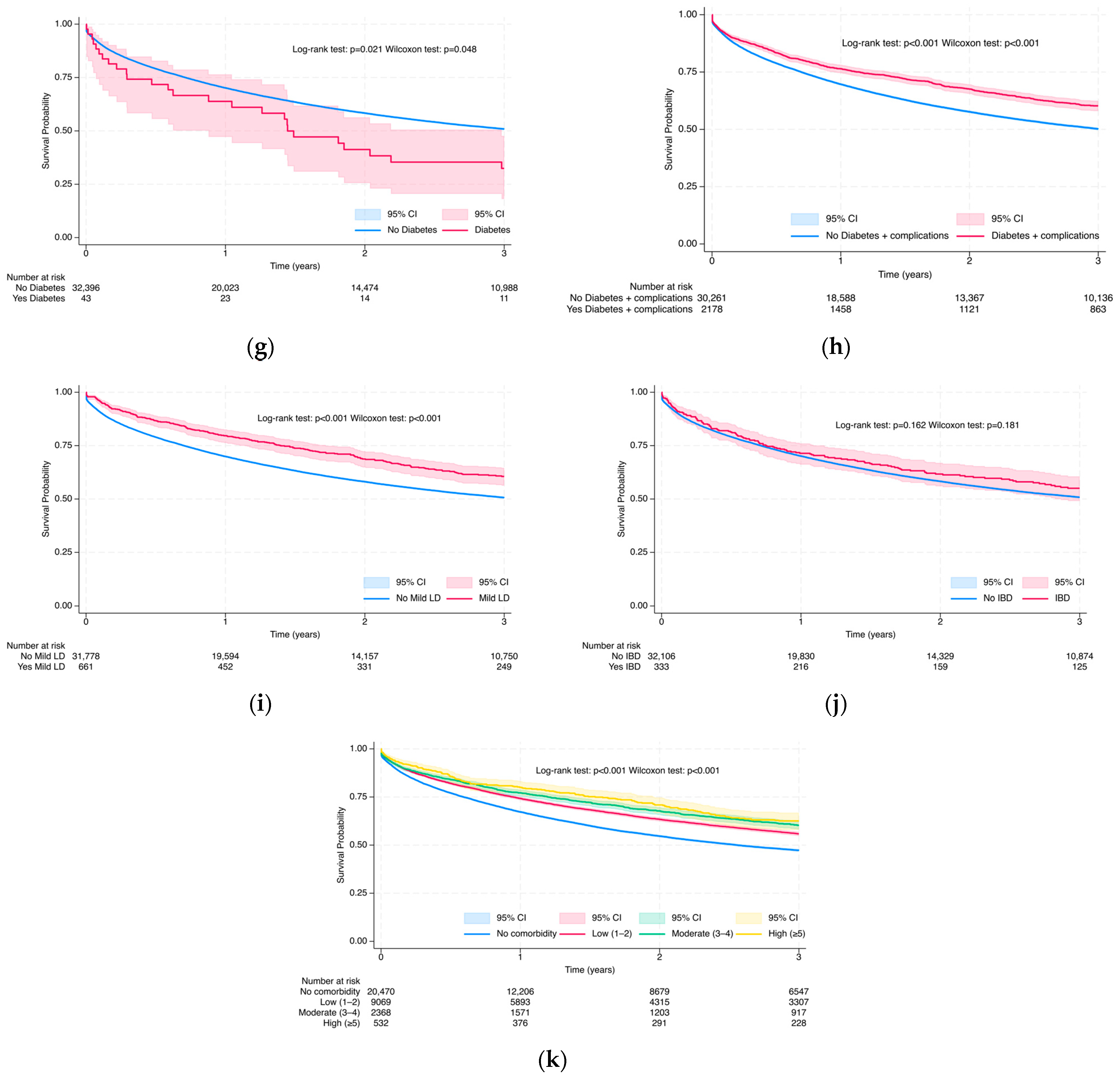
3.3.1. Overall Survival Probability at 3 and 5 Years via Kaplan–Meier Analysis
3.3.2. Multivariable Cox Proportional Hazards Regression Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aHR | Adjusted Hazard Ratio |
| ASIR | Age-Standardized Incidence Rate |
| ASMR | Age-Standardized Mortality Rate |
| ASPR | Age-Standardized Prevalence Rate |
| ASR | Age-Standardized Rate |
| CAC | Classical Adenocarcinoma |
| CCI | Charlson Comorbidity Index |
| CEVD | Cerebrovascular Disease |
| CHF | Congestive Heart Failure |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CP | Other Chronic Pulmonary Diseases |
| CRC | Colorectal Cancer |
| EN | Epithelial Neoplasms |
| EROP | Electronic Registry of Oncological Patients |
| HDI | Human Development Index |
| IBD | Inflammatory Bowel Disease |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| ICD-O-3 | International Classification of Disease for Oncology by 3rd Edition |
| IQR | Interquartile Range |
| IPW | Inverse Probability Weighting |
| LC | Left Colon |
| LD | Liver Disease |
| MAC | Mucinous Adenocarcinoma |
| NOS | Not Otherwise Specified |
| OS | Overall Survival |
| PH | Proportional Hazards |
| PY | Person-Years |
| PVD | Peripheral Vascular Disease |
| R | Rectum |
| RC | Right Colon |
| RD | Rheumatic Disease |
| RJ | Rectosigmoid Junction |
| RPN ID | Resident Population Number |
| SCC | Squamous Cell Carcinoma |
| SRCC | Signet Ring Cell Carcinoma |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TC | Transverse Colon |
| TVC | Time-Varying Cox |
| UNEHS | Unified National Electronic Health System |
| VIF | Variance Inflation Factor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Mauyenova, D.; Zhadykova, Y.; Khozhayev, A.; Turebayev, D.; Kulmirzayeva, D.; Urazova, S.; Nurtazinova, G.; Kuandykov, Y.; Amanshayeva, A.; Sakhanov, S.; et al. Trends of Colorectal Cancer Incidence in Kazakhstan. Asian Pac. J. Cancer Prev. 2021, 22, 3405–3415. [Google Scholar] [CrossRef]
- Mauyenova, D.; Axarin, A.; Telmanova, Z.; Baibusunova, A.; Bilyalova, Z.; Igissinova, G.; Bukeyeva, Z.; Zhantureyeva, A.; Kozhakhmetova, Z.; Kuandykov, Y.; et al. Colorectal Cancer Mortality in Kazakhstan: Spatio-Temporal Epidemiological Assessment. Asian Pac. J. Cancer Prev. 2022, 23, 953–960. [Google Scholar] [CrossRef]
- Akhmedullin, R.; Aimyshev, T.; Zhakhina, G.; Yerdessov, S.; Beyembetova, A.; Ablayeva, A.; Biniyazova, A.; Seyil, T.; Abdukhakimova, D.; Segizbayeva, A.; et al. In-Depth Analysis and Trends of Cancer Mortality in Kazakhstan: A Joinpoint Analysis of Nationwide Healthcare Data 2014–2022. BMC Cancer 2024, 24, 1340. [Google Scholar] [CrossRef]
- Zhylkaidarova, A.; Kaidarova, D.; Batyrbekov, K.; Shatkovskaya, O.; Begimbetova, D. Trends of Colorectal Cancer Prevalence in Kazakhstan Related to Screening. Clin. Endosc. 2021, 54, 32–37. [Google Scholar] [CrossRef]
- Beyembetova, A.; Ablayeva, A.; Akhmedullin, R.; Abdukhakimova, D.; Biniyazova, A.; Gaipov, A. National Electronic Oncology Registry in Kazakhstan: Patient’s Journey. Epidemiol. Health Data Insights 2025, 1, ehdi004. [Google Scholar] [CrossRef]
- Gusmanov, A.; Zhakhina, G.; Yerdessov, S.; Sakko, Y.; Mussina, K.; Alimbayev, A.; Syssoyev, D.; Sarria-Santamera, A.; Gaipov, A. Review of the Research Databases on Population-Based Registries of Unified Electronic Healthcare System of Kazakhstan (UNEHS): Possibilities and Limitations for Epidemiological Research and Real-World Evidence. Int. J. Med. Inform. 2023, 170, 104950. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Appelros, P.; Askling, J.; Byberg, L.; Carrero, J.-J.; Ekström, A.M.; Ekström, M.; Smedby, K.E.; Hagström, H.; James, S.; et al. Adaptation of the Charlson Comorbidity Index for Register-Based Research in Sweden. Clin. Epidemiol. 2021, 13, 21–41. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Van Der Hulst, H.C.; Van Der Bol, J.M.; Bastiaannet, E.; Portielje, J.E.A.; Dekker, J.W.T. Age-Specific Impact of Comorbidity on Postoperative Outcomes in Older Patients with Colorectal Cancer. J. Geriatr. Oncol. 2024, 15, 101836. [Google Scholar] [CrossRef] [PubMed]
- Luhn, P.; Kuk, D.; Carrigan, G.; Nussbaum, N.; Sorg, R.; Rohrer, R.; Tucker, M.G.; Arnieri, B.; Taylor, M.D.; Meropol, N.J. Validation of Diagnosis Codes to Identify Side of Colon in an Electronic Health Record Registry. BMC Med. Res. Methodol. 2019, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Majek, O.; Gondos, A.; Jansen, L.; Emrich, K.; Holleczek, B.; Katalinic, A.; Nennecke, A.; Eberle, A.; Brenner, H.; The GEKID Cancer Survival Working Group. Sex Differences in Colorectal Cancer Survival: Population-Based Analysis of 164,996 Colorectal Cancer Patients in Germany. PLoS ONE 2013, 8, e68077. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.B.; Shahjehan, F.; Merchea, A.; Li, Z.; Bekaii-Saab, T.S.; Grothey, A.; Colibaseanu, D.T.; Kasi, P.M. Impact of Tumor Location and Variables Associated with Overall Survival in Patients with Colorectal Cancer: A Mayo Clinic Colon and Rectal Cancer Registry Study. Front. Oncol. 2019, 9, 76. [Google Scholar] [CrossRef]
- Demographic Statistics Bureau of National Statistics of the Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. 2025. Available online: https://stat.gov.kz/ru/industries/social-statistics/demography/publications/377512/ (accessed on 1 May 2025).
- Ahmad, O.B.; Boschi Pinto, C.; Lopez, A.D. Age Standardization of Rates: A New WHO Standard; GPE Discussion Paper Series: No. 31; World Health Organization: Geneva, Switzerland, 2001; pp. 10–12. [Google Scholar]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of Patients with Peritoneal Metastatic Colorectal Cancer given Systemic Therapy: An Analysis of Individual Patient Data from Prospective Randomised Trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) Database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Klaver, Y.L.B.; Simkens, L.H.J.; Lemmens, V.E.P.P.; Koopman, M.; Teerenstra, S.; Bleichrodt, R.P.; De Hingh, I.H.J.T.; Punt, C.J.A. Outcomes of Colorectal Cancer Patients with Peritoneal Carcinomatosis Treated with Chemotherapy with and without Targeted Therapy. Eur. J. Surg. Oncol. (EJSO) 2012, 38, 617–623. [Google Scholar] [CrossRef]
- Furukawa, S.; Hiraki, M.; Kimura, N.; Okuyama, K.; Kohya, N.; Sakai, M.; Kawaguchi, A.; Ikubo, A.; Samejima, R. The Clinical Impact of Intraoperative Bleeding on Peritoneal Recurrence after Surgery for Stage II to III Colorectal Cancer. Asian J. Surg. 2025, 48, 363–369. [Google Scholar] [CrossRef]
- Bhattacharya, S. Colorectal Cancer Diagnosis and Therapeutic Updates; Bentham Science Publishers: Singapore, 2022; ISBN 9789815040944. [Google Scholar]
- Emile, S.H.; Horesh, N.; Strassmann, V.; Garoufalia, Z.; Gefen, R.; Zhou, P.; Wexner, S.D. A National Cancer Database Analysis of the Characteristics and Outcome of Colon Cancer According to Type of Preexisting Adenoma. Clin. Color. Cancer 2025, 24, 56–63. [Google Scholar] [CrossRef]
- Wu, X.; Lin, H.; Li, S. Prognoses of Different Pathological Subtypes of Colorectal Cancer at Different Stages: A Population-Based Retrospective Cohort Study. BMC Gastroenterol. 2019, 19, 164. [Google Scholar] [CrossRef]
- Morishima, T.; Matsumoto, Y.; Koeda, N.; Shimada, H.; Maruhama, T.; Matsuki, D.; Nakata, K.; Ito, Y.; Tabuchi, T.; Miyashiro, I. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J. Epidemiol. 2019, 29, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulou, E.; Matthes, K.L.; Karavasiloglou, N.; Wanner, M.; Limam, M.; Korol, D.; Held, L.; Rohrmann, S. Impact of Comorbidities at Diagnosis on the 10-Year Colorectal Cancer Net Survival: A Population-Based Study. Cancer Epidemiol. 2021, 73, 101962. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.J.; Alvares, D.; Redondo-Sanchez, D.; Marcos-Gragera, R.; Sánchez, M.-J.; Luque-Fernandez, M.A. Bayesian Variable Selection and Survival Modeling: Assessing the Most Important Comorbidities That Impact Lung and Colorectal Cancer Survival in Spain. BMC Med. Res. Methodol. 2022, 22, 95. [Google Scholar] [CrossRef]
- Tamraz, M.; Al Ghossaini, N.; Temraz, S. Optimization of Colorectal Cancer Screening Strategies: New Insights. World J. Gastroenterol. 2024, 30, 3361–3366. [Google Scholar] [CrossRef]
- Suenghataiphorn, T.; Kulthamrongsri, N.; Danpanichkul, P.; Saowapa, S.; Polpichai, N.; Thongpiya, J. Impact of Dementia in Colorectal Cancer Patients: United States Population-Based Cohort Study. Korean J. Gastroenterol. 2024, 84, 17–23. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, H.S.; Kang, J. Synergistic Prognostic Impact of Hemoglobin and Skeletal Muscle Index in Patients with Colorectal Cancer. Clin. Nutr. ESPEN 2024, 63, 371–377. [Google Scholar] [CrossRef]
- Balboa-Barreiro, V.; Pértega-Díaz, S.; García-Rodríguez, T.; González-Martín, C.; Pardeiro-Pértega, R.; Yáñez-González-Dopeso, L.; Seoane-Pillado, T. Colorectal Cancer Recurrence and Its Impact on Survival after Curative Surgery: An Analysis Based on Multistate Models. Dig. Liver Dis. 2024, 56, 1229–1236. [Google Scholar] [CrossRef]
- Kang, J.H.; Son, I.T.; Yoon, S.N.; Ihm, J.S.; Kang, B.M.; Kim, J.W. Impact of COVID-19 Pandemic on the Clinical and Pathologic Characteristics of Colorectal Cancer: A Retrospective Multicenter Study in South Korea. Cancer Manag. Res. 2024, 16, 1131–1139. [Google Scholar] [CrossRef]
- Loroña, N.C.; Himbert, C.; Ose, J.; Cohen, S.A.; Strehli, I.; Ulrich, C.M.; Cobos, S.; Jean-Baptiste, E.; Bloomer, A.M.; Figueiredo, J.C.; et al. Alcohol Consumption and Smoking History at the Time of Diagnosis and the Risk of Colorectal Cancer Recurrence and Mortality: Results from the ColoCare Study. Cancer Epidemiol. Biomark. Prev. 2025, 34, 59–66. [Google Scholar] [CrossRef]
- Sada, H.; Hinoi, T.; Niitsu, H.; Ohdan, H.; Yamamoto, S.; Endo, S.; Hida, K.; Kinugasa, Y.; Enomoto, T.; Maruyama, S.; et al. Right-Sided Versus Left-Sided Colorectal Cancer in Elderly Patients: A Sub-Analysis of a Large Multicenter Case–Control Study in Japan. Surg. Today 2024, 54, 1173–1183. [Google Scholar] [CrossRef]
- Osterman, E.; Syriopoulou, E.; Martling, A.; Andersson, T.M.-L.; Nordenvall, C. Despite Multi-Disciplinary Team Discussions the Socioeconomic Disparities Persist in the Oncological Treatment of Non-Metastasized Colorectal Cancer. Eur. J. Cancer 2024, 199, 113572. [Google Scholar] [CrossRef] [PubMed]
- Katayama, E.S.; Woldesenbet, S.; Tsilimigras, D.; Munir, M.M.; Endo, Y.; Huang, E.; Cunningham, L.; Harzman, A.; Gasior, A.; Husain, S.; et al. Inflammatory Bowel Disease-Associated Colorectal Cancer Negatively Affects Surgery Outcomes and Health Care Costs. Surgery 2024, 176, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Asghari-Jafarabadi, M.; Wilkins, S.; Plazzer, J.P.; Yap, R.; McMurrick, P.J. Prognostic Factors and Survival Disparities in Right-Sided Versus Left-Sided Colon Cancer. Sci. Rep. 2024, 14, 12306. [Google Scholar] [CrossRef] [PubMed]
- Schmocker, R.K.; Enomoto, L.M.; Low, G.K.; McLoughlin, J.M.; Casillas, M.A.; Antill, A.C.; Heidel, R.E.; Russ, A.J. Further Distance from Treating Facility Is Associated with Advanced Colon Cancer at Presentation and Increased Mortality. Am. Surg. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Alese, O.B.; Zhou, W.; Jiang, R.; Zakka, K.; Huang, Z.; Okoli, C.; Shaib, W.L.; Akce, M.; Diab, M.; Wu, C.; et al. Predictive and Prognostic Effects of Primary Tumor Size on Colorectal Cancer Survival. Front. Oncol. 2021, 11, 728076. [Google Scholar] [CrossRef]
- Al Khashali, H.; Ray, R.; Darweesh, B.; Wozniak, C.; Haddad, B.; Goel, S.; Seidu, I.; Khalil, J.; Lopo, B.; Murshed, N.; et al. Amyloid Beta Leads to Decreased Acetylcholine Levels and Non-Small Cell Lung Cancer Cell Survival via a Mechanism That Involves P38 Mitogen-Activated Protein Kinase and Protein Kinase C in a P53-Dependent and -Independent Manner. Int. J. Mol. Sci. 2024, 25, 5033. [Google Scholar] [CrossRef]
- Kolobova, E.; Petrushanko, I.; Mitkevich, V.; Makarov, A.A.; Grigorova, I.L. β-Amyloids and Immune Responses Associated with Alzheimer’s Disease. Cells 2024, 13, 1624. [Google Scholar] [CrossRef]
- Shi, J.; Hubbard, A.E.; Fong, N.; Pirracchio, R. Implicit Bias in Critical Care Data: Factors Affecting Sampling Frequencies and Missingness Patterns of Clinical and Biological Variables in ICU Patients. medRXiv 2024. [Google Scholar] [CrossRef]
- Beaulieu-Jones, B.K.; Lavage, D.R.; Snyder, J.W.; Moore, J.H.; Pendergrass, S.A.; Bauer, C.R. Characterizing and Managing Missing Structured Data in Electronic Health Records: Data Analysis. JMIR Med. Inf. 2018, 6, e11. [Google Scholar] [CrossRef]
- Beaulieu-Jones, B.K.; Moore, J.H. The Pooled Resource Open-Access Als Clinical Trials Consortium Missing Data Imputation in the Electronic Health Record Using Deeply Learned Autoencoders. In Proceedings of the Biocomputing 2017, Kohala Coast, HI, USA, 4–8 January 2017; World Scientific: Singapore, 2017; pp. 207–218. [Google Scholar]
- Botsis, T.; Hartvigsen, G.; Chen, F.; Weng, C. Secondary Use of EHR: Data Quality Issues and Informatics Opportunities. Summit Transl. Bioinform. 2010, 2010, 1–5. [Google Scholar]
- Kharrazi, H.; Wang, C.; Scharfstein, D. Prospective EHR-Based Clinical Trials: The Challenge of Missing Data. J. Gen. Intern. Med. 2014, 29, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Wells, B.J.; Nowacki, A.S.; Chagin, K.; Kattan, M.W. Strategies for Handling Missing Data in Electronic Health Record Derived Data. eGEMs 2013, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharafoddini, A.; Dubin, J.A.; Maslove, D.M.; Lee, J. A New Insight Into Missing Data in Intensive Care Unit Patient Profiles: Observational Study. JMIR Med. Inf. 2019, 7, e11605. [Google Scholar] [CrossRef]
- Seaman, S.R.; White, I.R. Review of Inverse Probability Weighting for Dealing with Missing Data. Stat. Methods Med. Res. 2013, 22, 278–295. [Google Scholar] [CrossRef]




| Total n = 37,871 | Alive n = 19,474 (51.42) | Dead n = 18,397 (48.58) | p-Value | Mortality Rate per 1000 PY [95%CI] | |
|---|---|---|---|---|---|
| Age, median (IQR) | 65.03 (57.54–72.50) | 63.46 (56.08–69.95) | 66.95 (59.20–75.28) | <0.001 | - |
| Age groups, n (%) | <0.001 | ||||
| 18–44 | 2350 (6.21) | 1430 (7.34) | 920 (5.00) | 130.1 [122.0; 138.8] | |
| 45–54 | 4512 (11.91) | 2687 (13.80) | 1825 (9.92) | 131.7 [125.8; 137.9] | |
| 55–64 | 11,321 (29.89) | 6434 (33.04) | 4887 (26.56) | 142.8 [138.8; 146.8] | |
| 65–74 | 12,045 (31.81) | 6284 (32.27) | 5761 (31.31) | 178.8 [174.3; 183.5] | |
| ≥75 | 7643 (20.18) | 2639 (13.55) | 5004 (27.20) | 293.3 [285.3; 301.5] | |
| Sex, n (%) | <0.001 | ||||
| Female | 19,524 (51.55) | 10,563 (54.24) | 8961 (48.71) | 159.2 [155.9; 162.5] | |
| Male | 18,335 (48.41) | 8905 (45.73) | 9430 (51.26) | 196.1 [192.2; 200.1] | |
| Not indicated | 12 (0.03) | 6 (0.03) | 6 (0.03) | 116.1 [52.1; 258.4] | |
| Ethnicity, n (%) | <0.001 | ||||
| Kazakh | 15,156 (40.02) | 8173 (41.97) | 6983 (37.96) | 171.8 [167.8; 175.9] | |
| Russian | 14,954 (39.49) | 7376 (37.88) | 7578 (41.19) | 182.3 [178.2; 186.4] | |
| Other | 7589 (20.04) | 3856 (19.80) | 3733 (20.29) | 171.9 [166.5; 177.6] | |
| Missing | 172 (0.45) | 69 (0.35) | 103 (0.56) | 203.7 [167.9; 247.1] | |
| Residence, n (%) | <0.001 | ||||
| Rural | 8545 (22.56) | 4158 (21.35) | 4387 (23.85) | 198.2 [192.4; 204.2] | |
| Urban | 23,710 (62.61) | 12,480 (64.09) | 11,230 (61.04) | 169.1 [166.0; 172.2] | |
| Missing | 5616 (14.83) | 2836 (14.56) | 2780 (15.11) | 175.1 [168.7; 181.8] | |
| Social Status, n (%) | <0.001 | ||||
| Disabled | 496 (1.31) | 239 (1.23) | 257 (1.40) | 185.8 [164.4; 209.9] | |
| Employed | 5148 (13.59) | 2974 (15.27) | 2174 (11.82) | 134.3 [128.8; 140.1] | |
| Housewife | 887 (2.34) | 512 (2.63) | 375 (2.04) | 149.5 [135.1; 165.5] | |
| Other | 3643 (9.62) | 1926 (9.89) | 1717 (9.33) | 172.9 [164.9; 181.2] | |
| Pensioner | 19,327 (51.03) | 8777 (45.07) | 10,550 (57.35) | 216.0 [211.9; 220.1] | |
| Unemployed | 2957 (7.81) | 1631 (8.38) | 1326 (7.21) | 166.1 [157.4; 175.3] | |
| Missing | 5413 (14.29) | 3415 (17.54) | 1998 (10.86) | 113.6 [108.7; 118.7] | |
| Total n = 37,871 | Alive n = 19,474 (51.42) | Dead n = 18,397 (48.58) | p-Value | Mortality Rate per 1000 PY [95%CI] | |
|---|---|---|---|---|---|
| Stages, n (%) | <0.001 | ||||
| I | 3402 (8.98) | 2669 (13.71) | 733 (3.98) | 57.9 [53.9; 62.3] | |
| II | 16,419 (43.36) | 9289 (47.70) | 7130 (38.76) | 142.3 [139.0; 145.6] | |
| III | 9025 (23.83) | 3945 (20.26) | 5080 (27.61) | 221.9 [215.9; 228.1] | |
| IV | 5893 (15.56) | 1278 (6.56) | 4615 (25.09) | 569.5 [553.3; 586.2] | |
| Not applicable | 268 (0.71) | 196 (1.01) | 72 (0.39) | 73.9 [58.6; 93.1] | |
| Missing | 2864 (7.56) | 2097 (10.77) | 767 (4.17) | 79.2 [73.8; 85.0] | |
| Histological subtypes, n (%) | <0.001 | ||||
| Classical adenocarcinoma | 25,996 (68.64) | 13,447 (69.05) | 12,549 (68.21) | 176.2 [173.1; 179.3] | |
| Epithelial neoplasms, NOS | 1840 (4.86) | 824 (4.23) | 1016 (5.52) | 256.9 [241.5; 273.2] | |
| Mucinous adenocarcinoma | 515 (1.36) | 250 (1.28) | 265 (1.44) | 191.6 [169.9; 216.1] | |
| Neoplasms, NOS | 2022 (5.34) | 625 (3.21) | 1397 (7.59) | 230.7 [218.9; 243.1] | |
| Other specified types | 1210 (3.20) | 489 (2.51) | 721 (3.92) | 359.3 [334.0; 386.5] | |
| Signet-ring cell carcinoma | 91 (0.24) | 28 (0.14) | 63 (0.34) | 444.8 [347.5; 569.4] | |
| Squamous cell carcinoma | 784 (2.07) | 396 (2.03) | 388 (2.11) | 187.1 [169.3; 206.6] | |
| Missing | 5413 (14.29) | 3415 (17.54) | 1998 (10.86) | 113.6 [108.7; 118.7] | |
| Primary tumor location, n (%) | <0.001 | ||||
| Left colon (descending colon) | 10,603 (28.00) | 5707 (29.31) | 4896 (26.61) | 159.5 [155.1; 164.0] | |
| Rectosigmoid junction | 4545 (12.00) | 2207 (11.33) | 2338 (12.71) | 197.2 [189.4; 205.4] | |
| Rectum | 12,664 (33.44) | 6194 (31.81) | 6470 (35.17) | 185.4 [180.9; 189.9] | |
| Right colon (ascending colon) | 5594 (14.77) | 2695 (13.84) | 2899 (15.76) | 201.6 [194.3; 209.0] | |
| Transverse colon | 1218 (3.22) | 588 (3.02) | 630 (3.42) | 209.8 [194.0; 226.8] | |
| Unspecified colon site | 1408 (3.72) | 649 (3.33) | 759 (4.13) | 162.8 [151.6; 174.8] | |
| Not applicable (benign, in situ, uncertain behavior neoplasms) | 1839 (4.86) | 1434 (7.36) | 405 (2.20) | 82.2 [74.5; 90.6] | |
| Total n = 37,871 | Alive n = 19,474 (51.42) | Dead n = 18,397 (48.58) | p-Value | Mortality Rate per 1000 PY [95%CI] | |
|---|---|---|---|---|---|
| Comorbidities | |||||
| Myocardial infarction, n (%) | 777 (2.05) | 408 (2.10) | 369 (2.01) | 0.540 | 161.5 [145.8; 178.8] |
| Congestive heart failure, n (%) | 5046 (13.32) | 2884 (14.81) | 2162 (11.75) | <0.001 | 138.4 [132.7; 144.4] |
| Peripheral vascular disease, n (%) | 764 (2.02) | 479 (2.46) | 285 (1.55) | <0.001 | 111.0 [98.8; 124.7] |
| Cerebrovascular disease, n (%) | 3003 (7.93) | 1910 (9.81) | 1093 (5.94) | <0.001 | 111.6 [105.2; 118.4] |
| Chronic obstructive pulmonary disease (COPD), n (%) | 1228 (3.24) | 727 (3.73) | 501 (2.72) | <0.001 | 130.9 [120.0; 142.9] |
| Other chronic pulmonary diseases, n (%) | 1210 (3.20) | 747 (3.84) | 463 (2.52) | <0.001 | 120.7 [110.2; 132.2] |
| Rheumatic disease, n (%) | 464 (1.23) | 298 (1.53) | 166 (0.90) | <0.001 | 119.8 [102.9; 139.5] |
| Dementia, n (%) | 95 (0.25) | 46 (0.24) | 49 (0.27) | 0.558 | 130.8 [98.9; 173.1] |
| Hemiplegia, tetraplegia, n (%) | 140 (0.37) | 69 (0.35) | 71 (0.39) | 0.612 | 198.5 [157.3; 250.5] |
| Diabetes, n (%) | 49 (0.13) | 16 (0.08) | 33 (0.18) | 0.009 | 313.3 [222.7; 440.7] |
| Diabetes with end-organ damage, n (%) | 2554 (6.74) | 1472 (7.56) | 1082 (5.88) | <0.001 | 137.1 [129.1; 145.5] |
| Moderate or severe kidney disease, n (%) | 3054 (8.06) | 1577 (8.10) | 1477 (8.03) | 0.804 | 160.5 [152.5; 168.9] |
| Mild liver disease, n (%) | 829 (2.19) | 499 (2.56) | 330 (1.79) | <0.001 | 135.6 [121.7; 151.0] |
| Moderate or severe liver disease, n (%) | 60 (0.16) | 21 (0.11) | 39 (0.21) | 0.011 | 240.2 [175.5; 328.8] |
| (Peptic) Ulcer disease, n (%) | 1148 (3.03) | 596 (3.06) | 552 (3.00) | 0.734 | 171.6 [157.8; 186.5] |
| Any malignancy, including leukemia and lymphoma *, n (%) | 2547 (6.73) | 1350 (6.93) | 1197 (6.51) | 0.098 | 187.6 [177.3; 198.5] |
| HIV/AIDS, n (%) | 4 (0.01) | 3 (0.01) | 1 (0.01) | 0.345 | 67.2 [9.5; 477.1] |
| Inflammatory bowel disease, n (%) | 429 (1.13) | 242 (1.24) | 187 (1.02) | 0.038 | 143.0 [123.9; 165.0] |
| CCI group | <0.001 | ||||
| No comorbidity (0) | 22,816 (60.25) | 11,101 (57.00) | 11,715 (63.68) | 195.0 [191.5; 198.5] | |
| Low (1–2) | 11,132 (29.39) | 6101 (31.33) | 5031 (27.35) | 156.2 [152.0; 160.6] | |
| Moderate (3–4) | 3163 (8.35) | 1835 (9.42) | 1328 (7.22) | 137.4 [130.3; 145.0] | |
| High (≥5) | 760 (2.01) | 437 (2.24) | 323 (1.76) | 130.0 [116.5; 145.0] |
| First Year of Follow-Up | Subsequent Years | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age group | ||||
| 18–44 | reference | reference | ||
| 45–54 | 0.92 (0.85–0.99) † | 0.033 † | 1.04 (0.92–1.17) | 0.521 |
| 55–64 | 0.89 (0.83–0.95) † | 0.001† | 1.15 (1.03–1.29) | 0.010 |
| 65–74 | 0.88 (0.83–0.94) † | 0.001 † | 1.38 (1.23–1.54) | <0.001 |
| ≥75 | 0.86 (0.81–0.93) † | <0.001 † | 2.10 (1.88–2.35) | <0.001 |
| Sex | ||||
| Female | reference | reference | ||
| Male | 1.15 (1.10–1.20) | <0.001 | 1.07 (0.98–1.17) † | 0.129 † |
| Ethnicity | ||||
| Kazakh | reference | reference | ||
| Other | 1.00 (0.90–1.01) § | 0.928 § | 0.98 (0.92–1.04) | 0.534 |
| Russian | 0.91 (0.89–0.93) † | <0.001 † | 1.03 (0.97–1.09) | 0.284 |
| Primary tumor location | ||||
| Left colon (descending colon) | reference | reference | ||
| Rectosigmoid junction | 1.09 (1.05–1.13) † | <0.001 † | 0.86 (0.75–1.00) § | 0.053 § |
| Rectum | 1.21 (1.17–1.25) † | <0.001 † | 0.86 (0.80–0.96) † | 0.006 † |
| Right colon (ascending colon) | 1.07 (1.04–1.11) † | <0.001† | 0.84 (0.72–0.98) § | 0.025 § |
| Transverse colon | 1.03 (0.98–1.09) § | 0.252 § | 0.85 (0.63–1.14) § | 0.273 § |
| Unspecified colon site | 0.94 (0.90–0.99) § | 0.020§ | 1.09 (0.85–1.40) § | 0.511 § |
| Histological subtypes | ||||
| Classical adenocarcinoma | reference | reference | ||
| Epithelial neoplasms, NOS | 0.89 (0.85–0.92) † | <0.001 † | 0.84 (0.66–1.06) § | 0.153 § |
| Mucinous adenocarcinoma | 1.21 (1.07–1.37) † | 0.002 † | 0.99 (0.72–1.38) § | 0.980 § |
| Neoplasms, NOS | 0.86 (0.83–0.89) † | <0.001 † | 0.80 (0.68–0.95) † | 0.012 † |
| Other specified types | 0.96 (0.92–1.01) † | 0.095 † | 0.76 (0.56–1.05) § | 0.101 § |
| Signet-ring cell carcinoma | 1.03 (0.88–1.22) § | 0.068 § | 0.48 (0.16–1.45) § | 0.196 § |
| Squamous cell carcinoma, NOS | 1.18 (1.02–1.36) § | 0.101 § | 0.91 (0.69–1.20) § | 0.521 § |
| Comorbidities | ||||
| Absent comorbidity | reference | reference | ||
| Congestive heart failure | 0.82 (0.76–0.87) | <0.001 | 1.39 (1.22–1.58) † | <0.001 † |
| Peripheral vascular disease | 0.76 (0.64–0.92) | 0.004 | 0.86 (0.72–1.03) | 0.102 |
| Cerebrovascular disease | 0.96 (0.92–1.01) † | 0.171 † | 1.28 (1.08–1.52) † | 0.005 † |
| Chronic obstructive pulmonary disease (COPD) | 0.95 (0.88–1.02) † | 0.193 † | 0.87 (0.76–1.00) | 0.058 |
| Other chronic pulmonary diseases | 0.79 (0.68–0.92) | 0.002 | 0.88 (0.76–1.01) | 0.077 |
| Rheumatic disease | 1.06 (0.86–1.31) | 0.555 | 0.86 (0.67–1.11) | 0.254 |
| Diabetes | 1.59 (0.96–2.65) | 0.071 | 2.45 (1.48–4.07) | 0.001 |
| Diabetes with end-organ damage | 0.89 (0.81–0.98) | 0.015 | 1.37 (1.15–1.63)† | <0.001 † |
| Mild liver disease | 0.79 (0.66–0.94) | 0.009 | 1.51 (1.12–2.03) † | 0.007 † |
| Inflammatory bowel disease | 1.16 (0.94–1.42) | 0.154 | 1.05 (0.84–1.33) | 0.659 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdukhakimova, D.; Beyembetova, A.; Ablayeva, A.; Akhmedullin, R.; Aimyshev, T.; Biniyazova, A.; Zhakhina, G.; Orazbekova, Z.; Orazova, G.; Gaipov, A. Epidemiological Insights into Colorectal Cancer Survival in Kazakhstan (2014–2023): A Retrospective Analysis Using the National Electronic Registry of Oncological Patients. Cancers 2025, 17, 2336. https://doi.org/10.3390/cancers17142336
Abdukhakimova D, Beyembetova A, Ablayeva A, Akhmedullin R, Aimyshev T, Biniyazova A, Zhakhina G, Orazbekova Z, Orazova G, Gaipov A. Epidemiological Insights into Colorectal Cancer Survival in Kazakhstan (2014–2023): A Retrospective Analysis Using the National Electronic Registry of Oncological Patients. Cancers. 2025; 17(14):2336. https://doi.org/10.3390/cancers17142336
Chicago/Turabian StyleAbdukhakimova, Diyora, Altynay Beyembetova, Ayana Ablayeva, Ruslan Akhmedullin, Temirgali Aimyshev, Aigerim Biniyazova, Gulnur Zhakhina, Zhanar Orazbekova, Galiya Orazova, and Abduzhappar Gaipov. 2025. "Epidemiological Insights into Colorectal Cancer Survival in Kazakhstan (2014–2023): A Retrospective Analysis Using the National Electronic Registry of Oncological Patients" Cancers 17, no. 14: 2336. https://doi.org/10.3390/cancers17142336
APA StyleAbdukhakimova, D., Beyembetova, A., Ablayeva, A., Akhmedullin, R., Aimyshev, T., Biniyazova, A., Zhakhina, G., Orazbekova, Z., Orazova, G., & Gaipov, A. (2025). Epidemiological Insights into Colorectal Cancer Survival in Kazakhstan (2014–2023): A Retrospective Analysis Using the National Electronic Registry of Oncological Patients. Cancers, 17(14), 2336. https://doi.org/10.3390/cancers17142336





