Advances in Doxorubicin Chemotherapy: Emerging Polymeric Nanocarriers for Drug Loading and Delivery
Simple Summary
Abstract
1. Introduction
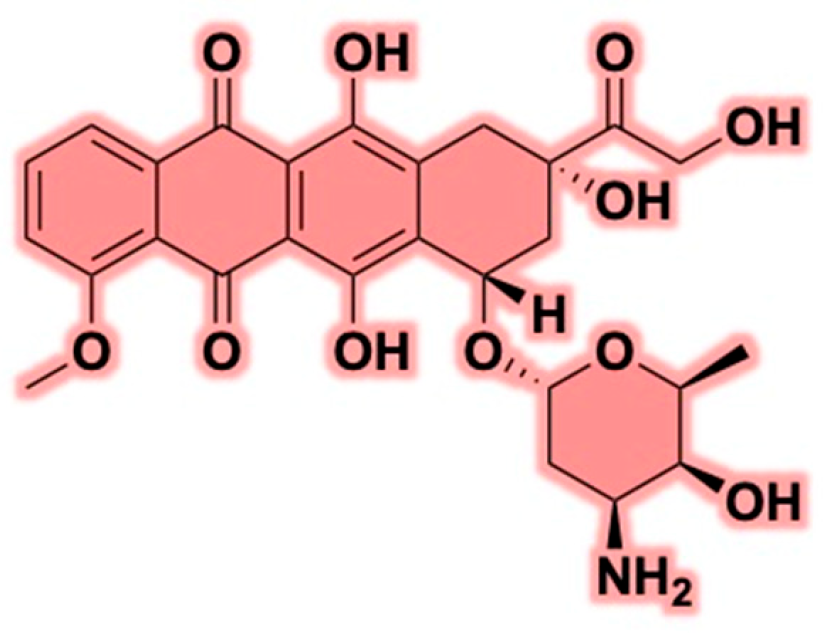
2. Doxorubicin
2.1. Mechanisms of Action
2.2. Clinical Challenges: Cardiotoxicity and Multidrug Resistance
2.3. Chemotherapeutic Applications and Combination Therapies
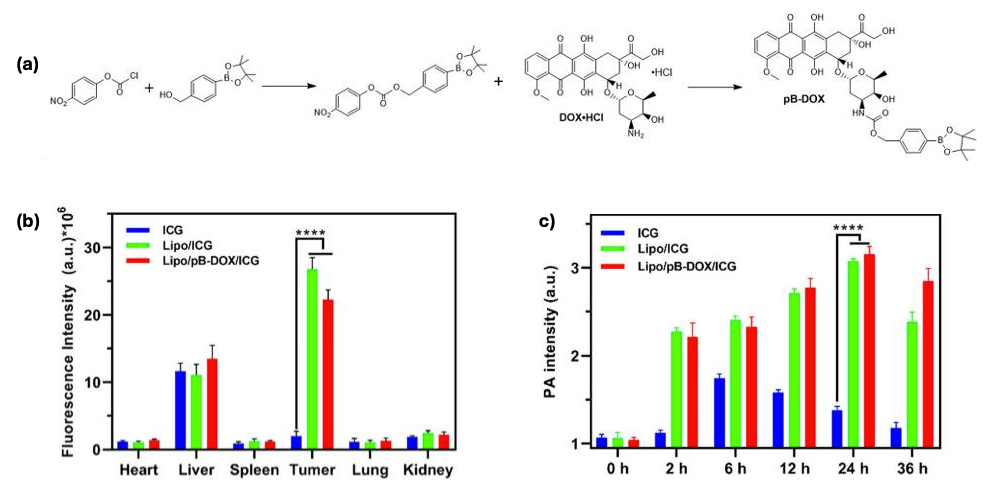
2.4. Innovations in Nanomedicine and Alternative Delivery Platforms
3. Advancements in Drug Delivery Systems: Polymeric Nanocarriers
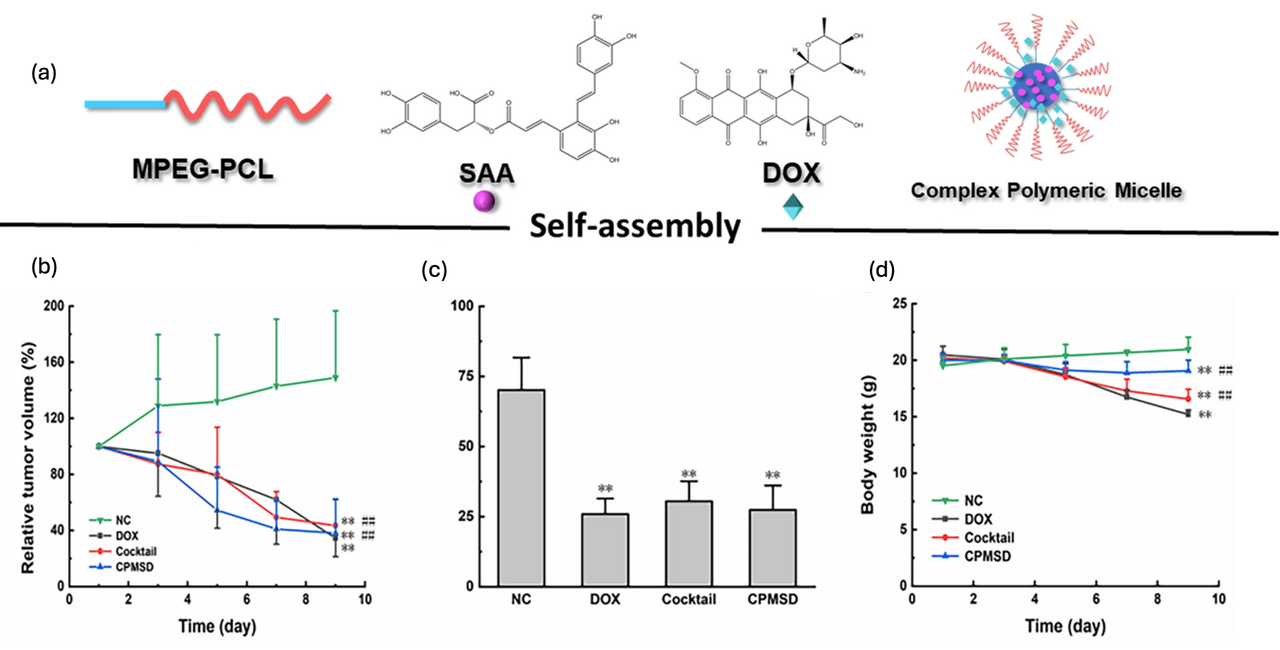
3.1. Polymeric Micelles
3.2. Hydrogels
3.3. Dendrimers
3.4. Polymersomes
3.5. Polymeric Drug Conjugates
3.6. Clinical Challenges
4. Future Directions in Doxorubicin Delivery Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Johnson-Arbor, K.; Dubey, R. Doxorubicin. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef]
- Ma, X.; Sun, R.; Cheng, J.; Liu, J.; Gou, F.; Xiang, H.; Zhou, X. Fluorescence aggregation-caused quenching versus aggregation-induced emission: A visual teaching technology for undergraduate chemistry students. J. Chem. Educ. 2016, 93, 345–350. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Gerber, M.C.; Weisberg, S.; York, M.; Spicer, D.; Jones, S.E.; Wadler, S.; Desai, A.; Vogel, C.; et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 1997, 15, 1318–1332. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Almajwal, A.; Al-Disi, D. Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by Acacia hydaspica tannin-rich ethyl acetate fraction. Saudi J. Biol. Sci. 2020, 27, 2251–2260. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Li, J.; Liu, X.; Wu, S.; Wang, B.; Wang, Y.; Jia, H. Doxorubicin-Induced Cognitive Impairment: The Mechanistic Insights. Front. Oncol. 2021, 11, 673340. [Google Scholar] [CrossRef]
- Kamińska, K.; Cudnoch-Jędrzejewska, A. A Review on the Neurotoxic Effects of Doxorubicin. Neurotox. Res. 2023, 41, 383–397. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2010, 7, 771–782. [Google Scholar] [CrossRef]
- Lammers, T.; Hennink, W.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef]
- Daglar, B.; Ozgur, E.; Corman, M.; Uzun, L.; Demirel, G. Polymeric nanocarriers for expected nanomedicine: Current challenges and future prospects. RSC Adv. 2014, 4, 48639–48659. [Google Scholar] [CrossRef]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical translation of nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.; Matthews, B.R.; Holan, G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–318. [Google Scholar] [CrossRef]
- Ghosh, R.; Malhotra, M.; Sathe, R.R.M.; Jayakannan, M. Biodegradable polymer theranostic fluorescent nanoprobe for direct visualization and quantitative determination of antimicrobial activity. Biomacromolecules 2020, 21, 2896–2912. [Google Scholar] [CrossRef]
- Svenson, S. The dendrimer paradox–high medical expectations but poor clinical translation. Chem. Soc. Rev. 2015, 44, 4131–4144. [Google Scholar] [CrossRef]
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 0063. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Yan, S.; Na, J.; Liu, X.; Wu, P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics 2024, 16, 248. [Google Scholar] [CrossRef]
- Liu, M.; Fang, X.; Yang, Y.; Wang, C. Peptide-Enabled Targeted Delivery Systems for Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 701504. [Google Scholar] [CrossRef]
- Ferris, R.L.; Jaffee, E.M.; Ferrone, S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: Clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 2010, 28, 4390–4399. [Google Scholar] [CrossRef]
- Khan, S.; Hussain, A.; Fahimi, H.; Aliakbari, F.; Haj Bloukh, S.; Edis, Z.; Mahdi Nejadi Babadaei, M.; Izadi, Z.; Shiri Varnamkhasti, B.; Jahanshahi, F.; et al. A review on the therapeutic applications of aptamers and aptamer-conjugated nanoparticles in cancer, inflammatory and viral diseases. Arab. J. Chem. 2022, 15, 103626. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Peng, S.; Tang, Z.; Tan, C.; Ling, J.; Lin, W.; Lin, X.; Zu, X.; Yi, G. pH/Reduction Dual-Stimuli-Responsive Cross-Linked Micelles Based on Multi-Functional Amphiphilic Star Copolymer: Synthesis and Controlled Anti-Cancer Drug Release. Polymers 2020, 12, 82. [Google Scholar] [CrossRef]
- Li, S.; Wu, W.; Xiu, K.; Xu, F.; Li, Z.; Li, J. Doxorubicin loaded pH-responsive micelles capable of rapid intracellular drug release for potential tumor therapy. J. Biomed. Nanotechnol. 2014, 10, 1480–1489. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, J.; Xiao, W.; Lin, W.; Chen, J.; Chen, Q.; Zhang, L.; Zhang, C.; Guo, J. Fabrication of PDEAEMA-based pH-responsive mixed micelles for application in controlled doxorubicin release. RSC Adv. 2017, 7, 27564–27573. [Google Scholar] [CrossRef]
- Alibolandi, M.; Sadeghi, F.; Abnous, K.; Atyabi, F.; Ramezani, M.; Hadizadeh, F. The chemotherapeutic potential of doxorubicin-loaded PEG-b-PLGA nanopolymersomes in mouse breast cancer model. Eur. J. Pharm. Biopharm. 2015, 94, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Guo, S.; Zhang, J.; Kim, W.Y.; Huang, L. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Adv. Funct. Mater. 2014, 24, 6601–6611. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipinska, J.; Tytman, K.; Lipinska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postep. Hig. Med. Dosw. 2016, 70, 1150–1165. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Attina, G.; Triarico, S.; Romano, A.; Maurizi, P.; Ruggiero, A. The DNA-topoisomerase inhibitors in cancer therapy. Biomed. Pharmacol. J. 2022, 15, 553–562. [Google Scholar] [CrossRef]
- Sinha, S.J.; Kumar, B.; Prasad, C.P.; Chauhan, S.S.; Kumar, M. Emerging Research and Future Directions on Doxorubicin: A Snapshot. Asian Pac. J. Cancer Prev. 2025, 26, 5–15. [Google Scholar] [CrossRef]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from S. Peucetius var. caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Rozencweig, M.; Layard, M.; Slavik, M.; Muggia, F.M. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am. J. Med. 1977, 62, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Alrushaid, S.; Sayre, C.L.; Yáñez, J.A.; Forrest, M.L.; Senadheera, S.N.; Burczynski, F.J.; Löbenberg, R.; Davies, N.M. Pharmacokinetic and Toxicodynamic Characterization of a Novel Doxorubicin Derivative. Pharmaceutics 2017, 9, 35. [Google Scholar] [CrossRef]
- Pushkaran, A.C.; Arabi, A.A. Accurate prediction of DNA-Intercalator binding energies: Ensemble of short or long molecular dynamics simulations? Int. J. Biol. Macromol. 2025, 306, 141408. [Google Scholar] [CrossRef]
- Lee, J.; Choi, M.-K.; Song, I.-S. Recent Advances in Doxorubicin Formulation to Enhance Pharmacokinetics and Tumor Targeting. Pharmaceuticals 2023, 16, 802. [Google Scholar] [CrossRef]
- Thotakura, N.; Panjeta, A.; Negi, P.; Preet, S.; Raza, K. Doxorubicin-Loaded Mixed Micelles for the Effective Management of Skin Carcinoma: In Vivo Anti-Tumor Activity and Biodistribution Studies. AAPS PharmSciTech 2021, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Imantay, A.; Mashurov, N.; Zhaisanbayeva, B.A.; Mun, E.A. Doxorubicin-Conjugated Nanoparticles for Potential Use as Drug Delivery Systems. Nanomaterials 2025, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardiooncology 2019, 5, 18. [Google Scholar] [CrossRef]
- Palvia, A.R.; Damera, A.R.; Nandi, A.R.; Magar, S.; Patidar, S.; Kasarla, S.; Ghantasala, V.; Shah, M.K.; Goyal, M. Cardio-Oncology’s Modern Approaches to Prevent Doxorubicin-Induced Cardiotoxicity: A Systematic Review. Cureus 2024, 16, e66215. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzi, N.; Kesharwani, P.; Pittala, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; Lopez Fernandez, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A review of the pathophysiological mechanisms of doxorubicin-induced cardiotoxicity and aging. NPJ Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Qiao, X.; Janssen, L.; Velds, A.; Groothuis, T.; Kerkhoven, R.; Nieuwland, M.; Ovaa, H.; Rottenberg, S.; van Tellingen, O.; et al. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat. Commun. 2013, 4, 1908. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Doroshow, J.H. Mechanisms of Anthracycline-Enhanced Reactive Oxygen Metabolism in Tumor Cells. Oxid. Med. Cell. Longev. 2019, 2019, 9474823. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecinska, A.; Mujwar, S.; Kolat, D.; Kaluzinska-Kolat, Z.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Solem, L.E.; Henry, T.R.; Wallace, K.B. Disruption of mitochondrial calcium homeostasis following chronic doxorubicin administration. Toxicol. Appl. Pharmacol. 1994, 129, 214–222. [Google Scholar] [CrossRef]
- Zhou, S.; Starkov, A.; Froberg, M.K.; Leino, R.L.; Wallace, K.B. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001, 61, 771–777. [Google Scholar] [PubMed]
- Haq, M.M.; Legha, S.S.; Choksi, J.; Hortobagyi, G.N.; Benjamin, R.S.; Ewer, M.; Ali, M. Doxorubicin-induced congestive heart failure in adults. Cancer 1985, 56, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, K.; Vaseghi, G.; Mansourian, M. Pharmacological interventions for preventing anthracycline-induced clinical and subclinical cardiotoxicity: A network meta-analysis of metastatic breast cancer. J. Oncol. Pharm. Pract. 2021, 27, 414–427. [Google Scholar] [CrossRef]
- Muzzammil, T.; Moore, M.J.; Hedley, D.; Ballinger, J.R. Comparison of 99mTc-sestamibi and doxorubicin to monitor inhibition of P-glycoprotein function. Br. J. Cancer 2001, 84, 367–373. [Google Scholar] [CrossRef]
- Shen, F.; Chu, S.; Bence, A.K.; Bailey, B.; Xue, X.; Erickson, P.A.; Montrose, M.H.; Beck, W.T.; Erickson, L.C. Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. J. Pharmacol. Exp. Ther. 2008, 324, 95–102. [Google Scholar] [CrossRef]
- Shchulkin, A.V.; Abalenikhina, Y.V.; Kosmachevskaya, O.V.; Topunov, A.F.; Yakusheva, E.N. Regulation of P-Glycoprotein during Oxidative Stress. Antioxidants 2024, 13, 215. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kawabata, S.; Yanagihara, Y.; Kezuka, C.; Kato, M.; Morikawa, Y.; Endo, S.; Chen, H.; Iguchi, K.; Ikari, A. Pathophysiological roles of autophagy and aldo-keto reductases in development of doxorubicin resistance in gastrointestinal cancer cells. Chem. Biol. Interact. 2019, 314, 108839. [Google Scholar] [CrossRef]
- Jamrozik, M.; Piska, K.; Bucki, A.; Koczurkiewicz-Adamczyk, P.; Sapa, M.; Wladyka, B.; Pekala, E.; Kolaczkowski, M. In Silico and In Vitro Assessment of Carbonyl Reductase 1 Inhibition Using ASP9521—A Potent Aldo-Keto Reductase 1C3 Inhibitor with the Potential to Support Anticancer Therapy Using Anthracycline Antibiotics. Molecules 2023, 28, 3767. [Google Scholar] [CrossRef] [PubMed]
- Pinzon-Daza, M.L.; Cuellar-Saenz, Y.; Nualart, F.; Ondo-Mendez, A.; Del Riesgo, L.; Castillo-Rivera, F.; Garzon, R. Oxidative Stress Promotes Doxorubicin-Induced Pgp and BCRP Expression in Colon Cancer Cells Under Hypoxic Conditions. J. Cell. Biochem. 2017, 118, 1868–1878. [Google Scholar] [CrossRef]
- Jurj, A.; Ionescu, C.; Berindan-Neagoe, I.; Braicu, C. The extracellular matrix alteration, implication in modulation of drug resistance mechanism: Friends or foes? J. Exp. Clin. Cancer Res. 2022, 41, 276. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Cheteh, E.H.; Sarne, V.; Ceder, S.; Bianchi, J.; Augsten, M.; Rundqvist, H.; Egevad, L.; Ostman, A.; Wiman, K.G. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Discov. 2020, 6, 42. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, L.; Chen, L.; Wang, X. Doxorubicin-Induced Cardiac Remodeling: Mechanisms and Mitigation Strategies. Cardiovasc. Drugs Ther. 2025, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Pondugula, S.R.; Salamat, J.M.; Abbott, K.L.; Flannery, P.C.; Majrashi, M.; Almaghrabi, M.; Govindarajulu, M.; Ramesh, S.; Sandey, M.; Onteru, S.K.; et al. A clinically relevant combination treatment with doxorubicin and cyclophosphamide does not induce hepatotoxicity in C57BL/6J mice. Liver Res. 2021, 5, 239–242. [Google Scholar] [CrossRef]
- Amgalan, D.; Garner, T.P.; Pekson, R.; Jia, X.F.; Yanamandala, M.; Paulino, V.; Liang, F.G.; Corbalan, J.J.; Lee, J.; Chen, Y.; et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat. Cancer 2020, 1, 315–328. [Google Scholar] [CrossRef]
- Garner, T.P.; Amgalan, D.; Reyna, D.E.; Li, S.; Kitsis, R.N.; Gavathiotis, E. Small-molecule allosteric inhibitors of BAX. Nat. Chem. Biol. 2019, 15, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, J.N.; Parson, S.K.; Buchsbaum, R.J.; Schlam, I.; Ruddy, K.J.; Durani, U.; Epperla, N.; Leong, D.P. Dexrazoxane to Prevent Cardiotoxicity in Adults Treated with Anthracyclines: JACC: CardioOncology Controversies in Cardio-Oncology. JACC CardioOncol. 2024, 6, 322–324. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Gutterman, J.U.; Blumenschein, G.R.; Tashima, C.K.; Burgess, M.A.; Einhorn, L.; Buzdar, A.U.; Richman, S.P.; Hersh, E.M. Combination chemoimmunotherapy of metastatic breast cancer with 5-fluorouracil, adriamycin, cyclophosphamide, and BCG. Cancer 1979, 43, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Nabholtz, J.; Mackey, J.; Smylie, M.; Paterson, A.; Noel, D.; Al-Tweigeri, T.; Tonkin, K.; North, S.; Azli, N.; Riva, A. Phase II study of docetaxel, doxorubicin, and cyclophosphamide as first-line chemotherapy for metastatic breast cancer. J. Clin. Oncol. 2001, 19, 314–321. [Google Scholar] [CrossRef]
- Santana-Krimskaya, S.E.; Franco-Molina, M.A.; Zarate-Trivino, D.G.; Prado-Garcia, H.; Zapata-Benavides, P.; Torres-Del-Muro, F.; Rodriguez-Padilla, C. IMMUNEPOTENT CRP plus doxorubicin/cyclophosphamide chemotherapy remodel the tumor microenvironment in an air pouch triple-negative breast cancer murine model. Biomed. Pharmacother. 2020, 126, 110062. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Tuscano, J.M.; Martin, S.M.; Ma, Y.; Zamboni, W.; O’Donnell, R.T. Efficacy, biodistribution, and pharmacokinetics of CD22-targeted pegylated liposomal doxorubicin in a B-cell non-Hodgkin’s lymphoma xenograft mouse model. Clin. Cancer Res. 2010, 16, 2760–2768. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Wang, J.; Shen, Y.; Bao, Q.; He, F.; Wang, H.; Gong, L.; Liu, Z.; Hu, F.; et al. Checkpoint Blockade in Combination with Doxorubicin Augments Tumor Cell Apoptosis in Osteosarcoma. J. Immunother. 2019, 42, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.; Lozano, T.; Casares, N.; Lana, H.; Troconiz, I.F.; Ten Hagen, T.L.M.; Kochan, G.; Berraondo, P.; Zalba, S.; Garrido, M.J. Dual activity of PD-L1 targeted Doxorubicin immunoliposomes promoted an enhanced efficacy of the antitumor immune response in melanoma murine model. J. Nanobiotechnol. 2021, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Bae, J.S.; Kim, K.M.; Moon, Y.J.; Park, S.-H.; Ha, S.H.; Hussein, U.K.; Zhang, Z.; Park, H.S.; Park, B.-H.; et al. The PARP inhibitor olaparib potentiates the effect of the DNA damaging agent doxorubicin in osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 107. [Google Scholar] [CrossRef]
- Gehl, J.; Boesgaard, M.; Paaske, T.; Vittrup Jensen, B.; Dombernowsky, P. Combined doxorubicin and paclitaxel in advanced breast cancer: Effective and cardiotoxic. Ann. Oncol. 1996, 7, 687–693. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Lukovic, D.; Zlabinger, K.; Spannbauer, A.; Gugerell, A.; Pavo, N.; Traxler, D.; Pils, D.; Maurer, G.; Jakab, A.; et al. Liposomal doxorubicin attenuates cardiotoxicity via induction of interferon-related DNA damage resistance. Cardiovasc. Res. 2020, 116, 970–982. [Google Scholar] [CrossRef]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Livingston, M.B.; Jagosky, M.H.; Robinson, M.M.; Ahrens, W.A.; Benbow, J.H.; Farhangfar, C.J.; Foureau, D.M.; Maxwell, D.M.; Baldrige, E.A.; Begic, X.; et al. Phase II Study of Pembrolizumab in Combination with Doxorubicin in Metastatic and Unresectable Soft-Tissue Sarcoma. Clin. Cancer Res. 2021, 27, 6424–6431. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Xiong, N.; Cheng, S.C.; Barry, W.T.; Penson, R.T.; Konstantinopoulos, P.A.; Hoffman, M.A.; Horowitz, N.; Dizon, D.S.; Stover, E.H.; et al. Combined pembrolizumab and pegylated liposomal doxorubicin in platinum resistant ovarian cancer: A phase 2 clinical trial. Gynecol. Oncol. 2020, 159, 72–78. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on Responsive Drug Delivery Based on Liposome Vehicles for Cancer Treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef]
- Yi, H.; Lu, W.; Liu, F.; Zhang, G.; Xie, F.; Liu, W.; Wang, L.; Zhou, W.; Cheng, Z. ROS-responsive liposomes with NIR light-triggered doxorubicin release for combinatorial therapy of breast cancer. J. Nanobiotechnol. 2021, 19, 134. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K.; et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar] [CrossRef]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Aloss, K.; Hamar, P. Recent Preclinical and Clinical Progress in Liposomal Doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Torchilin, V.P. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012, 14, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pramanik, S.; Losada-Pérez, P.; Reekmans, G.; Carleer, R.; D’Olieslaeger, M.; Vanderzande, D.; Adriaensens, P.; Ethirajan, A. Physicochemical characterizations of functional hybrid liposomal nanocarriers formed using photo-sensitive lipids. Sci. Rep. 2017, 7, 46257. [Google Scholar] [CrossRef]
- Garcia-Pinel, B.; Porras-Alcala, C.; Ortega-Rodriguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; Lopez-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Fobian, S.F.; Cheng, Z.; Ten Hagen, T.L.M. Smart Lipid-Based Nanosystems for Therapeutic Immune Induction against Cancers: Perspectives and Outlooks. Pharmaceutics 2021, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. In Liposomes; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1522, pp. 17–22. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Abenojar, E.; De Leon, A.; Wegierak, D.; Exner, A.A. Increasing Doxorubicin Loading in Lipid-Shelled Perfluoropropane Nanobubbles via a Simple Deprotonation Strategy. Front. Pharmacol. 2020, 11, 644. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Kovrigina, E.; Chubarov, A.; Dmitrienko, E. High Drug Capacity Doxorubicin-Loaded Iron Oxide Nanocomposites for Cancer Therapy. Magnetochemistry 2022, 8, 54. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Nguyen Tran, D.H.; Bach, L.G.; Vu-Quang, H.; Nguyen, D.C.; Park, K.D.; Nguyen, D.H. Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery. Pharmaceutics 2019, 11, 120. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. Doxorubicin Loading on Functional Graphene as a Promising Nanocarrier Using Ternary Deep Eutectic Solvent Systems. ACS Omega 2020, 5, 1656–1668. [Google Scholar] [CrossRef]
- Mishra, P.; Dey, R.K. Co-delivery of docetaxel and doxorubicin using biodegradable PEG-PLA micelles for treatment of breast cancer with synergistic anti-tumour effects. J. Macromol. Sci. Part A 2018, 55, 310–316. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Meng, F.; Deng, C.; Cheng, R.; Feijen, J.; Zhong, Z. cRGD-functionalized reduction-sensitive shell-sheddable biodegradable micelles mediate enhanced doxorubicin delivery to human glioma xenografts in vivo. J. Control. Release 2016, 233, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Nittayacharn, P.; Nasongkla, N. Development of self-forming doxorubicin-loaded polymeric depots as an injectable drug delivery system for liver cancer chemotherapy. J. Mater. Sci. Mater. Med. 2017, 28, 101. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Chen, Y.; Zhao, W.; Zhai, Q.; Rathod, S.; Huang, Y.; Tang, S.; Kwon, Y.T.; Fernandez, C.; et al. Doxorubicin delivered by a redox-responsive dasatinib-containing polymeric prodrug carrier for combination therapy. J. Control. Release 2017, 258, 43–55. [Google Scholar] [CrossRef]
- Babanyinah, G.K.; Bhadran, A.; Polara, H.; Wang, H.; Shah, T.; Biewer, M.C.; Stefan, M.C. Maleimide functionalized polycaprolactone micelles for glutathione quenching and doxorubicin delivery. Chem. Sci. 2024, 15, 9987–10001. [Google Scholar] [CrossRef]
- Quiram, G.; Montagner, F.; Palmer, K.L.; Stefan, M.C.; Washington, K.E.; Rodrigues, D.C. Novel Chlorhexidine-Loaded Polymeric Nanoparticles for Root Canal Treatment. J. Funct. Biomater. 2018, 9, 29. [Google Scholar] [CrossRef]
- Bhadran, A.; Polara, H.; Calubaquib, E.L.; Wang, H.; Babanyinah, G.K.; Shah, T.; Anderson, P.A.; Saleh, M.; Biewer, M.C.; Stefan, M.C. Reversible Cross-linked Thermoresponsive Polycaprolactone Micelles for Enhanced Stability and Controlled Release. Biomacromolecules 2023, 24, 5823–5835. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Du, J.; Gillings, M.J.; Webb, J.C.; Doan, N.C.; Biewer, M.C.; Stefan, M.C. Synthesis of linear and star-like poly(ε-caprolactone)-b-poly{γ-2-[2-(2-methoxy-ethoxy)ethoxy]ethoxy-ε-caprolactone} amphiphilic block copolymers using zinc undecylenate. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3601–3608. [Google Scholar] [CrossRef]
- Yang, F.; Xu, J.; Fu, M.; Ji, J.; Chi, L.; Zhai, G. Development of stimuli-responsive intelligent polymer micelles for the delivery of doxorubicin. J. Drug Target. 2020, 28, 993–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, Z.; Zhang, X.; Zhao, Z.; Ma, G.; Pei, Y.; Zhao, W.; Wan, D.; Pan, J. Dual-Triggered Peptide-Based Polymeric Micelles Enhance Doxorubicin Delivery for Targeted Cancer Therapy. ACS Appl. Nano Mater. 2024, 7, 14380–14391. [Google Scholar] [CrossRef]
- Xu, D.Z.; Sun, X.Y.; Liang, Y.X.; Huang, H.W.; Liu, R.; Lu, Z.L.; He, L. Esterase-Responsive Polymeric Micelles Containing Tetraphenylethene and Poly(ethylene glycol) Moieties for Efficient Doxorubicin Delivery and Tumor Therapy. Bioconjug. Chem. 2023, 34, 248–256. [Google Scholar] [CrossRef]
- Veeren, A.; Bhaw-Luximon, A.; Mukhopadhyay, D.; Jhurry, D. Mixed poly(vinyl pyrrolidone)-based drug-loaded nanomicelles shows enhanced efficacy against pancreatic cancer cell lines. Eur. J. Pharm. Sci. 2017, 102, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Han, J.; Tian, B.; Shi, Y.; Ding, Y.; Wang, L.; Han, J. Galactose-Containing Polymer-DOX Conjugates for Targeting Drug Delivery. AAPS PharmSciTech 2017, 18, 749–758. [Google Scholar] [CrossRef]
- Chen, K.; Cai, H.; Zhang, H.; Zhu, H.; Gu, Z.; Gong, Q.; Luo, K. Stimuli-responsive polymer-doxorubicin conjugate: Antitumor mechanism and potential as nano-prodrug. Acta Biomater. 2019, 84, 339–355. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, L.; Sheng, Y.; Sun, Y.; Deng, L.; Li, X.; Bradley, M.; Zhang, R. Biodegradable pH-responsive hydrogels for controlled dual-drug release. J. Mater. Chem. B 2018, 6, 510–517. [Google Scholar] [CrossRef]
- Chittasupho, C.; Angklomklew, J.; Thongnopkoon, T.; Senavongse, W.; Jantrawut, P.; Ruksiriwanich, W. Biopolymer Hydrogel Scaffolds Containing Doxorubicin as A Localized Drug Delivery System for Inhibiting Lung Cancer Cell Proliferation. Polymers 2021, 13, 3580. [Google Scholar] [CrossRef]
- Chung, C.K.; Garcia-Couce, J.; Campos, Y.; Kralisch, D.; Bierau, K.; Chan, A.; Ossendorp, F.; Cruz, L.J. Doxorubicin Loaded Poloxamer Thermosensitive Hydrogels: Chemical, Pharmacological and Biological Evaluation. Molecules 2020, 25, 2219. [Google Scholar] [CrossRef]
- Wen, Y.; Li, F.; Li, C.; Yin, Y.; Li, J. High mechanical strength chitosan-based hydrogels cross-linked with poly(ethylene glycol)/polycaprolactone micelles for the controlled release of drugs/growth factors. J. Mater. Chem. B 2017, 5, 961–971. [Google Scholar] [CrossRef]
- Chao, Y.; Liang, Y.; Fang, G.; He, H.; Yao, Q.; Xu, H.; Chen, Y.; Tang, X. Biodegradable Polymersomes as Nanocarriers for Doxorubicin Hydrochloride: Enhanced Cytotoxicity in MCF-7/ADR Cells and Prolonged Blood Circulation. Pharm. Res. 2017, 34, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.J.C.; Sincari, V.; Jager, A.; Kucka, J.; Humajova, J.; Pankrac, J.; Paral, P.; Heizer, T.; Janouskova, O.; Davidovich, I.; et al. pH-responsive polymersome-mediated delivery of doxorubicin into tumor sites enhances the therapeutic efficacy and reduces cardiotoxic effects. J. Control. Release 2021, 332, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.; Kairys, V.; Rodrigues, J.; Tomas, H. Polyester Dendrimers Based on Bis-MPA for Doxorubicin Delivery. Biomacromolecules 2022, 23, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Ferrari, M. Emerging nanotherapeutic strategies in breast cancer. Breast 2014, 23, 10–18. [Google Scholar] [CrossRef]
- Valle, J.W.; Lawrance, J.; Brewer, J.; Clayton, A.; Corrie, P.; Alakhov, V.; Ranson, M. A phase II, window study of SP1049C as first-line therapy in inoperable metastatic adenocarcinoma of the oesophagus. J. Clin. Oncol. 2004, 22, 4195. [Google Scholar] [CrossRef]
- Braccini, S.; Tacchini, C.; Chiellini, F.; Puppi, D. Polymeric Hydrogels for In Vitro 3D Ovarian Cancer Modeling. Int. J. Mol. Sci. 2022, 23, 3265. [Google Scholar] [CrossRef]
- Parashar, A.K.; Saraogi, G.K.; Jain, P.K.; Kurmi, B.; Shrivastava, V.; Arora, V. Polymer-drug conjugates: Revolutionizing nanotheranostic agents for diagnosis and therapy. Discov. Oncol. 2024, 15, 641. [Google Scholar] [CrossRef]
- Kansız, S.; Elçin, Y.M. Advanced liposome and polymersome-based drug delivery systems: Considerations for physicochemical properties, targeting strategies and stimuli-sensitive approaches. Adv. Colloid Interface Sci. 2023, 317, 102930. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Minehan, R.L.; Del Borgo, M.P. Controlled release of therapeutics from enzyme-responsive biomaterials. Front. Biomater. Sci. 2022, 1, 916985. [Google Scholar] [CrossRef]
- Wang, H.; Polara, H.; Bhadran, A.; Shah, T.; Babanyinah, G.K.; Ma, Z.; Calubaquib, E.L.; Miller, J.T.; Biewer, M.C.; Stefan, M.C. Effect of aromatic substituents on thermoresponsive functional polycaprolactone micellar carriers for doxorubicin delivery. Front. Pharmacol. 2024, 15, 1356639. [Google Scholar] [CrossRef] [PubMed]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef]
- Fentahun Darge, H.; Yibru Hanurry, E.; Simegniew Birhan, Y.; Worku Mekonnen, T.; Tizazu Andrgie, A.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem. Eng. J. 2021, 406, 126879. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef]
- Cai, K.; He, X.; Song, Z.; Yin, Q.; Zhang, Y.; Uckun, F.M.; Jiang, C.; Cheng, J. Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency. J. Am. Chem. Soc. 2015, 137, 3458–3461. [Google Scholar] [CrossRef]
- Liao, C.; Chen, Y.; Yao, Y.; Zhang, S.; Gu, Z.; Yu, X. Cross-Linked Small-Molecule Micelle-Based Drug Delivery System: Concept, Synthesis, and Biological Evaluation. Chem. Mater. 2016, 28, 7757–7764. [Google Scholar] [CrossRef]
- Della Rocca, J.; Liu, D.; Lin, W. Are high drug loading nanoparticles the next step forward for chemotherapy? Nanomedicine 2012, 7, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Yang, C.; Attia, A.B.; Tan, J.P.; Ke, X.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials 2012, 33, 2971–2979. [Google Scholar] [CrossRef]
- McLaughlin, C.K.; Logie, J.; Shoichet, M.S. Core and Corona Modifications for the Design of Polymeric Micelle Drug-Delivery Systems. Isr. J. Chem. 2013, 53, 670–679. [Google Scholar] [CrossRef]
- Zhuang, W.R.; Wang, Y.; Cui, P.F.; Xing, L.; Lee, J.; Kim, D.; Jiang, H.L.; Oh, Y.K. Applications of π-π stacking interactions in the design of drug-delivery systems. J. Control. Release 2019, 294, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, T.; Li, S.; Lai, Y.; He, B.; Gu, Z. Polymeric micelles with π-π conjugated moiety on glycerol dendrimer as lipophilic segments for anticancer drug delivery. Biomater. Sci. 2014, 2, 775–783. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, J.; Mei, H.; Cai, S.; Li, S.; Cheng, F.; Cao, J.; He, B. High-drug-loading capacity of redox-activated biodegradable nanoplatform for active targeted delivery of chemotherapeutic drugs. Regen. Biomater. 2020, 7, 359–369. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Sun, Z.; Li, Y.; Yu, B.; Zhao, F.; Wang, H.; Xu, H. Nanomicelles co-loaded with doxorubicin and salvianolic acid A for breast cancer chemotherapy. Cancer Nanotechnol. 2022, 13, 21. [Google Scholar] [CrossRef]
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 1977. [Google Scholar] [CrossRef]
- Wang, H.; Calubaquib, E.L.; Bhadran, A.; Ma, Z.; Miller, J.T.; Zhang, A.; Biewer, M.C.; Stefan, M.C. Self-assembly behavior of thermoresponsive difunctionalized γ-amide polycaprolactone amphiphilic diblock copolymers. Polym. Chem. 2023, 14, 514–522. [Google Scholar] [CrossRef]
- Hao, J.; Cheng, Y.; Ranatunga, R.J.K.U.; Senevirathne, S.; Biewer, M.C.; Nielsen, S.O.; Wang, Q.; Stefan, M.C. A Combined Experimental and Computational Study of the Substituent Effect on Micellar Behavior of γ-Substituted Thermoresponsive Amphiphilic Poly(ε-caprolactone)s. Macromolecules 2013, 46, 4829–4838. [Google Scholar] [CrossRef]
- Shah, T.; Polara, H.; Babanyinah, G.; Bhadran, A.; Wang, H.; Castillo, C.C.; Grabowski, G.; Biewer, M.C.; Torabifard, H.; Stefan, M.C. Computational design to experimental validation: Molecular dynamics-assisted development of polycaprolactone micelles for drug delivery. J. Mater. Chem. B 2025, 13, 4166–4178. [Google Scholar] [CrossRef]
- Babanyinah, G.K.; Bhadran, A.; Polara, H.; Shah, T.; Biewer, M.C.; Stefan, M.C. Fluorescent Poly(ε-Caprolactone)s Micelles for Anticancer Drug Delivery and Bioimaging. Biomacromolecules 2025, 26, 3651–3665. [Google Scholar] [CrossRef]
- Polara, H.; Shah, T.; Babanyinah, G.; Wang, H.; Bhadran, A.; Biewer, M.C.; Stefan, M.C. Improved Drug Delivery through Amide-Functionalized Polycaprolactones: Enhanced Loading Capacity and Sustained Drug Release. Biomacromolecules 2025, 26, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Washington, K.E.; Kularatne, R.N.; Biewer, M.C.; Stefan, M.C. Combination Loading of Doxorubicin and Resveratrol in Polymeric Micelles for Increased Loading Efficiency and Efficacy. ACS Biomater. Sci. Eng. 2018, 4, 997–1004. [Google Scholar] [CrossRef]
- Soltantabar, P.; Calubaquib, E.L.; Mostafavi, E.; Biewer, M.C.; Stefan, M.C. Enhancement of loading efficiency by coloading of doxorubicin and quercetin in thermoresponsive polymeric micelles. Biomacromolecules 2020, 21, 1427–1436. [Google Scholar] [CrossRef]
- Zeng, W.; Luo, Y.; Gan, D.; Zhang, Y.; Deng, H.; Liu, G. Advances in Doxorubicin-based nano-drug delivery system in triple negative breast cancer. Front. Bioeng. Biotechnol. 2023, 11, 1271420. [Google Scholar] [CrossRef]
- Sun, W.; Fan, J.; Wang, S.; Kang, Y.; Du, J.; Peng, X. Biodegradable Drug-Loaded Hydroxyapatite Nanotherapeutic Agent for Targeted Drug Release in Tumors. ACS Appl. Mater. Interfaces 2018, 10, 7832–7840. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Aguirre, L.; Morales-Avila, E.; Ocampo-Garcia, B.E.; Medina, L.A.; Lopez-Tellez, G.; Gibbens-Bandala, B.V.; Izquierdo-Sanchez, V. Biodegradable poly(D,L-lactide-co-glycolide)/poly(L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, S.A.; Washington, K.E.; Miller, J.B.; Biewer, M.C.; Oupicky, D.; Siegwart, D.J.; Stefan, M.C. HDAC Inhibitor Conjugated Polymeric Prodrug Micelles for Doxorubicin Delivery. J. Mater. Chem. B 2017, 5, 2106–2114. [Google Scholar] [CrossRef]
- Calubaquib, E.L.; Soltantabar, P.; Wang, H.; Shin, H.; Flores, A.; Biewer, M.C.; Stefan, M.C. Self-assembly behavior of oligo(ethylene glycol) substituted polycaprolactone homopolymers. Polym. Chem. 2021, 12, 3544–3550. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Nasr, M.; Hashem, F.; Teiama, M.; Tantawy, N.; Abdelmoniem, R. Folic acid grafted mixed polymeric micelles as a targeted delivery strategy for tamoxifen citrate in treatment of breast cancer. Drug Deliv. Transl. Res. 2024, 14, 945–958. [Google Scholar] [CrossRef]
- An, H.; Yang, Y.; Zhou, Z.; Bo, Y.; Wang, Y.; He, Y.; Wang, D.; Qin, J. Pectin-based injectable and biodegradable self-healing hydrogels for enhanced synergistic anticancer therapy. Acta Biomater 2021, 131, 149–161. [Google Scholar]
- Cao, J.; Du, X.; Zhao, H.; Zhu, C.; Li, C.; Zhang, X.; Wei, L.; Ke, X. Sequentially degradable hydrogel-microsphere loaded with doxorubicin and pioglitazone synergistically inhibits cancer stemness of osteosarcoma. Biomed. Pharmacother. 2023, 165, 115096. [Google Scholar] [CrossRef] [PubMed]
- Alipournazari, P.; Pourmadadi, M.; Abdouss, M.; Rahdar, A.; Pandey, S. Enhanced delivery of doxorubicin for breast cancer treatment using pH-sensitive starch/PVA/g-C3N4 hydrogel. Int. J. Biol. Macromol. 2024, 265, 130901. [Google Scholar] [CrossRef]
- Akkaya, B.; Akkaya, R.; Celikkaya, S.I.; Sarıaydin, N.; Raheem, K.Y. Doxorubucin loaded pH-responsive chitosan-poly(acrylamide-maleic acid) composite hydrogel for anticancer targeting. J. Mol. Struct. 2023, 1274, 134536. [Google Scholar] [CrossRef]
- Nikzamir, M.; Hanifehpour, Y.; Akbarzadeh, A.; Panahi, Y. Applications of dendrimers in nanomedicine and drug delivery: A review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2246–2261. [Google Scholar] [CrossRef]
- Kaurav, M.; Ruhi, S.; Al-Goshae, H.A.; Jeppu, A.K.; Ramachandran, D.; Sahu, R.K.; Sarkar, A.K.; Khan, J.; Ashif Ikbal, A.M. Dendrimer: An update on recent developments and future opportunities for the brain tumors diagnosis and treatment. Front. Pharmacol. 2023, 14, 1159131. [Google Scholar] [CrossRef]
- Soltany, P.; Miralinaghi, M.; Shariati, F.P. Folic acid conjugated poly (Amidoamine) dendrimer grafted magnetic chitosan as a smart drug delivery platform for doxorubicin: In-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2024, 257, 127564. [Google Scholar] [CrossRef]
- Szota, M.; Jachimska, B. Effect of Alkaline Conditions on Forming an Effective G4. 0 PAMAM Complex with Doxorubicin. Pharmaceutics 2023, 15, 875. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, W.; Qu, X.; Wu, H.; Qu, L.; Zhang, X.; Mäkilä, E.; Salonen, J.; Zhu, Y.; Yang, Z.; et al. Photothermal-responsive nanosized hybrid polymersome as versatile therapeutics codelivery nanovehicle for effective tumor suppression. Proc. Natl. Acad. Sci. USA 2019, 116, 7744–7749. [Google Scholar] [CrossRef]
- Ferrero, C.; Casas, M.; Caraballo, I. Redox-Responsive Polymersomes as Smart Doxorubicin Delivery Systems. Pharmaceutics 2022, 14, 1724. [Google Scholar] [CrossRef] [PubMed]
- Nehate, C.; Nayal, A.; Koul, V. Redox responsive polymersomes for enhanced doxorubicin delivery. ACS Biomater. Sci. Eng. 2018, 5, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Bobde, Y.; Patel, T.; Paul, M.; Biswas, S.; Ghosh, B. PEGylated N-(2 hydroxypropyl) methacrylamide polymeric micelles as nanocarriers for the delivery of doxorubicin in breast cancer. Colloids Surf. B: Biointerfaces 2021, 204, 111833. [Google Scholar] [CrossRef] [PubMed]
- Janani, S.; John, J.; Viswanad, V. Poly (N-(2-hydroxypropyl) methacrylamide). In Synthetic Polymers in Drug and Biotherapeutics Delivery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 297–313. [Google Scholar]
- Braunová, A.; Chytil, P.; Laga, R.; Šírová, M.; Machová, D.; Parnica, J.; Říhová, B.; Janoušková, O.; Etrych, T. Polymer nanomedicines based on micelle-forming amphiphilic or water-soluble polymer-doxorubicin conjugates: Comparative study of in vitro and in vivo properties related to the polymer carrier structure, composition, and hydrodynamic properties. J. Control. Release 2020, 321, 718–733. [Google Scholar] [CrossRef]
- Ou, Y.; Chen, K.; Cai, H.; Zhang, H.; Gong, Q.; Wang, J.; Chen, W.; Luo, K. Enzyme/pH-sensitive polyHPMA–DOX conjugate as a biocompatible and efficient anticancer agent. Biomater. Sci. 2018, 6, 1177–1188. [Google Scholar] [CrossRef]
- Bajwa, N.; Mahal, S.; Singh, P.A.; Jyoti, K.; Dewangan, P.; Madan, J.; Baldi, A. Drug–polymer conjugates: Challenges, opportunities, and future prospects in clinical trials. Polym. Drug Conjug. 2023, 389–469. [Google Scholar]
- Parshad, B.; Arora, S.; Singh, B.; Pan, Y.; Tang, J.; Hu, Z.; Patra, H.K. Towards precision medicine using biochemically triggered cleavable conjugation. Commun. Chem. 2025, 8, 100. [Google Scholar]
- Singh, D. Dynamic Covalent Macromolecular Networks for Adaptive Drug Delivery Systems: An Informative Review. J. Macromol. Sci. Part B 2024, 1–14. [Google Scholar] [CrossRef]
- Utterström, J.; Naeimipour, S.; Selegård, R.; Aili, D. Coiled coil-based therapeutics and drug delivery systems. Adv. Drug Deliv. Rev. 2021, 170, 26–43. [Google Scholar] [CrossRef]
- Kwon, G.S. Polymeric micelles for delivery of poorly water-soluble compounds. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 357–403. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lammers, T.; Storm, G.; Hennink, W.E. Physico-Chemical Strategies to Enhance Stability and Drug Retention of Polymeric Micelles for Tumor-Targeted Drug Delivery. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- D’Addio, S.M.; Saad, W.; Ansell, S.M.; Squiers, J.J.; Adamson, D.H.; Herrera-Alonso, M.; Wohl, A.R.; Hoye, T.R.; Macosko, C.W.; Mayer, L.D.; et al. Effects of block copolymer properties on nanocarrier protection from in vivo clearance. J. Control. Release 2012, 162, 208–217. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhadran, A.; Polara, H.; Babanyinah, G.K.; Baburaj, S.; Stefan, M.C. Advances in Doxorubicin Chemotherapy: Emerging Polymeric Nanocarriers for Drug Loading and Delivery. Cancers 2025, 17, 2303. https://doi.org/10.3390/cancers17142303
Bhadran A, Polara H, Babanyinah GK, Baburaj S, Stefan MC. Advances in Doxorubicin Chemotherapy: Emerging Polymeric Nanocarriers for Drug Loading and Delivery. Cancers. 2025; 17(14):2303. https://doi.org/10.3390/cancers17142303
Chicago/Turabian StyleBhadran, Abhi, Himanshu Polara, Godwin K. Babanyinah, Sruthy Baburaj, and Mihaela C. Stefan. 2025. "Advances in Doxorubicin Chemotherapy: Emerging Polymeric Nanocarriers for Drug Loading and Delivery" Cancers 17, no. 14: 2303. https://doi.org/10.3390/cancers17142303
APA StyleBhadran, A., Polara, H., Babanyinah, G. K., Baburaj, S., & Stefan, M. C. (2025). Advances in Doxorubicin Chemotherapy: Emerging Polymeric Nanocarriers for Drug Loading and Delivery. Cancers, 17(14), 2303. https://doi.org/10.3390/cancers17142303





