Preliminary Experience with Electronic Brachytherapy in the Treatment of Locally Advanced Cervical Carcinoma
Simple Summary
Abstract
1. Introduction
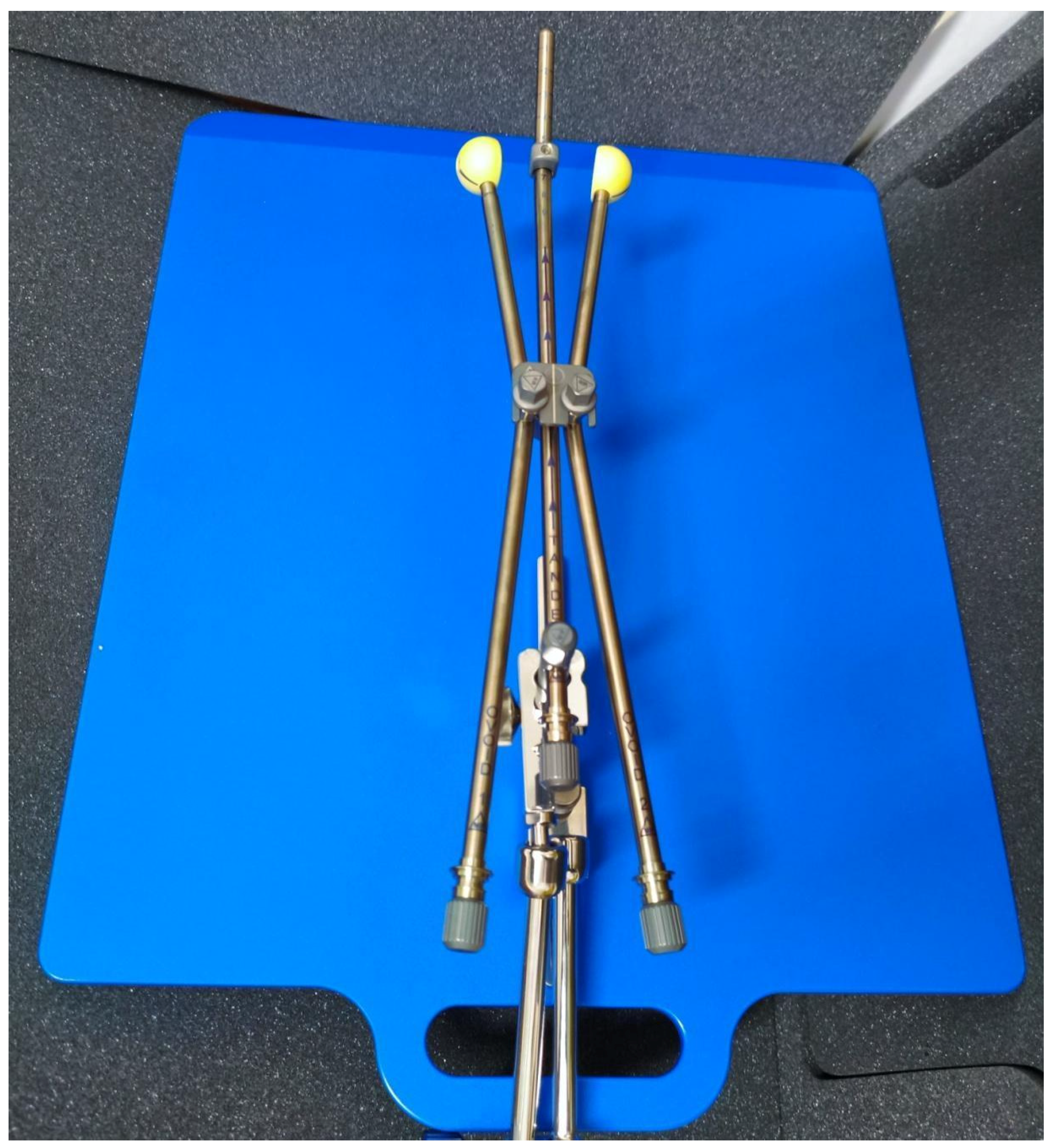
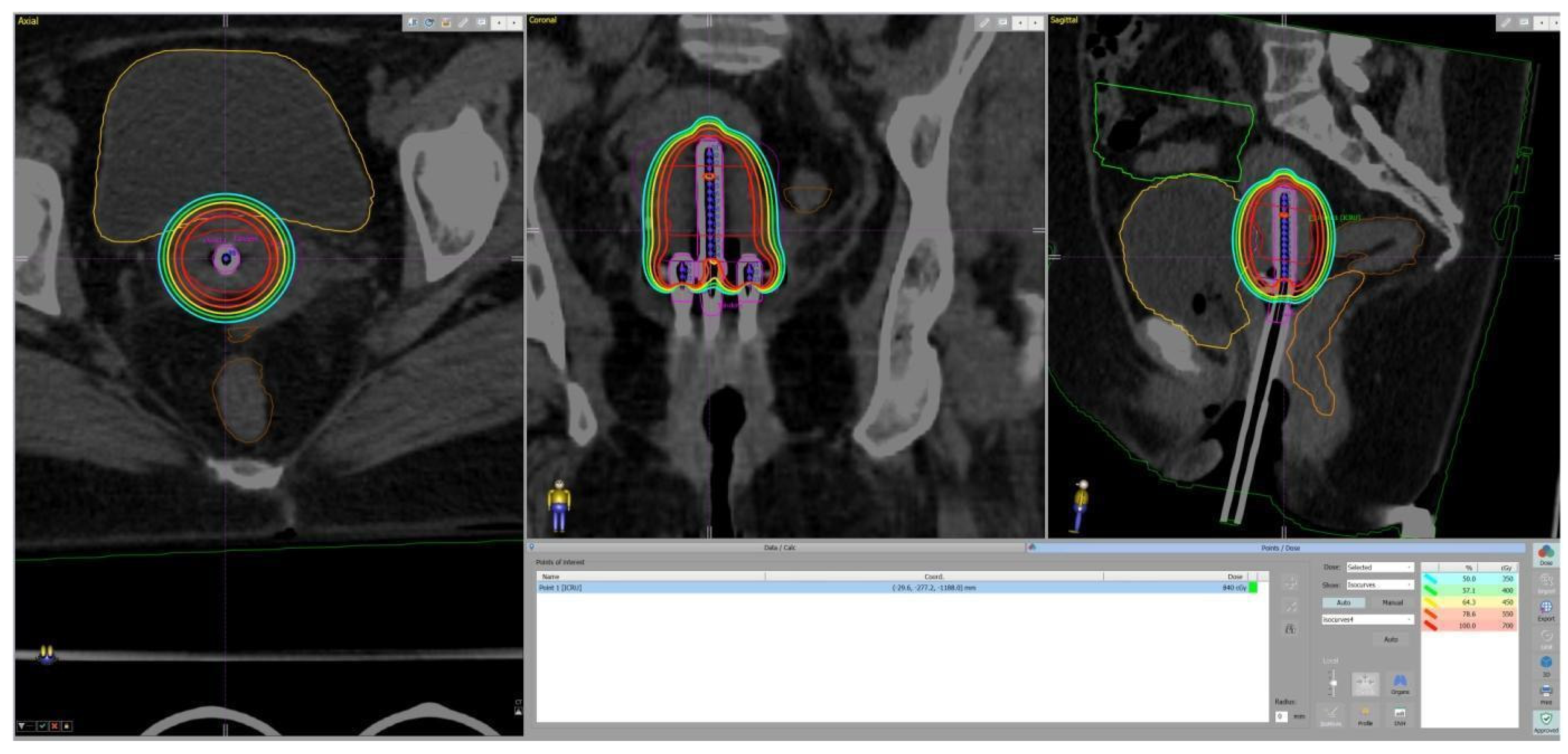
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 26 May 2024).
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Virchows Archiv 2023, 482, 935–966. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines® insights: Cervical cancer, version 1.2024: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2023, 21, 1224–1233. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef]
- Tanderup, K.; Fokdal, L.U.; Sturdza, A.; Haie-Meder, C.; Mazeron, R.; Van Limbergen, E.; Jürgenliemk-Schulz, I.; Petric, P.; Hoskin, P.; Dörr, W.; et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother. Oncol. 2016, 120, 441–446. [Google Scholar] [CrossRef]
- Swain, M.; Budrukkar, A.; Rembielak, A.; Kron, T.; Agarwal, J.P. Challenges in the sustainability of brachytherapy service in contemporary radiotherapy. Clin. Oncol. 2023, 35, 489–496. [Google Scholar] [CrossRef]
- Guedea, F.; Venselaar, J.; Hoskin, P.; Hellebust, T.P.; Peiffert, D.; Londres, B.; Ventura, M.; Mazeron, J.J.; Van Limbergen, E.; Pötter, R.; et al. Patterns of care for brachytherapy in Europe: Updated results. Radiother. Oncol. 2010, 97, 514–520. [Google Scholar] [CrossRef]
- Guedea, F.; Ellison, T.; Venselaar, J.; Borras, J.M.; Hoskin, P.; Poetter, R.; Heeren, G.; Nisin, R.; François, G.; Mazeron, J.J.; et al. Overview of brachytherapy resources in Europe: A survey of patterns of care study for brachytherapy in Europe. Radiother. Oncol. 2007, 82, 50–54. [Google Scholar] [CrossRef]
- Hadjieva, T. Pattern of radiotherapy care in Bulgaria. Rep. Pract. Oncol. Radiother. 2015, 20, 340–350. [Google Scholar] [CrossRef]
- Available online: https://dirac.iaea.org/Query/Countries (accessed on 13 May 2025).
- Ramachandran, P. New era of electronic brachytherapy. World J. Radiol. 2017, 9, 148. [Google Scholar] [CrossRef]
- Lozares-Cordero, S.; Font-Gómez, J.A.; Gandía-Martínez, A.; Miranda-Burgos, A.; Méndez-Villamón, A.; Villa-Gazulla, D.; Alba-Escorihuela, V.; Jiménez-Puertas, S.; González-Pérez, V. Treatment of cervical cancer with electronic brachytherapy. J. Appl. Clin. Med. Phys. 2019, 20, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Lozares-Cordero, S.; Gonzalez-Perez, V.; Pellejero-Pellejero, S.; Rodriguez-Ruiz, L.; Guinot-Rodriguez, J.L.; Villafranca-Iturre, E.; Méndez-Villamón, A.; Gandía-Martínez, A.; Fuentemilla-Urío, N.; Ruggeri, R. Feasibility of electronic brachytherapy in cervix cancer–A dosimetric comparison of different brachytherapy techniques. Brachytherapy 2022, 21, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, J.C.; Lang, S.; Kirisits, C.; Fidarova, E.F.; Berger, D.; Georg, P.; Dörr, W.; Pötter, R. Dose–volume histogram parameters and local tumor control in magnetic resonance image–guided cervical cancer brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.E.; Pötter, R.; Kossmeier, M.; Kirchheiner, K.; Mahantshetty, U.; Haie-Meder, C.; Lindegaard, J.C.; Jurgenliemk-Schulz, I.; Tan, L.T.; Hoskin, P.; et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with radiochemotherapy including image-guided brachytherapy: A retro-EMBRACE study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 168–177. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Cvek, J.; Randall, L.; de Santana Gomes, A.J.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 404, 1321–1332. [Google Scholar] [CrossRef]
- Kang, H.B.; Kim, S.H.; Lee, J.H.; Lee, H.C.; Kang, N.K.; Lee, J.H. MRI-based volumetric tumor parameters before and during chemoradiation predict tumor recurrence and patient survival in locally advanced cervical cancer: A subgroup analysis of a phase II prospective trial. Int. J. Clin. Oncol. 2024, 29, 620–628. [Google Scholar] [CrossRef]
- Skipar, K.; Hompland, T.; Lund, K.V.; Lindemann, K.; Hellebust, T.P.; Bruheim, K.; Lyng, H. MRI-guided dynamic risk assessment in cervical cancer based on tumor hypoxia at diagnosis and volume response at brachytherapy. Radiother. Oncol. 2024, 195, 110263. [Google Scholar] [CrossRef]
- Mazeron, R.; Castelnau-Marchand, P.; Dumas, I.; del Campo, E.R.; Kom, L.K.; Martinetti, F.; Farha, G.; Tailleur, A.; Morice, P.; Chargari, C.; et al. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother. Oncol. 2015, 114, 257–263. [Google Scholar] [CrossRef]
- Peters, M.; de Leeuw, A.A.; Nomden, C.N.; Tanderup, K.; Kirchheiner, K.; Lindegaard, J.C.; Kirisits, C.; Haie-Meder, C.; Sturdza, A.; Fokdal, L.; et al. Risk factors for nodal failure after radiochemotherapy and image guided brachytherapy in locally advanced cervical cancer: An EMBRACE analysis. Radiother. Oncol. 2021, 163, 150–158. [Google Scholar] [CrossRef]
- Song, S.; Kim, J.Y.; Kim, Y.J.; Yoo, H.J.; Kim, S.H.; Kim, S.K.; Lim, M.C.; Kang, S.; Seo, S.S.; Park, S.Y. The size of the metastatic lymph node is an independent prognostic factor for the patients with cervical cancer treated by definitive radiotherapy. Radiother. Oncol. 2013, 108, 168–173. [Google Scholar] [CrossRef]
- Vargo, J.A.; Kim, H.; Choi, S.; Sukumvanich, P.; Olawaiye, A.B.; Kelley, J.L.; Edwards, R.P.; Comerci, J.T.; Beriwal, S. Extended field intensity modulated radiation therapy with concomitant boost for lymph node–positive cervical cancer: Analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Ramlov, A.; Kroon, P.S.; Jürgenliemk-Schulz, I.M.; De Leeuw, A.A.; Gormsen, L.C.; Fokdal, L.U.; Tanderup, K.; Lindegaard, J.C. Impact of radiation dose and standardized uptake value of (18) FDG PET on nodal control in locally advanced cervical cancer. Acta Oncol. 2015, 54, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Ch, P.N.; Gurram, L.; Chopra, S.; Mahantshetty, U. The management of locally advanced cervical cancer. Curr. Opin. Oncol. 2018, 30, 323–329. [Google Scholar] [CrossRef]
- Schmid, M.P.; Franckena, M.; Kirchheiner, K.; Sturdza, A.; Georg, P.; Dörr, W.; Pötter, R. Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy. Gynecol. Oncol. 2014, 133, 256–262. [Google Scholar] [CrossRef]
- Duska, L.R.; Petroni, G.R.; Thaker, P.H.; Crane, E.K.; Holman, L.L.; Armstrong, D.K.; Romano, K.; Scalici, J. Update on safety and feasibility of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer. Cancer 2025, 131, e35757. [Google Scholar] [CrossRef]
- Tanderup, K.; Nesvacil, N.; Kirchheiner, K.; Serban, M.; Spampinato, S.; Jensen, N.B.; Schmid, M.; Smet, S.; Westerveld, H.; Ecker, S.; et al. Evidence-based dose planning aims and dose prescription in image-guided brachytherapy combined with radiochemotherapy in locally advanced cervical cancer. In Seminars in Radiation Oncology; WB Saunders: Philadelphia, PA, USA, 2020; Volume 30, pp. 311–327. [Google Scholar] [CrossRef]
- Spampinato, S.; Fokdal, L.U.; Pötter, R.; Haie-Meder, C.; Lindegaard, J.C.; Schmid, M.P.; Sturdza, A.; Jürgenliemk-Schulz, I.M.; Mahantshetty, U.; Segedin, B.; et al. Risk factors and dose-effects for bladder fistula, bleeding and cystitis after radiotherapy with imaged-guided adaptive brachytherapy for cervical cancer: An EMBRACE analysis. Radiother. Oncol. 2021, 158, 312–320. [Google Scholar] [CrossRef]
- Spampinato, S.; Fokdal, L.U.; Pötter, R.; Haie-Meder, C.; Lindegaard, J.C.; Schmid, M.P.; Sturdza, A.; Jürgenliemk-Schulz, I.M.; Mahantshetty, U.; Segedin, B.; et al. Importance of the ICRU bladder point dose on incidence and persistence of urinary frequency and incontinence in locally advanced cervical cancer: An EMBRACE analysis. Radiother. Oncol. 2021, 158, 300–308. [Google Scholar] [CrossRef]
- Van Dyk, S.; Khaw, P.; Lin, M.Y.; Chang, D.; Bernshaw, D. Ultrasound-guided brachytherapy for cervix cancer. Clin. Oncol. 2021, 33, e403–e411. [Google Scholar] [CrossRef]
- Manea, E.; Chitoran, E.; Rotaru, V.; Ionescu, S.; Luca, D.; Cirimbei, C.; Alecu, M.; Capsa, C.; Gafton, B.; Prutianu, I.; et al. Integration of Ultrasound in Image-Guided Adaptive Brachytherapy in Cancer of the Uterine Cervix. Bioengineering 2024, 11, 506. [Google Scholar] [CrossRef]
- Mazeron, R.; Fokdal, L.U.; Kirchheiner, K.; Georg, P.; Jastaniyah, N.; Šegedin, B.; Mahantshetty, U.; Hoskin, P.; Jürgenliemk-Schulz, I.; Kirisits, C.; et al. Dose–volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: Results from the prospective multicenter EMBRACE study. Radiother. Oncol. 2016, 120, 412–419. [Google Scholar] [CrossRef]
- Jensen, N.B.; Pötter, R.; Spampinato, S.; Fokdal, L.U.; Chargari, C.; Lindegaard, J.C.; Schmid, M.P.; Sturdza, A.; Jürgenliemk-Schulz, I.M.; Mahantshetty, U.; et al. Dose-volume effects and risk factors for late diarrhea in cervix cancer patients after radiochemotherapy with image guided adaptive brachytherapy in the EMBRACE I study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 688–700. [Google Scholar] [CrossRef]
- Vittrup, A.S.; Kirchheiner, K.; Pötter, R.; Fokdal, L.U.; Jensen, N.B.; Spampinato, S.; Haie-Meder, C.; Schmid, M.P.; Sturdza, A.E.; Mahantshetty, U.; et al. Overall severe morbidity after chemo-radiation therapy and magnetic resonance imaging-guided adaptive brachytherapy in locally advanced cervical cancer: Results from the EMBRACE-I study. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 807–824. [Google Scholar] [CrossRef]
| Patient | Histology | TNM Stage | FIGO 2018 Stage | Number of Lymph Node Metastases | Short-Axis Diameter of the Largest Node (cm) |
|---|---|---|---|---|---|
| 1 | KSCC G2 | T4N1 | IVA | 4 | 1 |
| 2 | NKSCC G2 | T4N0 | IVA | 0 | |
| 3 | KSCC G2 | T2aN0 | IIA | 0 | |
| 4 | NKSCC G2 | T2bN1 | IIIC1 | 6 | 2 |
| 5 | NKSCC G2 | T1b3N0 | IB3 | 0 | |
| 6 | KSCC G2 | T3bN0 | IIIB | 0 | |
| 7 | NKSCC G2 | T3bN0 | IIIB | 0 | |
| 8 | NKSCC G2 | T2bN1 | IIIC1 | 8 | 2.8 |
| 9 | NKSCC G2 | T2bN0 | IIB | 0 | |
| 10 | NKSCC G2 | T3bN1 | IIIC2r | 11 | 2.8 |
| 11 | NKSCC G2 | T2bN1 | IIIC1 | 2 | 1 |
| 12 | NKSCC G2 | T3bN1 | IIIC2r | 8 | 1.9 |
| 13 | KSCC G2 | T2bN0 | IIB | 0 | |
| 14 | KSCC G2 | T2bN1 | IIIC1 | 2 | 0.8 |
| 15 | KSCC G2 | T2bN0 | IIB | 4 | 1.4 |
| 16 | KSCC G2 | T2aN1 | IIIC1 | 3 | 2.2 |
| 17 | NKSCC G3 | T2bN1 | IIIC1 | 2 | 2 |
| 18 | NKSCC G2 | T2bN0 | IIB | 0 | |
| 19 | NKSCC G2 | T2bN1 | IIIC1 | 2 | 0.9 |
| 20 | NKSCC G2 | T2bN0 | IIB | 0 | |
| 21 | NKSCC G3 | T2bN0 | IIB | 0 | |
| 22 | NKSCC G3 | T2aN0 | IIA | 0 | |
| 23 | STCC | T2bN0 | IIB | 0 | |
| 24 | NKSCC G3 | T4N0 | IVA | 0 | |
| 25 | NKSCC G2 | T2aN1 | IIIC1 | 5 | 1.5 |
| Structure | Dose Constraint |
|---|---|
| CTV HR | D90% > 700 cGy |
| CTV HR | D95% > 585 cGy |
| CTV HR | D90% < 840 cGy |
| Bladder | D2cc ≤ 550 cGy |
| Rectum | D2cc ≤ 400 cGy |
| Sigmoid | D2cc ≤ 450 cGy |
| n | CTV HR V700cGy (%) | CTV HR D90 EQD2 (Gy) | Bladder D2cc (Gy) | Bladder D2cc EQD2 (Gy) | Rectum D2cc (Gy) | Rectum D2cc EQD2 (Gy) | Sigmoid D2cc (Gy) | Sigmoid D2cc EQD2 (Gy) |
|---|---|---|---|---|---|---|---|---|
| 1 | 90 | 86 | 6.20 | 92.8 | 8.01 | 115.9 | 7.03 | 102.7 |
| 2 | 92 | 89 | 5.23 | 81.2 | 3.13 | 62.6 | 4.52 | 73.2 |
| 3 | 93 | 89 | 4.95 | 77.8 | 1.89 | 53.9 | 4.11 | 68.4 |
| 4 | 88 | 86 | 9.78 | 147.9 | 2.83 | 59.4 | 2.68 | 57.2 |
| 5 | 91 | 88 | 5.62 | 85.0 | 3.03 | 60.2 | 4.29 | 70.3 |
| 6 | 93 | 89 | 3.30 | 61.6 | 4.91 | 76.9 | 3.64 | 63.9 |
| 7 | 95 | 91 | 2.88 | 59.9 | 3.85 | 67.8 | 3.73 | 66.7 |
| 8 | 96 | 92 | 4.29 | 71.1 | 2.66 | 58.6 | 2.51 | 58.5 |
| 9 | 93 | 89 | 3.91 | 68.3 | 1.88 | 51.6 | 4.99 | 76.6 |
| 10 | 96 | 91 | 4.10 | 71.9 | 2.80 | 58.8 | 2.55 | 57.1 |
| 11 | 95 | 91 | 3.68 | 66.5 | 1.55 | 51.6 | 2.32 | 56.4 |
| 12 | 94 | 90 | 2.97 | 59.8 | 2.30 | 55.6 | 3.14 | 55.7 |
| 13 | 97 | 92 | 2.39 | 56.1 | 7.5 | 48.4 | 2.52 | 56.0 |
| 14 | 93 | 88 | 5.34 | 82.4 | 1.29 | 48.7 | 1.71 | 51.2 |
| 15 | 93 | 89 | 5.14 | 80.0 | 1.16 | 49.6 | 1.57 | 51.3 |
| 16 | 95 | 90 | 3.71 | 66.1 | 0.58 | 46.1 | 2.32 | 55.9 |
| 17 | 94 | 90 | 4.13 | 70.6 | 1.50 | 51.9 | 1.04 | 47.9 |
| 18 | 95 | 91 | 3.95 | 69.5 | 1.82 | 53.8 | 4.03 | 68.0 |
| 19 | 97 | 93 | 3.22 | 62.2 | 0.65 | 47.8 | 2.03 | 48.7 |
| 20 | 94 | 89 | 4.26 | 71.4 | 1.14 | 48.8 | 2.42 | 52.9 |
| 21 | 97 | 93 | 3.51 | 64.4 | 1.89 | 51.8 | 2.69 | 51.0 |
| 22 | 95 | 91 | 2.45 | 57.7 | 0.39 | 46.1 | 1.48 | 46.2 |
| 23 | 97 | 93 | 2.85 | 60.2 | 2.04 | 53.9 | 2.03 | 41.0 |
| 24 | 93 | 89 | 5.05 | 80.7 | 2.63 | 59.3 | 2.96 | 61.6 |
| 25 | 96 | 91 | 4.43 | 72.0 | 2.11 | 53.9 | 1.89 | 51.5 |
| Patient Number | Event | Time to Event Detection | Treatment | Outcome |
|---|---|---|---|---|
| 4 | boosted lymph node persistence | 3 months | chemotherapy, stereotactic radiotherapy | partial response, developed bone metastasis 18 months after treatment |
| 7 | local failure, liver metastases | 3 months | chemotherapy, stereotactic radiotherapy, immunotherapy | complete remission |
| 8 | lower third vaginal recurrence, cervical recurrence | 18 months | chemotherapy | stable disease |
| 10 | local failure, lymph node metastases outside the treatment field, lung metastases | 3 months | refused any treatment | death 20 months after treatment completion |
| 11 | local failure, lymph node metastases outside the treatment field | 2 months | refused immediate treatment | death 13.5 months after completion of treatment |
| 12 | lymph node metastases outside the treatment field | 9 months | chemotherapy, immunotherapy | complete remission |
| 16 | boosted lymph node persistence | 3 months | stereotactic radiotherapy, surgery | partial response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitova-Topkarova, D.; Payakova, V.; Yordanov, A.; Kostova-Lefterova, D.; Ivanova, M.; Iliev, I.; Valkov, M.; Mutkurov, N.; Kostov, S.; Encheva, E. Preliminary Experience with Electronic Brachytherapy in the Treatment of Locally Advanced Cervical Carcinoma. Cancers 2025, 17, 2286. https://doi.org/10.3390/cancers17142286
Hitova-Topkarova D, Payakova V, Yordanov A, Kostova-Lefterova D, Ivanova M, Iliev I, Valkov M, Mutkurov N, Kostov S, Encheva E. Preliminary Experience with Electronic Brachytherapy in the Treatment of Locally Advanced Cervical Carcinoma. Cancers. 2025; 17(14):2286. https://doi.org/10.3390/cancers17142286
Chicago/Turabian StyleHitova-Topkarova, Desislava, Virginia Payakova, Angel Yordanov, Desislava Kostova-Lefterova, Mirela Ivanova, Ilko Iliev, Marin Valkov, Nikolay Mutkurov, Stoyan Kostov, and Elitsa Encheva. 2025. "Preliminary Experience with Electronic Brachytherapy in the Treatment of Locally Advanced Cervical Carcinoma" Cancers 17, no. 14: 2286. https://doi.org/10.3390/cancers17142286
APA StyleHitova-Topkarova, D., Payakova, V., Yordanov, A., Kostova-Lefterova, D., Ivanova, M., Iliev, I., Valkov, M., Mutkurov, N., Kostov, S., & Encheva, E. (2025). Preliminary Experience with Electronic Brachytherapy in the Treatment of Locally Advanced Cervical Carcinoma. Cancers, 17(14), 2286. https://doi.org/10.3390/cancers17142286







