The Real-World Efficacy and Side Effects of Different Nivolumab Regimens in Japanese Patients with Advanced Melanoma: A Single-Center Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Efficacy Assessment
2.3. Ethics Statement
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Objective Response
3.3. Progression-Free Survivals and Overall Survivals
3.4. Multivariate Analysis of Potential Prognostic Factors for Progression-Free Survival and Overall Survival
3.5. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRAF/MEK | B-rapidly accelerated fibrosarcoma and mitogen-activated protein kinase |
| CR | Complete response |
| ICI | Immune-checkpoint inhibitor |
| irAE | Immune-related adverse event |
| ORR | Objective response rate |
| OS | Overall survival |
| PFS | Progression-free survival |
| PR | Partial response |
| SD | Stable disease |
References
- Cybulska-Stopa, B.; Piejko, K.; Pacholczak, R.; Domagała-Haduch, M.; Drosik-Kwaśniewska, A.; Rolski, J.; Wiktor-Mucha, P.; Zemełka, T. Real-world treatment practice in patients with advanced melanoma. Contemp. Oncol. 2020, 24, 118–124. [Google Scholar] [CrossRef]
- Malmberg, R.; Zietse, M.; Dumoulin, D.W.; Hendrikx, J.J.M.A.; Aerts, J.G.J.V.; van der Veldt, A.A.M.; Koch, B.C.P.; Sleijfer, S.; van Leeuwen, R.W.F. Alternative dosing strategies for immune checkpoint inhibitors to improve cost-effectiveness: A special focus on nivolumab and pembrolizumab. Lancet Oncol. 2022, 23, e552–e561. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Kiyohara, Y.; Uhara, H.; Uehara, J.; Fujimoto, M.; Takenouchi, T.; Otsuka, M.; Uchi, H.; Ihn, H.; Minami, H. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: A phase II study. Cancer Sci. 2017, 108, 1223–1230. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Pacholczak-Madej, R.; Grela-Wojewoda, A.; Puskulluoglu, M.; Lompart, J.; Las-Jankowska, M.; Krawczak, K.; Wrona, E.; Zaręba, L.; Żubrowska, J.; Walocha, J.; et al. Early Effects of Nivolumab and Ipilimumab Combined Immunotherapy in the Treatment of Metastatic Melanoma in Poland: A Multicenter Experience. Biomedicines 2022, 10, 2528. [Google Scholar] [CrossRef]
- Sznol, M.; Ferrucci, P.F.; Hogg, D.; Atkins, M.B.; Wolter, P.; Guidoboni, M.; Lebbé, C.; Kirkwood, J.M.; Schachter, J.; Daniels, G.A.; et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J. Clin. Oncol. 2017, 35, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Takenouchi, T.; Nakamura, Y.; Takahashi, A.; Namikawa, K.; Kitano, S.; Fujita, T.; Kubota, K.; Yamanaka, T.; Kawakami, Y. Prospective observational study of the efficacy of nivolumab in Japanese patients with advanced melanoma (CREATIVE study). Jpn. J. Clin. Oncol. 2021, 51, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in acral and mucosal melanoma: Current status and future directions. Front. Immunol. 2021, 12, 680407. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, K.; Ito, T.; Yoshikawa, S.; Yoshino, K.; Kiniwa, Y.; Ohe, S.; Isei, T.; Takenouchi, T.; Kato, H.; Mizuhashi, S.; et al. Systemic therapy for Asian patients with advanced BRAF V600-mutant melanoma in a real-world setting: A multi-center retrospective study in Japan (B-CHECK-RWD study). Cancer Med. 2023, 12, 17967–17980. [Google Scholar] [CrossRef]
- Bei, D.; Osawa, M.; Uemura, S.; Ohno, T.; Gobburu, J.; Roy, A.; Hasegawa, M. Benefit-risk assessment of nivolumab 240 mg flat dose relative to 3 mg/kg Q2W regimen in Japanese patients with advanced cancers. Cancer Sci. 2020, 111, 528–535. [Google Scholar] [CrossRef]
- Staender, H.F.; Langan, E.A. Fixed-dose versus weight-adapted immune checkpoint inhibitor therapy in melanoma: A retrospective monocentric analysis of efficacy and immune-related adverse events. Cancers 2025, 17, 1147. [Google Scholar] [CrossRef]
- Campo Le Brun, I.; Dalle, S.; Mortier, L.; Dereure, O.; Dalac Rat, S.; Dutriaux, C.; Leccia, M.T.; Legoupil, D.; Montaudié, H.; De Quatrebarbes, J.; et al. Methods of nivolumab administration in advanced melanoma: A comparison of patients’ clinical outcomes treated with flat dose or weight-adjusted dose, a multicenter observational study. Cancer 2025, 131, e35679. [Google Scholar] [CrossRef]
- Truong, J.; Yeung, S.S.T.; Kletas, V.; de Lemos, M.; Schaff, K.; Nakashima, L. Utilization and toxicity patterns of 2-weekly (Q2W) versus 4-weekly (Q4W) nivolumab for treatment of adjuvant and metastatic melanoma at BC Cancer. J. Oncol. Pharm. Pract. 2024, 30, 1016–1022. [Google Scholar] [CrossRef]
- Samlowski, W.; Robert, N.J.; Chen, L.; Schenkel, B.; Davis, C.; Moshyk, A.; Kotapati, S.; Poretta, T.; Weber, J.S. Real-world nivolumab dosing patterns and safety outcomes in patients receiving adjuvant therapy for melanoma. Cancer Med. 2023, 12, 2378–2388. [Google Scholar] [CrossRef]
- Simeone, E.; Mallardo, D.; Giannarelli, D.; Festino, L.; Vanella, V.; Trojaniello, C.; Vitale, M.G.; Palla, M.; Scarpato, L.; Capone, M.; et al. Correlation of nivolumab 480 mg Q4W with better survival than other nivolumab monotherapy schedule in metastatic melanoma patients. J. Clin. Oncol. 2020, 38, e22008. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Shah, S. Common Terminology Criteria for Adverse Events, version 5.0; National Cancer Institute: Bethesda, MD, USA, 2022; pp. 784–785. [Google Scholar]
- Leroy, M.; Desmedt, E.; Deramoudt, L.; Vasseur, M.; Odou, P.; Béhal, H.; Décaudin, B.; Mortier, L.; Simon, N. Retrospective comparison of a weight-based dose every 2 weeks with a fixed dose every month: A real-life analysis of nivolumab in the treatment of advanced melanoma. Melanoma Res. 2024, 34, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Murashima, Y.; Yamamoto, S.; Hirose, T.; Kadono, T.; Ikeda, G.; Ohara, A.; Itoyama, M.; Yokoyama, K.; Honma, Y.; Ishiyama, K.; et al. Efficacy and safety of salvage-line nivolumab monotherapy for advanced esophageal squamous cell carcinoma: Comparison of 240 mg versus 480 mg doses. J. Gastrointest. Cancer 2024, 55, 1345–1351. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshikawa, S.; Takenouchi, T.; Mori, S.; Asai, J.; Uhara, H.; Ichigosaki, Y.; Fujimura, T.; Nakamura, Y.; Nakamura, Y.; et al. Melanoma skin cancer statistics derived from 7442 Japanese patients: Japanese melanoma study. Int. J. Clin. Oncol. 2025, 30, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Fan, J.; Misra, S. Acral Lentiginous Melanoma: Incidence and Survival in the United States, 2006–2015, an Analysis of the SEER Registry. J. Surg. Res. 2020, 251, 329–339. [Google Scholar] [CrossRef]
- Sergi, M.C.; Filoni, E.; Triggiano, G.; Cazzato, G.; Internò, V.; Porta, C.; Tucci, M. Mucosal Melanoma: Epidemiology, Clinical Features, and Treatment. Curr. Oncol. Rep. 2023, 25, 1247–1258. [Google Scholar] [CrossRef]
- Boer, F.L.; Ho, V.K.Y.; Louwman, M.W.J.; Schrader, A.M.R.; Zuur, C.L.; Blank, C.U.; van Poelgeest, M.I.E.; Kapiteijn, E.H.W. Trends in Incidence and Survival of 1496 Patients with Mucosal Melanoma in The Netherlands (1990–2019). Cancers 2023, 15, 1541. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination with Ipilimumab in Patients with Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef]
- Nathan, P.; Ascierto, P.A.; Haanen, J.; Espinosa, E.; Demidov, L.; Garbe, C.; Guida, M.; Lorigan, P.; Chiarion-Sileni, V.; Gogas, H.; et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: A single-arm, open-label, phase II study (CheckMate 172). Eur. J. Cancer 2019, 119, 168–178. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. 2023, 41, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Elijah, J.; Puzanov, I.; Cresanti, B.; Hamad, L.; Attwood, K.; Catalfamo, K.; Riebandt, G. Evaluation of safety outcomes between nivolumab regimens with differing dosing patterns. J. Oncol. Pharm. Pract. 2024, 10781552241264817. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Day, D.; Chen, T.W.W.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hu, J.; Wang, Y.; Wang, Z. The role of tumor types in immune-related adverse events. In Clinical and Translational Oncology; Springer: Milan, Italy, 2024. [Google Scholar]
- Chatziioannou, E.; Leiter, U.; Thomas, I.; Keim, U.; Seeber, O.; Meiwes, A.; Boessenecker, I.; Gonzalez, S.S.; Torres, F.M.; Niessner, H.; et al. Features and Long-Term Outcomes of Stage IV Melanoma Patients Achieving Complete Response Under Anti-PD-1-Based Immunotherapy. Am. J. Clin. Dermatol. 2023, 24, 453–467. [Google Scholar] [CrossRef]
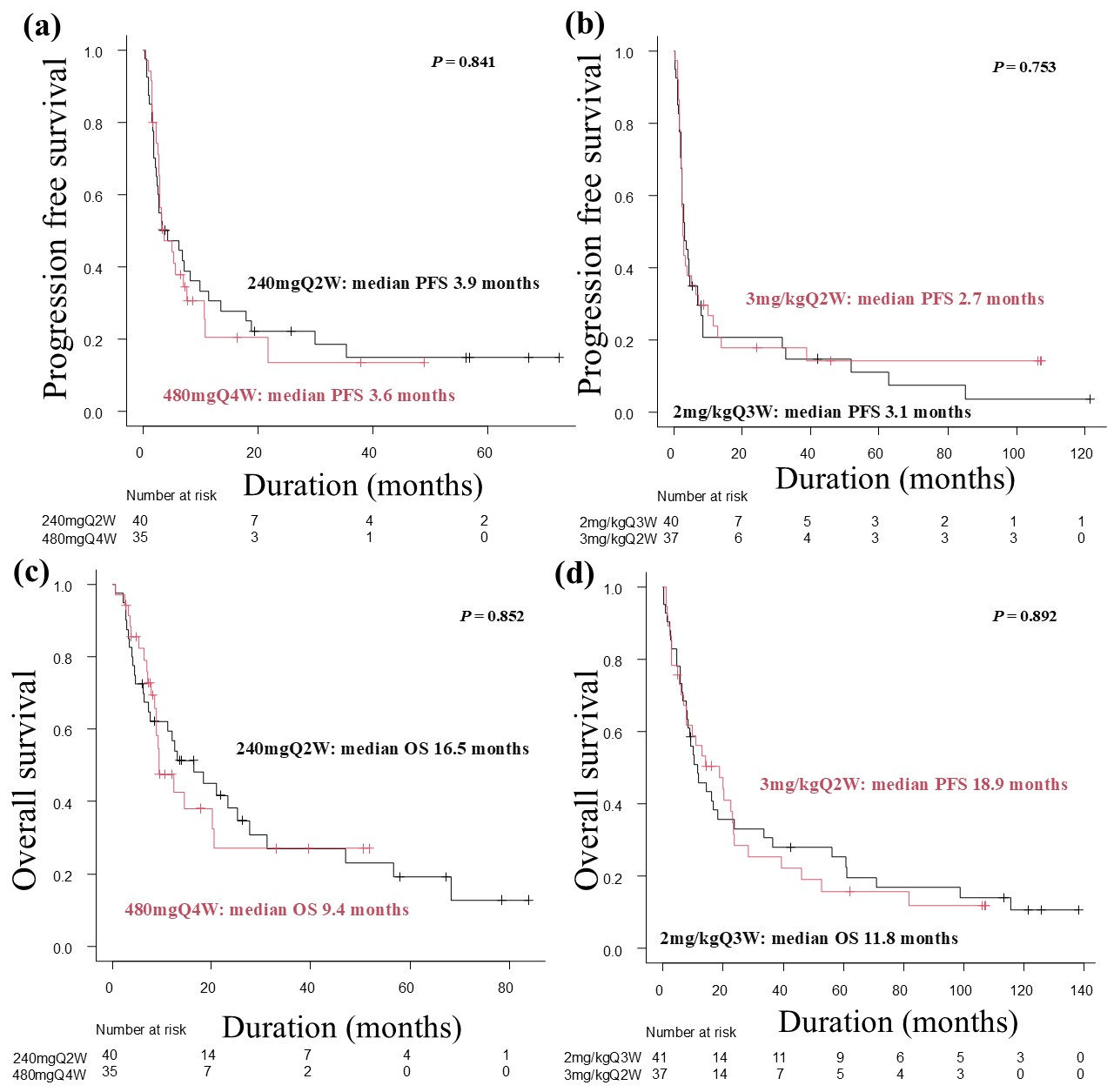
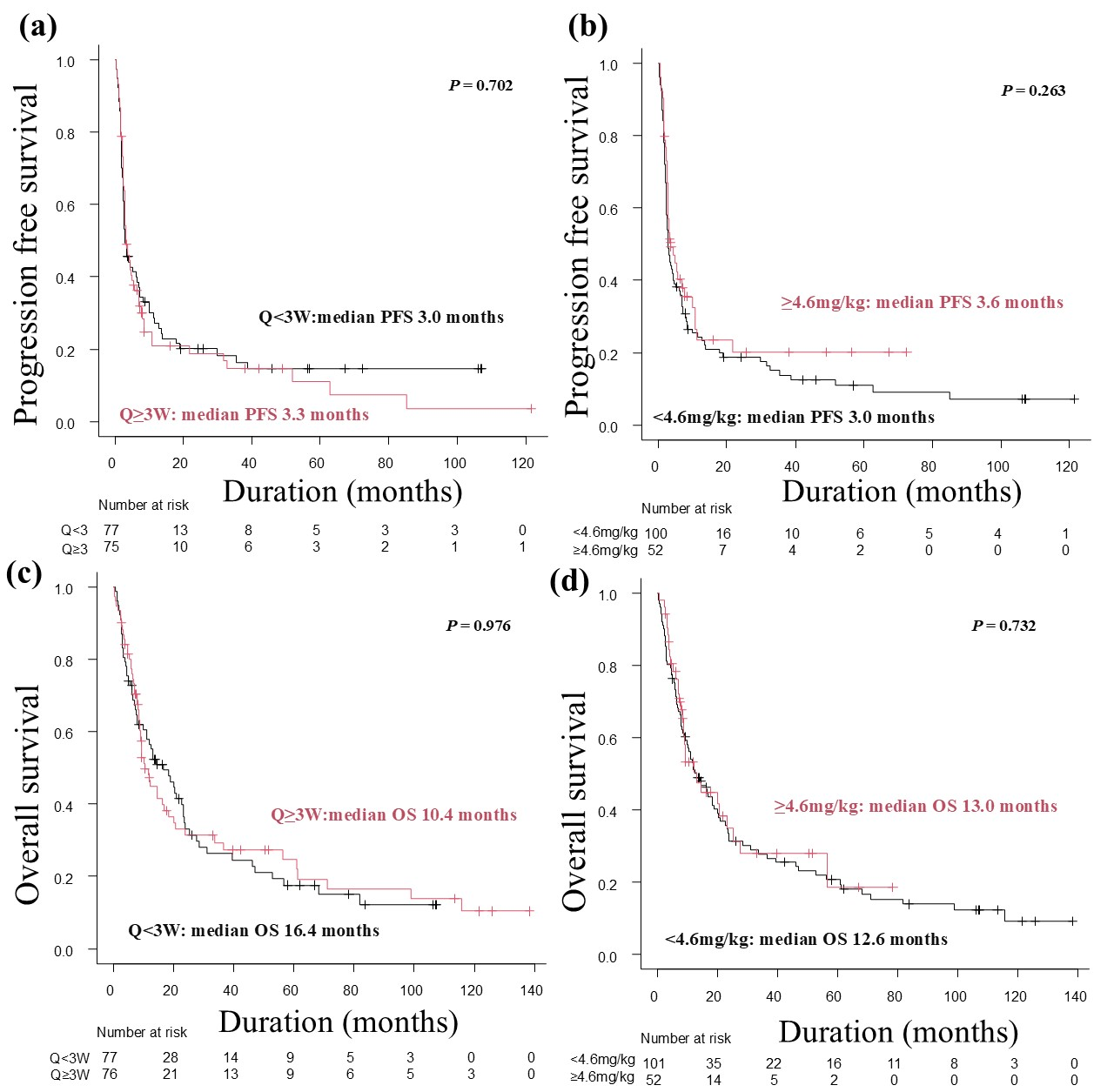
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Characteristic | 240mgQ2W | 480mgQ4W | 2mg/kgQ3W | 3mg/kgQ2W | ||
| Patients, n (%) | 40 (53.3) | 35 (46.7) | 41 (52.6) | 37 (47.4) | 0.908 | |
| Age, years, Median [range] | 69.5 [34.0, 92.0] | 67.0 [34.0, 84.0] | 0.718 | 66.0 [28.0, 88.0] | 66.0 [27.0, 87.0] | |
| Sex | 0.819 | 0.656 | ||||
| Male | 21 (52.5) | 17 (48.6) | 21 (51.2) | 21 (56.8) | ||
| Female | 19 (47.5) | 18 (51.4) | 20 (48.8) | 16 (43.2) | ||
| ECOG-PS | 0.364 | 0.553 | ||||
| 0–1 | 36 (90.0) | 34 (97.1) | 33 (80.5) | 32 (86.5) | ||
| ≥2 | 4 (10.0) | 1 (2.9) | 8 (19.5) | 5 (13.5) | ||
| Primary site | 0.853 | 0.459 | ||||
| Cutaneous | 16 (40.0) | 11 (31.4) | 19 (46.3) | 11 (29.7) | ||
| Acral | 8 (20.0) | 6 (17.1) | 4 (9.8) | 8 (21.6) | ||
| Mucosal | 13 (32.5) | 13 (37.1) | 13 (31.7) | 14 (37.8) | ||
| Uveal | 2 (5.0) | 3 (8.6) | 2 (4.9) | 1 (2.7) | ||
| Unknown | 1 (2.5) | 2 (5.7) | 3 (7.3) | 3 (8.1) | ||
| Details of stage Ⅳ | 0.542 | 0.622 | ||||
| M1a | 7 (17.5) | 7 (20.0) | 6 (14.6) | 6 (16.2) | ||
| M1b | 9 (22.5) | 4 (11.4) | 4 (9.8) | 7 (18.9) | ||
| M1c | 21 (52.5) | 19 (54.3) | 27 (65.9) | 22 (59.5) | ||
| M1d | 3 (7.5) | 5 (14.3) | 4 (9.8) | 2 (5.4) | ||
| LDH value | 0.252 | |||||
| <ULN | 24 (60.0) | 16 (45.7) | 18 (43.9) | 17 (45.9) | ||
| ≥ULN | 16 (40.0) | 19 (54.3) | 23 (56.1) | 20 (54.1) | ||
| BRAF | 0.158 | 0.031 * | ||||
| Mutant | 3 (7.5) | 8 (22.9) | 7 (17.1) | 5 (13.5) | ||
| Wild | 31 (77.5) | 24 (68.6) | 16 (39.0) | 25 (67.6) | ||
| Not investigated | 6 (15.0) | 3 (8.6) | 18 (43.9) | 7 (18.9) | ||
| Number of organs involved | 0.795 | 0.191 | ||||
| 1 | 21 (52.5) | 16 (45.7) | 16 (39.0) | 20 (54.1) | ||
| 2 | 9 (22.5) | 10 (28.6) | 14 (34.1) | 6 (16.2) | ||
| ≥3 | 10 (25.0) | 9 (25.7) | 11 (26.8) | 11 (29.7) | ||
| Surgery for primary site | 0.611 | 0.812 | ||||
| Yes | 30 (75.0) | 24 (68.6) | 28 (68.3) | 24 (64.9) | ||
| Adjuvant therapy | 1.000 | 0.066 | ||||
| Yes | 10 (25.0) | 8 (22.9) | 13 (31.7) | 5 (13.5) | ||
| Number of previous treatment lines (for metastatic/unresectable) | 0.476 | 0.882 | ||||
| 0 | 26 (65.0) | 18 (51.4) | 36 (87.8) | 32 (86.5) | ||
| 1 | 11 (27.5) | 13 (37.1) | 4 (9.8) | 3 (8.1) | ||
| ≥2 | 3 (7.5) | 4 (11.4) | 1 (2.4) | 2 (5.4) | ||
| Previous systematic therapy | ||||||
| BRAF/MEK inhibitor | 1 (2.5) | 5 (14.3) | 0.092 | 0 (0) | 1 (2.7) | 0.474 |
| Immune-checkpoint inhibitor | 14 (35.0) | 15 (42.9) | 0.635 | 3 (7.3) | 4 (10.8) | 0.702 |
| Cytotoxic anticancer drugs | 0 (0) | 1 (2.9) | 0.467 | 3 (7.3) | 1 (2.7) | 0.617 |
| Median follow-up time (months) [IQR] | 12.8 [4.5–25.5] | 8.8 [5.8–13.4] | 0.276 | 10.4 [5.9–42.4] | 14.2 [5.1–23.8] | 0.837 |
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Characteristic | Q < 3W | Q ≥ 3W | <4.6mg/kg | ≥4.6mg/kg | ||
| Patients, n (%) | 77 (50.3) | 76 (49.7) | 101 (66.0) | 52 (34.0) | 0.304 | |
| Age, years, Median [range] | 68.0 [27.0, 92.0] | 67.0 [28.0, 88.0] | 0.693 | 68.0 [27.0, 88.0] | 68.0 [34.0, 92.0] | |
| Sex | 0.629 | 0.174 | ||||
| Male | 42 (54.5) | 38 (50.0) | 57 (56.4) | 23 (44.2) | ||
| Female | 35 (45.5) | 38 (50.0) | 44 (43.6) | 29 (55.8) | ||
| ECOG-PS | 1.000 | 0.118 | ||||
| 0–1 | 68 (88.3) | 67 (88.2) | 86 (85.1) | 49 (94.2) | ||
| ≥2 | 9 (11.7) | 9 (11.8) | 15 (14.9) | 3 (5.8) | ||
| Primary site | 0.717 | 0.711 | ||||
| Cutaneous | 27 (35.1) | 30 (39.5) | 40 (39.6) | 17 (32.7) | ||
| Acral | 16 (20.8) | 10 (13.2) | 17 (16.8) | 9 (17.3) | ||
| Mucosal | 27 (35.1) | 26 (34.2) | 33 (32.7) | 20 (38.5) | ||
| Uveal | 3 (3.9) | 5 (6.6) | 4 (4.0) | 4 (7.7) | ||
| Unknown | 4 (5.2) | 5 (6.6) | 7 (6.9) | 2 (3.8) | ||
| Details of stage Ⅳ | 0.275 | 0.285 | ||||
| M1a | 13 (16.9) | 13 (16.9) | 17 (16.8) | 9 (17.3) | ||
| M1b | 16 (20.8) | 8 (10.5) | 16 (15.8) | 8 (15.4) | ||
| M1c | 43 (55.8) | 46 (60.5) | 62 (61.4) | 27 (51.9) | ||
| M1d | 5 (6.5) | 9 (11.8) | 6 (5.9) | 8 (15.4) | ||
| LDH value | 0.333 | 0.614 | ||||
| <ULN | 41 (53.2) | 34 (44.7) | 48 (47.5) | 27 (51.9) | ||
| ≥ULN | 36 (46.8) | 42 (55.3) | 53 (52.5) | 25 (48.1) | ||
| BRAF | 0.036 * | 0.354 | ||||
| Mutant | 8 (10.4) | 15 (19.7) | 14 (13.9) | 9 (17.3) | ||
| Wild | 56 (72.7) | 40 (52.6) | 61 (60.4) | 35 (67.3) | ||
| Not investigated | 13 (16.9) | 21 (27.6) | 26 (25.7) | 8 (15.4) | ||
| Number of organs involved | 0.202 | 0.837 | ||||
| 1 | 41 (53.2) | 32 (42.1) | 50 (49.5) | 23 (44.2) | ||
| 2 | 15 (19.5) | 24 (31.6) | 25 (24.8) | 14 (26.9) | ||
| ≥3 | 21 (27.3) | 20 (26.3) | 26 (25.7) | 15 (28.8) | ||
| Surgery for primary site | 0.862 | 0.853 | ||||
| Yes | 54 (70.1) | 52 (68.4) | 69 (68.3) | 37 (71.2) | ||
| Adjuvant therapy | 0.258 | 0.547 | ||||
| Yes | 15 (19.5) | 21 (27.6) | 22 (21.8) | 14 (26.9) | ||
| Number of previous treatment lines (for metastatic/unresectable) | 0.851 | <0.001 * | ||||
| 0 | 58 (75.3) | 54 (71.1) | 84 (83.2) | 28 (53.8) | ||
| 1 | 14 (18.2) | 17 (22.4) | 11 (10.9) | 20 (38.5) | ||
| ≥2 | 5 (6.5) | 5 (6.6) | 6 (5.9) | 4 (7.7) | ||
| Previous systematic therapy | ||||||
| BRAF/MEK inhibitor | 2 (2.6) | 5 (6.6) | 0.276 | 2 (2.0) | 5 (9.6) | 0.045 |
| Immune-checkpoint inhibitor | 18 (23.4) | 18 (23.7) | 1.000 | 14 (13.9) | 22 (42.3) | <0.001 |
| Cytotoxic anticancer drugs | 1 (1.3) | 4 (5.3) | 0.209 | 4 (4.0) | 1 (1.9) | 0.662 |
| Median follow-up time (months) [IQR] | 13.1 [5.0–25.2] | 9.3 [5.9–21.3] | 0.602 | 12.6 [5.6–31.2] | 8.8 [5.2–20.3] | 0.252 |
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| 240mgQ2W n = 40 | 480mgQ4W n = 35 | 2mg/kgQ3W n = 41 | 3mg/kgQ2W n = 37 | |||
| Best overall response | 0.095 | 0.228 | ||||
| Complete response | 1 (2.5) | 2 (5.7) | 0 (0.0) | 2 (5.4) | ||
| Partial response | 10 (25.0) | 2 (5.7) | 6 (14.6) | 7 (18.9) | ||
| Stable response | 8 (20.0) | 11 (31.4) | 13 (31.7) | 6 (16.2) | ||
| Progressive disease | 21 (52.5) | 20 (57.1) | 22 (53.7) | 22 (59.5) | ||
| Objective response rate | 27.5% | 11.4% | 0.082 | 14.6% | 24.3% | 0.278 |
| Disease control rate | 47.5% | 42.8% | 0.687 | 46.3% | 40.5% | 0.606 |
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Q < 3W n = 77 | Q ≥ 3W n = 76 | <4.6mg/kg n= 101 | ≥4.6mg/kg n = 52 | |||
| Best overall response | 0.103 | 0.446 | ||||
| Complete response | 3 (3.9) | 2 (2.6) | 2 (2.0) | 3 (5.8) | ||
| Partial response | 17 (22.1) | 8 (10.5) | 19 (18.8) | 6 (11.5) | ||
| Stable response | 14 (18.2) | 24 (31.6) | 24 (23.8) | 14 (26.9) | ||
| Progressive disease | 43 (55.8) | 42 (55.3) | 56 (55.4) | 29 (55.8) | ||
| Objective response rate | 26.0% | 13.1% | 0.045 * | 20.8% | 17.3% | 0.607 |
| Disease control rate | 44.2% | 44.7% | 0.910 | 44.6% | 44.2% | 0.970 |
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| 240mgQ2W n = 37 | 480mgQ4W n = 31 | 2mg/kgQ3W n = 41 | 3mg/kgQ2W n = 36 | |||
| Reason for discontinuing nivolumab | 0.663 | 0.108 | ||||
| Complete response | 1 (2.7) | 1 (3.2) | 0 (0.0) | 1 (2.8) | ||
| Immune-related adverse events | 5 (13.5) | 2 (6.5) | 8 (19.5) | 4 (11.1) | ||
| Progressive disease or patient deterioration | 31 (83.8) | 27 (87.1) | 33 (80.5) | 28 (77.8) | ||
| Patient decision | 0 (0.0) | 1 (3.2) | 0 (0.0) | 3 (8.3) | ||
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| 240mgQ2W n = 40 | 480mgQ4W n = 35 | 2mg/kgQ3W n = 41 | 3mg/kgQ2W n = 37 | |||
| 6-month PFS rate | 17 (42.5) | 12 (34.3) | 0.487 | 13 (31.7) | 13 (35.1) | 0.812 |
| 12-month PFS rate | 11 (27.5) | 4 (11.4) | 0.147 | 7 (17.1) | 8 (21.6) | 0.775 |
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value * | Hazard Ratio | 95% CI | p-Value * | |
| Age | 1.009 | 0.994–1.025 | 0.249 | 1.013 | 0.996–1.030 | 0.145 |
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 1.168 | 0.806–1.693 | 0.412 | 1.350 | 0.912–1.999 | 0.133 |
| Primary site | ||||||
| Cutaneous | Reference | Reference | ||||
| Non-cutaneous | 1.283 | 0.818–2.014 | 0.278 | 1.628 | 1.032–2.568 | 0.036 |
| BRAF | ||||||
| Wild | Reference | Reference | ||||
| Mutant | 1.002 | 0.528–1.901 | 0.996 | 0.619 | 0.325–1.179 | 0.145 |
| Not investigated | 0.828 | 0.511–1.343 | 0.445 | 1.060 | 0.656–1.711 | 0.813 |
| Nivolumab regimens | ||||||
| 240mgQ2W | Reference | Reference | ||||
| 480mgQ4W | 0.959 | 0.561–1.642 | 0.881 | 1.028 | 0.572–1.847 | 0.928 |
| 2mg/kgQ3W | 1.460 | 0.848–2.513 | 0.172 | 1.316 | 0.758–2.287 | 0.329 |
| 3mg/kgQ2W | 1.282 | 0.761–2.160 | 0.350 | 1.299 | 0.759–2.222. | 0.3294 |
| Nivolumab treatment line | ||||||
| 1st line | Reference | Reference | ||||
| 2nd and subsequent lines | 1.647 | 0.975–2.782 | 0.062 | 2.388 | 1.435–3.974 | <0.001 |
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| 240mgQ2W n = 40 | 480mgQ4W n = 35 | 2mg/kgQ3W n = 41 | 240mgQ2W n = 40 | |||
| Any-grade immune-related adverse events | 0.819 | 1.000 | ||||
| Yes | 17 (42.5) | 16 (45.7) | 19 (46.3) | 18 (48.6) | ||
| No | 23 (57.5) | 19 (54.3) | 22 (53.7) | 19 (51.4) | ||
| ≥3 grade immune-related adverse events | 0.618 | 0.356 | ||||
| Yes | 3 (7.5) | 1 (2.9) | 8 (19.5) | 4 (10.8) | ||
| No | 37 (92.5) | 34 (97.1) | 33 (80.5) | 33 (89.2) | ||
| Patient Group (%) | p-Value | Patient Group (%) | p-Value | |||
|---|---|---|---|---|---|---|
| Q < 3W n = 77 | Q ≥ 3W n = 76 | <4.6mg/kg n = 101 | ≥4.6mg/kg n = 52 | |||
| Any-grade immune-related adverse events | 1.000 | 0.864 | ||||
| Yes | 35 (45.5) | 35 (46.1) | 47 (46.5) | 23 (44.2) | ||
| No | 42 (54.5) | 41 (53.9) | 54 (53.5) | 29 (55.8) | ||
| ≥3 grade immune-related adverse events | 0.608 | 0.265 | ||||
| Yes | 7 (9.1) | 9 (11.8) | 13 (12.9) | 3 (5.8) | ||
| No | 70 (90.9) | 67 (88.2) | 88 (87.1) | 49 (94.2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horisaki, K.; Yoshikawa, S.; Omata, W.; Tsutsumida, A.; Kiyohara, Y. The Real-World Efficacy and Side Effects of Different Nivolumab Regimens in Japanese Patients with Advanced Melanoma: A Single-Center Retrospective Study. Cancers 2025, 17, 2299. https://doi.org/10.3390/cancers17142299
Horisaki K, Yoshikawa S, Omata W, Tsutsumida A, Kiyohara Y. The Real-World Efficacy and Side Effects of Different Nivolumab Regimens in Japanese Patients with Advanced Melanoma: A Single-Center Retrospective Study. Cancers. 2025; 17(14):2299. https://doi.org/10.3390/cancers17142299
Chicago/Turabian StyleHorisaki, Ken, Shusuke Yoshikawa, Wataru Omata, Arata Tsutsumida, and Yoshio Kiyohara. 2025. "The Real-World Efficacy and Side Effects of Different Nivolumab Regimens in Japanese Patients with Advanced Melanoma: A Single-Center Retrospective Study" Cancers 17, no. 14: 2299. https://doi.org/10.3390/cancers17142299
APA StyleHorisaki, K., Yoshikawa, S., Omata, W., Tsutsumida, A., & Kiyohara, Y. (2025). The Real-World Efficacy and Side Effects of Different Nivolumab Regimens in Japanese Patients with Advanced Melanoma: A Single-Center Retrospective Study. Cancers, 17(14), 2299. https://doi.org/10.3390/cancers17142299






