Efficacy, Toxicity and Effect of Pretreatment Cardiologic Consultation on Outcomes of Ibrutinib Therapy for Chronic Lymphocytic Leukemia—A KroHem Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
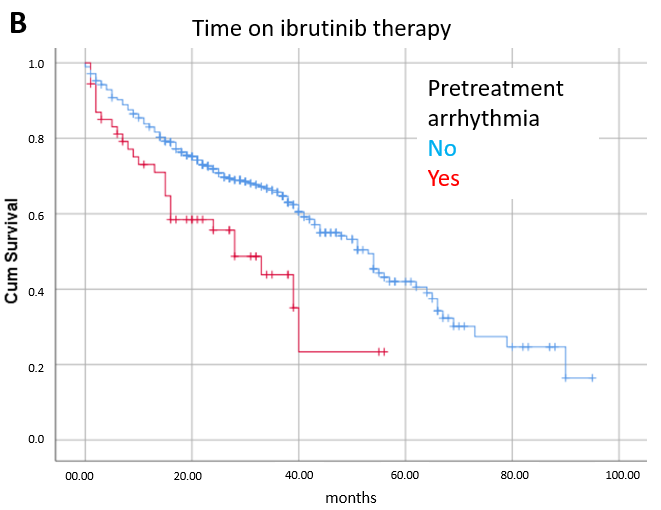
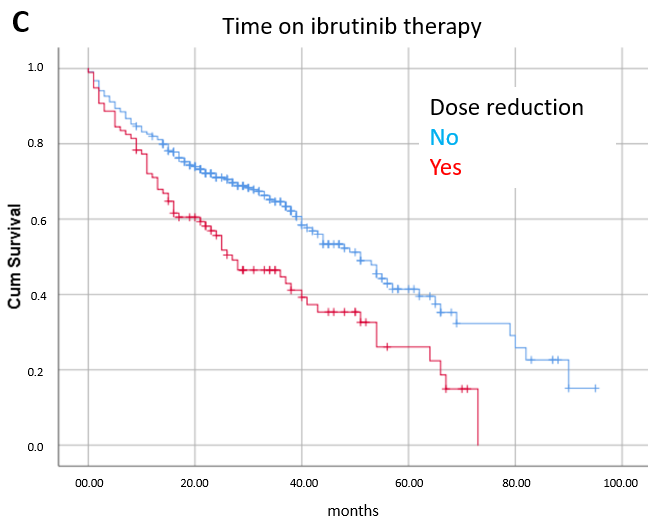
3.1. Toxicity
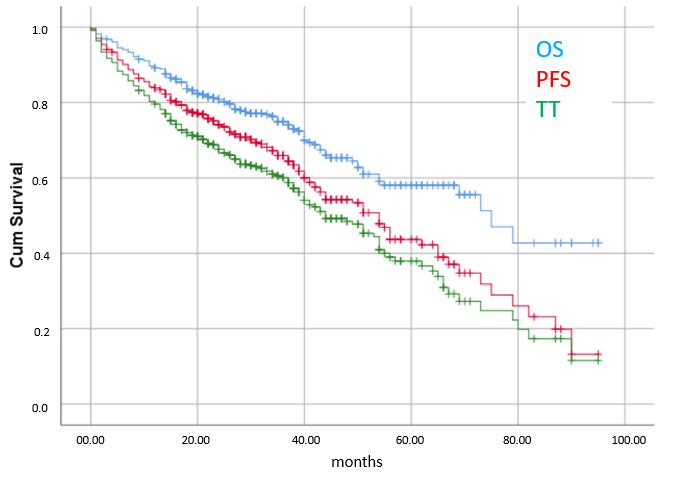
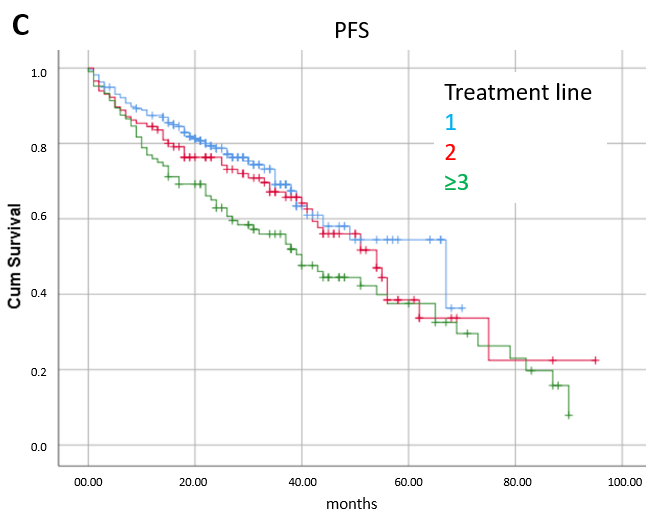
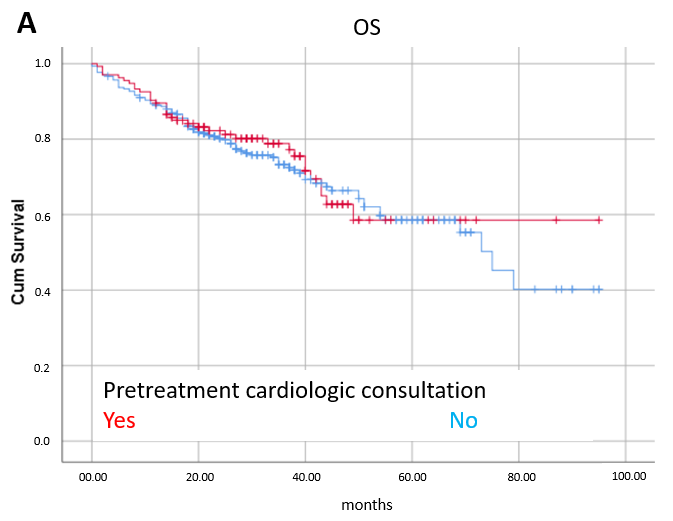
3.2. Efficacy
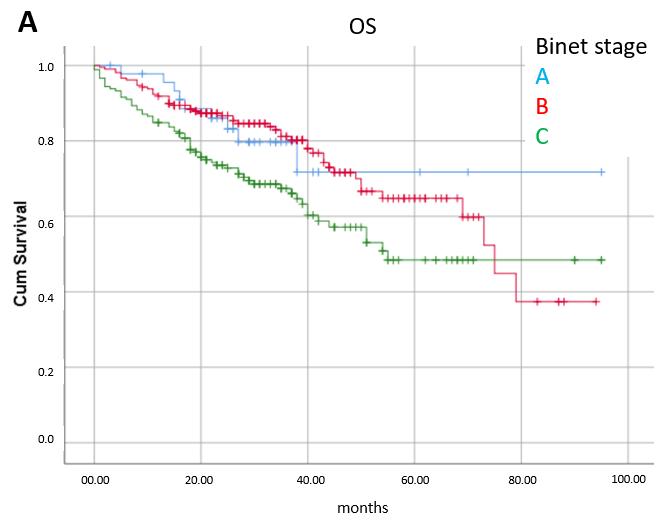
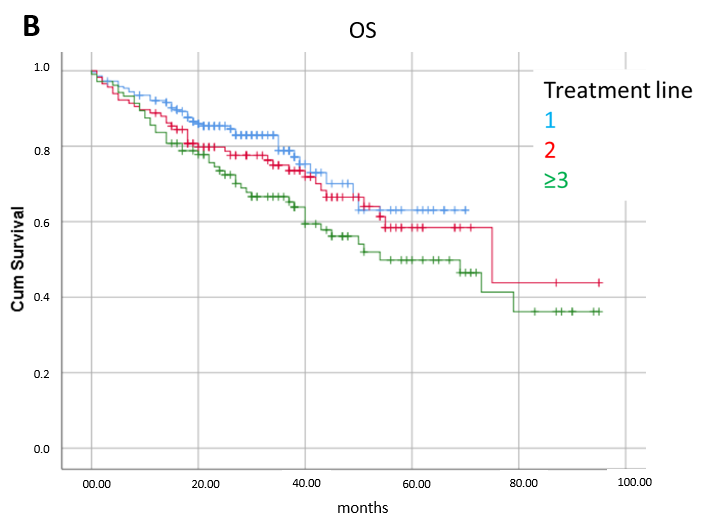
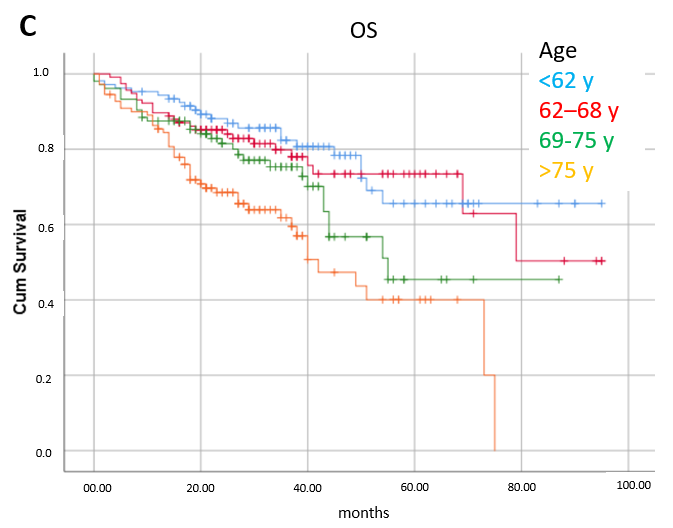
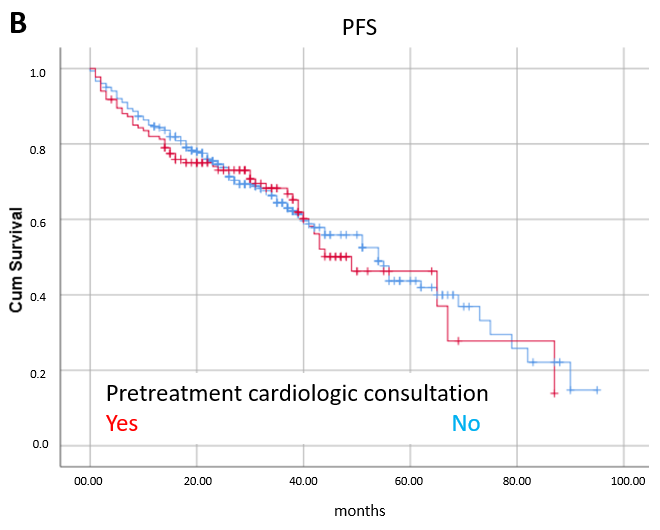
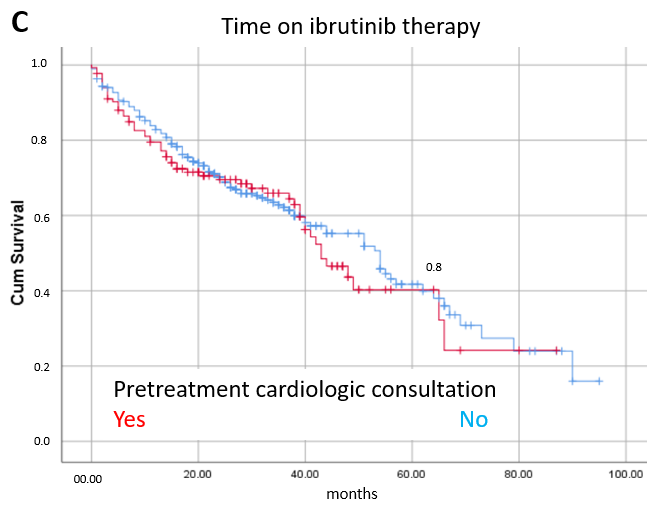
3.3. Prognostic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alu, A.; Lei, H.; Han, X.; Wei, Y.; Wei, X. BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: Mechanisms and clinical studies. J. Hematol. Oncol. 2022, 15, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Bewarder, M.; Stilgenbauer, S.; Thurner, L.; Kaddu-Mulindwa, D. Current treatment options in CLL. Cancers 2021, 13, 2468. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yan, Y.; Zeng, X.; Lin, N.; Tan, B. Ibrutinib-associated cardiotoxicity: From the pharmaceutical to the clinical. Drug Des. Dev. Ther. 2022, 16, 3225–3239. [Google Scholar] [CrossRef] [PubMed]
- Dartigeas, C.; Slama, B.; Doyle, M.; Tapprich, C.; Albrecht, C.; Dupuis, S.; Wapenaar, R.; Schmidt-Hieber, C.; Leblond, V. FIRE study: Real-world effectiveness and safety of ibrutinib in clinical practice in patients with CLL and MCL. Clin. Hematol. Int. 2022, 4, 65–74. [Google Scholar] [CrossRef]
- Mauro, F.R.; Scalzulli, P.R.; Scarfò, L.; Minoia, C.; Murru, R.; Sportoletti, P.; Frigeri, F.; Albano, F.; Di Renzo, N.; Sanna, A.; et al. Real-world outcome of treatment with single-agent ibrutinib in Italian patients with chronic lymphocytic leukemia: Final results of the EVIdeNCE study. Cancers 2024, 16, 1228. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Novak, J.; Strugov, V.; Gill, D.; et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: Final analysis of the randomized, phase III iLLUMINATE trial. Haematologica 2022, 107, 2108–2120. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef]
- Patel, K.; Pagel, J.M. Current and future treatment strategies in chronic lymphocytic leukemia. J. Hematol. Oncol. 2021, 14, 1–20. [Google Scholar] [CrossRef]
- Stühlinger, M.C.; Weltermann, A.; Staber, P.; Heintel, D.; Nösslinger, T.; Steurer, M. Recommendations for ibrutinib treatment in patients with atrial fibrillation and/or elevated cardiovascular risk. Wien. Klin. Wochenschr. 2020, 132, 97–109. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Sorang Kang Inhye, E. Ahn. Prognostic markers in the era of targeted therapies. Acta Haematol. 2024, 147, 33–46. [Google Scholar]
- Mato, A.R.; Nabhan, C.; Thompson, M.C.; Lamanna, N.; Brander, D.M.; Hill, B.; Howlett, C.; Skarbnik, A.; Cheson, B.D.; Zent, C.; et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica 2018, 103, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Winqvist, M.; Andersson, P.O.; Asklid, A.; Karlsson, K.; Karlsson, C.; Lauri, B.; Lundin, J.; Mattsson, M.; Norin, S.; Sandstedt, A.; et al. Long-term real-world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: 30-month follow up of the Swedish compassionate use cohort. Haematologica 2019, 104, e209–e210. [Google Scholar] [CrossRef]
- Abrisqueta, P.; Loscertales, J.; Terol, M.J.; Payer, A.R.; Ortiz, M.; Pérez, I.; Cuellar-García, C.; de la Mata, M.F.; Rodríguez, A.; Lario, A.; et al. Real-world characteristics and outcome of patients treated with single-agent ibrutinib for chronic lymphocytic leukemia in Spain (IBRORS-LLC Study). Clin. Lymphoma Myeloma Leuk. 2021, 21, e985–e999. [Google Scholar] [CrossRef]
- Tombak, A.; Tanrıkulu, F.P.; Durusoy, S.S.; Dinçyürek, H.D.; Kaya, E.; Ümit, E.G.; Yavaşoğlu, İ.; Mehtap, Ö.; Deveci, B.; Özcan, M.A.; et al. Efficacy and safety of ibrutinib therapy in patients with chronic lymphocytic leukemia: Retrospective analysis of real-life data. Turk. J. Hematol. 2021, 38, 273–285. [Google Scholar] [CrossRef]
- Špaček, M.; Smolej, L.; Šimkovič, M.; Nekvindová, L.; Křístková, Z.; Brychtová, Y.; Panovská, A.; Mašlejová, S.; Bezděková, L.; Écsiová, D.; et al. Idelalisib plus rituximab versus ibrutinib in the treatment of relapsed/refractory chronic lymphocytic leukaemia: A real-world analysis from the Chronic Lymphocytic Leukemia Patients Registry (CLLEAR). Br. J. Haematol. 2023, 202, 40–47. [Google Scholar] [CrossRef]
- Pula, B.; Iskierka-Jazdzewska, E.; Dlugosz-Danecka, M.; Szymczyk, A.; Hus, M.; Szeremet, A.; Drozd-Sokolowska, J.; Waszczuk-Gajda, A.; Zaucha, J.M.; Holojda, J.; et al. Long-term efficacy of ibrutinib in relapsed or refractory chronic lymphocytic leukemia: Results of the Polish Adult Leukemia Study Group observational study. Anticancer. Res. 2020, 40, 4059–4066. [Google Scholar] [CrossRef]
- Aarup, K.; Curovic Rotbain, E.; Enggaard, L.; Schou Pedersen, R.; Bergmann, O.J.; Thomsen, R.H.; Frederiksen, M.; Frederiksen, H.; Nielsen, T.; Christiansen, I.; et al. Real-world outcomes for 205 patients with chronic lymphocytic leukemia treated with ibrutinib. Eur. J. Haematol. 2020, 105, 646–654. [Google Scholar] [CrossRef]
- Hillmen, P.; Xie, J.; Yong, A.S.M.; Waweru, C.; Sorof, T.A.; Goyal, R.K.; Davis, K.L. Real-world treatment patterns, adverse events and clinical outcomes in patientswith chronic lymphocytic leukaemia treatedwith ibrutinib in the UK. Eur. J. Haematol. 2021, 2, 219–227. [Google Scholar]
- Visentin, A.; Mauro, F.R.; Cibien, F.; Vitale, C.; Reda, G.; Fresa, A.; Ciolli, S.; Pietrasanta, D.; Marchetti, M.; Murru, R.; et al. Continuous treatment with ibrutinib in 100 untreated patients with TP53 disrupted chronic lymphocytic leukemia: A real-life campus CLL study. Am. J. Hematol. 2021, 97, E95–E99. [Google Scholar] [CrossRef] [PubMed]
- Janssens, A.; Berneman, Z.N.; Offner, F.; Snauwaert, S.; Mineur, P.; Vanstraelen, G.; Meers, S.; Spoormans, I.; Bron, D.; Broek, I.V.; et al. Effectiveness and safety of ibrutinib for chronic lymphocytic leukemia in routine clinical practice: 3-year follow-up of the Belgian Ibrutinib Real-World Data (BIRD) study. Clin. Hematol. Int. 2022, 4, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Khelifi, R.S.; Huang, S.J.; Savage, K.J.; Villa, D.; Scott, D.W.; Ramadan, K.; Connors, J.M.; Sehn, L.H.; Toze, C.L.; Gerrie, A.S. Population-level impact of ibrutinib for chronic lymphocytic leukemia in British Columbia, Canada. Leuk. Lymphoma 2023, 64, 1129–1138. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Gordon, M.J.; Jones, J.E.; George, B.; Peterson, C.; Burger, J.A.; Jain, N.; Keating, M.; Wierda, W.G.; Durand, J.; Ferrajoli, A. Long-term outcomes in patients with chronic lymphocytic leukemia treated with ibrutinib: Focus on hypertension and cardiovascular toxicity. Cancer 2023, 129, 2192–2200. [Google Scholar] [CrossRef]
- Parikh, S.A.; Achenbach, S.J.; Call, T.G.; Rabe, K.G.; Ding, W.; Leis, J.F.; Kenderian, S.S.; Chanan-Khan, A.A.; Koehler, A.B.; Schwager, S.M.; et al. The impact of dose modification and temporary interruption of ibrutinib on outcomes of chronic lymphocytic leukemia patients in routine clinical practice. Cancer Med. 2020, 9, 3390–3399. [Google Scholar] [CrossRef]
- Ganatra, S.; Sharma, A.; Shah, S.; Chaudhry, G.M.; Martin, D.T.; Neilan, T.G.; Mahmood, S.S.; Barac, A.; Groarke, J.D.; Hayek, S.S.; et al. Ibrutinib-associated atrial fibrillation. JACC Clin. Electrophysiol. 2018, 4, 1491–1500. [Google Scholar] [CrossRef]
- Diamond, A.; Bensken, W.P.; Vu, L.; Dong, W.; Koroukian, S.M.; Caimi, P. Ibrutinib is associated with increased cardiovascular events and major bleeding in older CLL patients. JACC CardioOncology 2023, 5, 233–243. [Google Scholar] [CrossRef]
- Jakobsen, C.B.; Lamberts, M.; Carlson, N.; Lock-Hansen, M.; Torp-Pedersen, C.; Gislason, G.H.; Schou, M. Incidence of atrial fibrillation in different major cancer subtypes: A Nationwide population-based 12 year follow up study. BMC Cancer 2019, 19, 1105. [Google Scholar] [CrossRef]
- Visentin, A.; Deodato, M.; Mauro, F.R.; Autore, F.; Reda, G.; Vitale, C.; Molica, S.; Rigolin, G.M.; Piazza, F.; Cesini, L.; et al. A scoring system to predict the risk of atrial fibrillation in chronic lymphocytic leukemia. Hematol. Oncol. 2019, 37, 508–512. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’BRien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]



| Patient’s Characteristic | No. (%) |
|---|---|
| Sex: M/F | 268 (68.5%)/168 (31.5%) |
| Age (median/range/IQR) | 68 years/36–87 years/62–76 years |
| Binet stage * (A/B/C) | 46 (10.6%)/209 (48.3%)/178 (41.1%) |
| FISH: not performed | 85 (19%) |
| FISH: normal/del 11/+12/del 13/del 17 | 112 (31.0%)/49 (13.6%)/22 (6.1%)/62 (17.2%)/116 (32.1%) |
| Immunoglobulin heavy chain mutation: not analyzed | 378 (86%) |
| Immunoglobulin heavy chain: mutated/unmutated | 10 (17%)/48 (83%) |
| Prior lines of treatment: 0/1/≥2 | 216 (49.5%)/116 (26.6%)/104 (23.9%) |
| Prior lines of treatment: (range) | 0–10 |
| Cardiology consultation before ibrutinib ** (yes/no) | 132 (30.3%)/303 (69.7%) |
| History or ECG finding of cardiac arrhythmia before ibrutinib ** (yes/no) | 54 (12.4%)/381 (87.6%) |
| Arterial hypertension before ibrutinib (yes/no) | 219 (50.2%)/217 (49.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandac Smoljanović, I.; Aurer, I.; Bulj, N.; Dreta, B.; Miljak, A.; Petričević, F.; Ivić, M.; Bašić-Kinda, S.; Zatezalo, V.; Madunić, S.; et al. Efficacy, Toxicity and Effect of Pretreatment Cardiologic Consultation on Outcomes of Ibrutinib Therapy for Chronic Lymphocytic Leukemia—A KroHem Study. Cancers 2025, 17, 2302. https://doi.org/10.3390/cancers17142302
Mandac Smoljanović I, Aurer I, Bulj N, Dreta B, Miljak A, Petričević F, Ivić M, Bašić-Kinda S, Zatezalo V, Madunić S, et al. Efficacy, Toxicity and Effect of Pretreatment Cardiologic Consultation on Outcomes of Ibrutinib Therapy for Chronic Lymphocytic Leukemia—A KroHem Study. Cancers. 2025; 17(14):2302. https://doi.org/10.3390/cancers17142302
Chicago/Turabian StyleMandac Smoljanović, Inga, Igor Aurer, Nikola Bulj, Barbara Dreta, Antonija Miljak, Fran Petričević, Marija Ivić, Sandra Bašić-Kinda, Viktor Zatezalo, Sanja Madunić, and et al. 2025. "Efficacy, Toxicity and Effect of Pretreatment Cardiologic Consultation on Outcomes of Ibrutinib Therapy for Chronic Lymphocytic Leukemia—A KroHem Study" Cancers 17, no. 14: 2302. https://doi.org/10.3390/cancers17142302
APA StyleMandac Smoljanović, I., Aurer, I., Bulj, N., Dreta, B., Miljak, A., Petričević, F., Ivić, M., Bašić-Kinda, S., Zatezalo, V., Madunić, S., Čaržavec, D., Sinčić-Petričević, J., Grohovac, D., Jakšić, O., Krečak, I., Morić-Perić, M., Coha, B., Berneš, P., Živković, N., & Pejša, V. (2025). Efficacy, Toxicity and Effect of Pretreatment Cardiologic Consultation on Outcomes of Ibrutinib Therapy for Chronic Lymphocytic Leukemia—A KroHem Study. Cancers, 17(14), 2302. https://doi.org/10.3390/cancers17142302






