A Narrative Review of the Role of Non-Viral Circulating Tumor DNA Profiling in Predicting the Treatment Response and Recurrence in Head and Neck Squamous Cell Carcinoma
Simple Summary
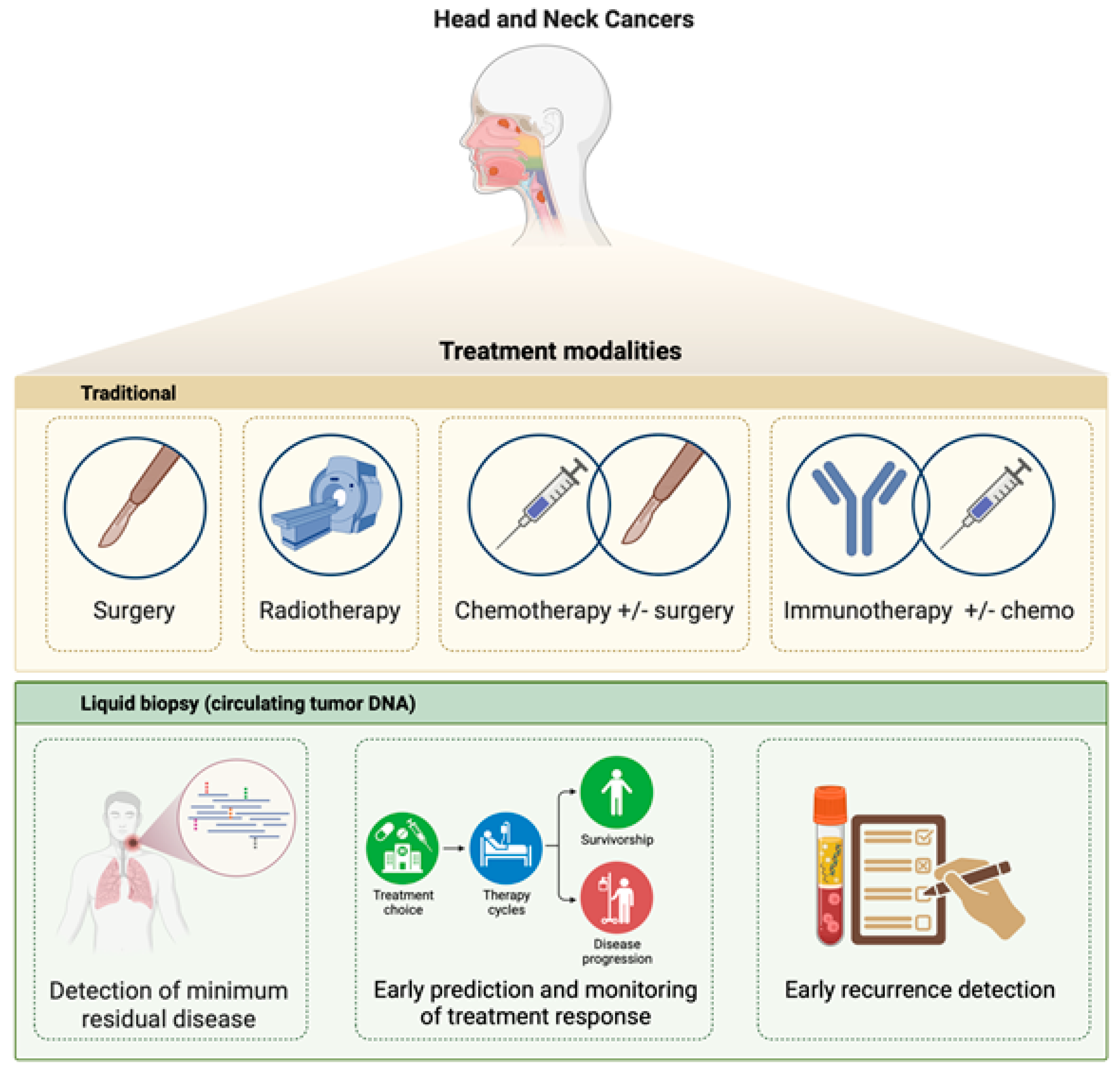
Abstract
1. Introduction
2. Liquid Biopsy
3. Circulating Tumor DNA Analysis in the Management of HNSCC Patients
3.1. Studies with Surgery Only
3.2. Studies with Radiotherapy Only
3.3. Studies with Multimodal Treatment Modality
3.4. Studies Including Immunotherapy
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vigneswaran, N.; Williams, M. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Economopoulou, P.; de Bree, R.; Kotsantis, I.; Psyrri, A. Diagnostic tumor markers in head and neck squamous cell carcinoma (HNSCC) in the clinical setting. Front. Oncol. 2019, 9, 827. [Google Scholar]
- Lo, T.; Wang, C.; Chen, C.; Yang, T.; Lou, P.; Ko, J.; Chang, Y.L.; Chen, T.C. Diagnostic performance of core needle biopsy for nodal recurrences in patients with head and neck squamous cell carcinoma. Sci. Rep. 2022, 12, 2048. [Google Scholar]
- Argiris, A.; Karamouzis, M.; Raben, D.; Ferris, R. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar]
- Pai, S.; Westra, W. Molecular pathology of head and neck cancer: Implications for diagnosis, prognosis, and treatment. Annu. Rev. Pathol. 2009, 4, 49–70. [Google Scholar] [PubMed]
- Pfister, D.; Spencer, S.; Brizel, D.; Burtness, B.; Busse, P.; Caudell, J.; Cmelak, A.; Colevas, A.; Dunphy, F.; Eisele, D.; et al. Head and neck cancers, Version 2.2014. Clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2014, 12, 1454–1487. [Google Scholar]
- Sahin, A.; Gilligan, T.; Caudell, J. Challenges with the 8th edition of the AJCC cancer staging manual for breast, testicular, and head and neck cancers. J. Natl. Compr. Canc. Netw. 2019, 17, 560–564. [Google Scholar]
- Wang, H.; Zheng, Z.; Zhang, Y.; Bian, C.; Bao, J.; Xin, Y.; Jiang, X. Locally advanced head and neck squamous cell carcinoma treatment efficacy and safety: A systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1269863. [Google Scholar]
- Bhat, G.; Hyole, R.; Li, J. Head and neck cancer: Current challenges and future perspectives. Adv. Cancer Res. 2021, 152, 67–102. [Google Scholar] [CrossRef]
- Pös, O.; Biró, O.; Szemes, T.; Nagy, B. Circulating cell-free nucleic acids: Characteristics and applications. Eur. J. Hum. Genet. 2018, 26, 937–945. [Google Scholar]
- Grabuschnig, S.; Bronkhorst, A.; Holdenrieder, S.; Rosales Rodriguez, I.; Schliep, K.; Schwendenwein, D.; Ungerer, V.; Sensen, C. Putative origins of cell-free DNA in humans: A review of active and passive nucleic acid release mechanisms. Int. J. Mol. Sci. 2020, 21, 8062. [Google Scholar]
- Dao, J.; Conway, P.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using cfDNA and ctDNA as oncologic markers: A path to clinical validation. Int. J. Mol. Sci. 2023, 24, 13219. [Google Scholar]
- Bellairs, J.; Hasina, R.; Agrawal, N. Tumor DNA: An emerging biomarker in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 515–523. [Google Scholar]
- Schwarzenbach, H.; Hoon, D.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [PubMed]
- McLaren, D.; Aitman, T. Redefining precision radiotherapy through liquid biopsy. Br. J. Cancer 2023, 129, 900–903. [Google Scholar] [PubMed]
- Telekes, A.; Horváth, A. The Role of Cell-Free DNA in Cancer Treatment Decision Making. Cancers 2022, 14, 6115. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.; Holdenrieder, S. The changing face of circulating tumor DNA (ctDNA) profiling: Factors that shape the landscape of methodologies, technologies, and commercialization. Med. Genet. 2023, 35, 201–235. [Google Scholar] [PubMed]
- Aulakh, S.; Silverman, D.; Young, K.; Dennis, S.; Birkeland, A. The Promise of Circulating Tumor DNA in Head and Neck Cancer. Cancers 2022, 14, 2968. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar]
- Mattox, A.K.; D’Souza, G.; Khan, Z.; Allen, H.; Henson, S.; Seiwert, T.Y.; Koch, W.; Pardoll, D.M.; Fakhry, C. Comparison of next generation sequencing, droplet digital PCR, and quantitative real-time PCR for the earlier detection and quantification of HPV in HPV-positive oropharyngeal cancer. Oral Oncol. 2022, 128, 105805. [Google Scholar] [PubMed]
- Nassar, S.I.; Suk, A.; Nguyen, S.A.; Adilbay, D.; Pang, J.; Nathan, C.O. The Role of ctDNA and Liquid Biopsy in the Diagnosis and Monitoring of Head and Neck Cancer: Towards Precision Medicine. Cancers 2024, 16, 3129. [Google Scholar] [CrossRef]
- Silvoniemi, A.; Laine, J.; Aro, K.; Nissi, L.; Bäck, L.; Schildt, J.; Hirvonen, J.; Hagström, J.; Irjala, H.; Aaltonen, L.M.; et al. Circulating tumor DNA in head and neck squamous cell carcinoma: Association with Metabolic Tumor Burden Determined with FDG-PET/CT. Cancers 2023, 15, 3970. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Myers, J.N. TP53 Mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J. Cell. Biochem. 2016, 117, 2682–2692. [Google Scholar] [PubMed]
- Wilson, H.L.; D’Agostino, R.B., Jr.; Meegalla, N.; Petro, R.; Commander, S.; Topaloglu, U.; Zhang, W.; Porosnicu, M. The Prognostic and therapeutic value of the mutational profile of blood and tumor tissue in head and neck squamous cell carcinoma. Oncologist 2021, 26, e279–e289. [Google Scholar]
- van Ginkel, J.H.; Huibers, M.M.H.; van Es, R.J.J.; de Bree, R.; Willems, S.M. Droplet digital PCR for detection and quantification of circulating tumor DNA in plasma of head and neck cancer patients. BMC Cancer 2017, 17, 428. [Google Scholar]
- Kampel, L.; Feldstein, S.; Tsuriel, S.; Hannes, V.; Neiderman, C.; Horowitz, G.; Warshavsky, A.; Leider-Trejo, L.; Hershkovitz, D.; Muhanna, N.; et al. Mutated TP53 in circulating tumor DNA as a risk level biomarker in head and neck squamous cell carcinoma patients. Biomolecules 2023, 13, 1418. [Google Scholar] [CrossRef]
- Wei, M.; Zhi, J.; Li, L.; Wang, W. Predicting therapeutic responses in head and neck squamous cell carcinoma from TP53 mutation detected by cell-free DNA. Transl. Cancer Res. 2023, 12, 3604–3617. [Google Scholar]
- Galot, R.; van Marcke, C.; Helaers, R.; Mendola, A.; Goebbels, R.M.; Caignet, X.; Ambroise, J.; Wittouck, K.; Vikkula, M.; Limaye, N.; et al. Liquid biopsy for mutational profiling of locoregional recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020, 104, 104631. [Google Scholar]
- Porter, A.; Natsuhara, M.; Daniels, G.A.; Patel, S.P.; Sacco, A.G.; Bykowski, J.; Banks, K.C.; Cohen, E.E.W. Next generation sequencing of cell free circulating tumor DNA in blood samples of recurrent and metastatic head and neck cancer patients. Transl. Cancer Res. 2020, 9, 203–209. [Google Scholar]
- Payne, K.F.B.; Brotherwood, P.; Suriyanarayanan, H.; Brooks, J.M.; Batis, N.; Beggs, A.D.; Gendoo, D.M.A.; Mehanna, H.; Nankivell, P. Circulating tumour DNA detects somatic variants contributing to spatial and temporal intra-tumoural heterogeneity in head and neck squamous cell carcinoma. Front. Oncol. 2024, 14, 1374816. [Google Scholar]
- Payne, K.; Suriyanarayanan, H.; Brooks, J.; Mehanna, H.; Nankivell, P.; Gendoo, D. Exploring the impact of intra-tumoural heterogeneity on liquid biopsy cell-free DNA methylation and copy number in head and neck squamous cell carcinoma. Oral Oncol. 2024, 158, 107011. [Google Scholar]
- Koukourakis, M.I.; Xanthopoulou, E.; Koukourakis, I.M.; Fortis, S.P.; Kesesidis, N.; Karakasiliotis, I.; Baxevanis, C.N. Circulating plasma cell-free DNA (cfDNA) as a predictive biomarker for radiotherapy: Results from a prospective trial in head and neck cancer. Cancer Diagn. Progn. 2023, 3, 551–557. [Google Scholar]
- Flach, S.; Howarth, K.; Hackinger, S.; Pipinikas, C.; Ellis, P.; McLay, K.; Marsico, G.; Forshew, T.; Walz, C.; Reichel, C.A.; et al. Liquid BIOpsy for MiNimal RESidual DiSease detection in head and meck squamous cell carcinoma (LIONESS)–a personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br. J. Cancer 2022, 126, 1186–1195. [Google Scholar]
- Marret, G.; Lamy, C.; Vacher, S.; Cabel, L.; Séné, M.; Ahmanache, L.; Courtois, L.; El Beaino, Z.; Klijanienko, J.; Martinat, C.; et al. Deciphering molecular relapse and intra-tumor heterogeneity in non-metastatic resectable head and neck squamous cell carcinoma using circulating tumor DNA. Oral Oncol. 2025, 160, 107111. [Google Scholar]
- Cho, W.K.; Lee, J.; Youn, S.M.; Oh, D.; Lim, D.H.; Yoon, H.G.; Cho, E.H.; Noh, J.M. Liquid biopsy using cfDNA to predict radiation therapy response in solid tumors. Radiat. Oncol. J. 2023, 41, 32–39. [Google Scholar] [PubMed]
- Janke, F.; Stritzke, F.; Dvornikovich, K.; Franke, H.; Angeles, A.K.; Riediger, A.L.; Ogrodnik, S.; Gerhardt, S.; Regnery, S.; Schröter, P.; et al. Early circulating tumor DNA changes predict outcomes in head and neck cancer patients under re-radiotherapy. Int. J. Cancer 2025, 156, 853–864. [Google Scholar] [PubMed]
- Lele, S.J.; Adilbay, D.; Lewis, E.; Pang, J.; Asarkar, A.A.; Nathan, C.O. ctDNA as an Adjunct to Posttreatment PET for Head and Neck Cancer Recurrence Risk Assessment. Otolaryngol. Head Neck Surg. 2024, 171, 439–444. [Google Scholar]
- Balázs, Z.; Balermpas, P.; Ivanković, I.; Willmann, J.; Gitchev, T.; Bryant, A.; Guckenberger, M.; Krauthammer, M.; Andratschke, N. Longitudinal cell-free DNA characterization by low-coverage whole-genome sequencing in patients undergoing high-dose radiotherapy. Radiother. Oncol. 2024, 197, 110364. [Google Scholar]
- Burgener, J.M.; Zou, J.; Zhao, Z.; Zheng, Y.; Shen, S.Y.; Huang, S.H.; Keshavarzi, S.; Xu, W.; Liu, F.F.; Liu, G.; et al. Tumor-naïve multimodal profiling of circulating tumor DNA in head and neck squamous cell carcinoma. Clin. Cancer Res. 2021, 27, 4230–4244. [Google Scholar]
- Orland, M.D.; Stewart, M.; Rajaram, S.N.; Kuzmanovic, T.; Karasik, N.; Campbell, S.R.; Miller, J.A.; Woody, N.M.; Silver, N.; Ku, J.; et al. Can circulating tumor DNA be utilized as a marker to guide high-risk head and neck squamous cell patients treatment? Int. J. Radiat. Oncol. Biol. Phys. 2024, 18, e33–e34. [Google Scholar]
- Chikuie, N.; Urabe, Y.; Ueda, T.; Hamamoto, T.; Taruya, T.; Kono, T.; Yumii, K.; Takeno, S. Utility of plasma circulating tumor DNA and tumor DNA profiles in head and neck squamous cell carcinoma. Sci. Rep. 2022, 12, 9316. [Google Scholar]
- Hilke, F.J.; Muyas, F.; Admard, J.; Kootz, B.; Nann, D.; Welz, S.; Rieß, O.; Zips, D.; Ossowski, S.; Schroeder, C.; et al. Dynamics of cell-free tumour DNA correlate with treatment response of head and neck cancer patients receiving radiochemotherapy. Radiother. Oncol. 2020, 151, 182–189. [Google Scholar]
- Sanz-Garcia, E.; Zou, J.; Avery, L.; Spreafico, A.; Waldron, J.; Goldstein, D.; Hansen, A.; Cho, B.C.J.; de Almeida, J.; Hope, A.; et al. Multimodal detection of molecular residual disease in high-risk locally advanced squamous cell carcinoma of the head and neck. Cell Death Differ. 2024, 31, 460–468. [Google Scholar]
- Kogo, R.; Manako, T.; Iwaya, T.; Nishizuka, S.; Hiraki, H.; Sasaki, Y.; Idogawa, M.; Tokino, T.; Koide, A.; Komune, N.; et al. Individualized circulating tumor DNA monitoring in head and neck squamous cell carcinoma. Cancer Med. 2022, 11, 3960–3968. [Google Scholar] [PubMed]
- Hanna, G.J.; Dennis, M.J.; Scarfo, N.; Mullin, M.S.; Sethi, R.K.V.; Sehgal, K.; Annino, D.J.; Goguen, L.A.; Haddad, R.I.; Tishler, R.B.; et al. Personalized ctDNA for monitoring disease status in head and neck squamous cell carcinoma. Clin. Cancer Res. 2024, 30, 3329–3336. [Google Scholar]
- Honoré, N.; van der Elst, A.; Dietz, A.; van Marcke, C.; Helaers, R.; Mendola, A.; Dahou, H.; Marbaix, E.; Poncin, R.; Seront, E.; et al. Tumour-agnostic plasma assay for circulating tumour DNA predicts outcome in recurrent and/or metastatic squamous cell carcinoma of the head and neck treated with a PD-1 inhibitor. Eur. J. Cancer 2023, 195, 113372. [Google Scholar]
- Koukourakis, M.I.; Xanthopoulou, E.; Koukourakis, I.M.; Fortis, S.P.; Kesesidis, N.; Kakouratos, C.; Karakasiliotis, I.; Baxevanis, C.N. Next-generation sequencing analysis of mutations in circulating tumor DNA from the plasma of patients with head-neck cancer undergoing chemo-radiotherapy using a pan-cancer cell-free assay. Curr. Oncol. 2023, 30, 8902–8915. [Google Scholar]
- Noji, R.; Tohyama, K.; Nakamura, S.; Naito, T.; Oikawa, Y.; Kuroshima, T.; Tomioka, H.; Michi, Y.; Ikeda, S.; Asakage, T.; et al. Dynamic changes in circulating tumor DNA during immunotherapy for head and neck cancer: SHIZUKU-HN study. Int. J. Mol. Sci. 2024, 26, 235. [Google Scholar]
- Taylor, K.; Zou, J.; Magalhaes, M.; Oliva, M.; Spreafico, A.; Hansen, A.R.; McDade, S.S.; Coyle, V.M.; Lawler, M.; Elimova, E.; et al. Circulating tumour DNA kinetics in recurrent/metastatic head and neck squamous cell cancer patients. Eur. J. Cancer 2023, 188, 29–38. [Google Scholar]
- Paolini, F.; Campo, F.; Iocca, O.; Manciocco, V.; De Virgilio, A.; De Pascale, V.; Moretto, S.; Dalfino, G.; Vidiri, A.; Blandino, G.; et al. It is time to improve the diagnostic workup of oropharyngeal cancer with circulating tumor HPV DNA: Systematic review and meta-analysis. Head Neck 2023, 45, 2945–2954. [Google Scholar] [PubMed]
- Campo, F.; Iocca, O.; Paolini, F.; Manciocco, V.; Moretto, S.; De Virgilio, A.; Moretti, C.; Vidiri, A.; Venuti, A.; Bossi, P.; et al. The landscape of circulating tumor HPV DNA and TTMV-HPV DNA for surveillance of HPV-oropharyngeal carcinoma: Systematic review and meta-analysis. J. Exp. Clin. Cancer Res. 2024, 43, 215. [Google Scholar]
- Rapado-González, Ó.; Rodríguez-Ces, A.M.; López-López, R.; Suárez-Cunqueiro, M.M. Liquid biopsies based on cell-free DNA as a potential biomarker in head and neck cancer. Jpn. Dent. Sci. Rev. 2023, 59, 289–302. [Google Scholar]
- Lakshmipathy, D.; Prasad, A.; Fritz, C.G.; Go, B.C.; Rajasekaran, K. Accuracy of salivary circulating tumor human papillomavirus DNA in detecting oropharyngeal cancer: A systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 580–586. [Google Scholar]
- Fung, S.Y.; Lam, J.W.; Chan, K.C. Clinical utility of circulating Epstein-Barr virus DNA analysis for the management of nasopharyngeal carcinoma. Chin. Clin. Oncol. 2016, 5, 18. [Google Scholar] [PubMed]
- Xue, F.; He, X. Epstein-Barr virus DNA in nasopharyngeal carcinoma: A brief review. Methods Mol. Biol. 2020, 2204, 99–107. [Google Scholar]
- Pascual, J.; Attard, G.; Bidard, F.C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [PubMed]
- Lam, W.K.J.; Kang, G.; Wong, W.S. Circulating tumour DNA for detection of minimal residual disease in head and neck squamous cell carcinoma: A new hope. Ann. Oncol. 2023, 34, 1080–1081. [Google Scholar]
- Chera, B.S.; Kumar, S.; Shen, C.; Amdur, R.; Dagan, R.; Green, R.; Goldman, E.; Weiss, J.; Grilley-Olson, J.; Patel, S.; et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J. Clin. Oncol. 2020, 38, 1050–1058. [Google Scholar]
- Lam, W.K.J.; Chan, K.C.A.; Lo, Y.M.D. Plasma Epstein-Barr virus DNA as an archetypal circulating tumour DNA marker. J. Pathol. 2019, 247, 641–649. [Google Scholar]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [PubMed]
- Machiels, J.P.; Leemans, C.R.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar]
- Chmura, S.J.; Milano, M.T.; Haraf, D.J. Reirradiation of recurrent head and neck cancers with curative intent. Semin. Oncol. 2004, 31, 816–821. [Google Scholar] [PubMed]
- Alterio, D.; Zaffaroni, M.; Bossi, P.; Dionisi, F.; Elicin, O.; Falzone, A.; Ferrari, A.; Jereczek-Fossa, B.A.; Sanguineti, G.; Szturz, P.; et al. Reirradiation of head and neck squamous cell carcinomas: A pragmatic approach-part I: Prognostic factors and indications to treatment. Radiol. Med. 2024, 129, 160–173. [Google Scholar] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar]
- Spencer, S.A.; Harris, J.; Wheeler, R.H.; Machtay, M.; Schultz, C.; Spanos, W.; Rotman, M.; Meredith, R.; Ang, K.-K. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008, 30, 281–288. [Google Scholar]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar]
- Braakhuis, B.J.; Brakenhoff, R.H.; Leemans, C.R. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: Biological risk factors. Ann. Oncol. 2012, 23, x173–x177. [Google Scholar]
- Honoré, N.; van Marcke, C.; Galot, R.; Helaers, R.; van Maanen, A.; Mendola, A.; Dahou, H.; Marbaix, E.; Van Eeckhout, P.; Longton, E.; et al. Tumor-agnostic plasma assay for circulating tumor DNA detects minimal residual disease and predicts outcome in locally advanced squamous cell carcinoma of the head and neck. Ann. Oncol. 2023, 34, 1175–1186. [Google Scholar]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The next decade of immune checkpoint therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar]
- Gavrielatou, N.; Doumas, S.; Economopoulou, P.; Foukas, P.G.; Psyrri, A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat. Rev. 2020, 84, 101977. [Google Scholar] [PubMed]
- Oliva, M.; Chepeha, D.; Araujo, D.V.; Diaz-Mejia, J.J.; Olson, P.; Prawira, A.; Spreafico, A.; Bratman, S.V.; Shek, T.; de Almeida, J.; et al. Antitumor immune effects of preoperative sitravatinib and nivolumab in oral cavity cancer: SNOW window-of-opportunity study. J. Immunother. Cancer 2021, 9, e003476. [Google Scholar] [PubMed]
- Wang, H.; Zhou, F.; Qiao, M.; Li, X.; Zhao, C.; Cheng, L.; Chen, X.; Zhou, C. The role of circulating tumor DNA in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: A aystematic review and Meta-Analysis. Front. Oncol. 2021, 11, 671874. [Google Scholar]
- Al-Showbaki, L.; Wilson, B.; Tamimi, F.; Molto, C.; Mittal, A.; Cescon, D.W.; Amir, E. Changes in circulating tumor DNA and outcomes in solid tumors treated with immune checkpoint inhibitors: A systematic review. J. Immunother. Cancer 2023, 11, e005854. [Google Scholar]
- Jiang, R.; Cheng, X.; Li, P.; Meng, E.; Wu, X.; Wu, H. Plasma circulating tumor DNA unveils the efficacy of PD-1 inhibitors and chemotherapy in advanced gastric cancer. Sci. Rep. 2024, 14, 14027. [Google Scholar]
| Tumor Sites | Method of ctDNA Analysis | Objective of Study | Treatment Modality | Main Findings | Reference |
|---|---|---|---|---|---|
| Oral cavity, oropharynx, larynx, hypopharynx (Total n = 17) | A sequencing-based personalized ctDNA assay (RaDaRTM) for mutation detection | Detection of MRD and recurrence | Surgery | Tumor-specific variants were detected in all pre-operative plasma samples. In patients who relapsed, ctDNA was detected before progression, making personalized ctDNA analysis feasible for detecting MRD and clinical recurrence | [34] |
| Oral cavity, oropharynx, larynx, hypopharynx (Total n = 43) | The targeted sequencing of a panel of genes | Relevance of ctDNA in MRD detection | Surgery | ctDNA-based MRD detection predicted clinical recurrence with a median lead-time of 9.9 months in 17 out of 27 patients. ctDNA in samples obtained within 14 weeks after surgery, correlated with disease recurrence | [35] |
| Oropharynx and non-HNC (lung and esophageal cancers) (Total n = 23) | The sequencing-based detection of copy number instability | MRD detection | Curative RT in most patients (total doses 30–68.4 Gy) | Copy number instability-based ctDNA score reflects tumor burden and is feasible for MRD detection | [36] |
| Oral cavity, oropharynx, nasal cavity, nasopharynx, hypopharynx, sinuses, and skull base (Total n = 16) | The whole-genome sequencing-based detection of copy number variation (CNV) | Correlation of ctDNA with disease outcome after re-irradiation in locally advanced HNSCC | Re-RT (51–60 Gy) | CNV-based ctDNA abundance reflects the initial response to re-radiation and recurrence | [37] |
| Oral cavity, larynx, oropharynx (Total n = 29) | Multiplex PCR NGS assay | Comparing PET and ctDNA for detection of high-risk patients after treatment | Surgery (34% of the patients) and definitive CRT (66%) | ctDNA detection after definitive treatment was associated with a higher risk of disease recurrence, with 100% specificity at a sensitivity rate of 78% | [38] |
| HNSCC (oropharynx, tonsillar carcinoma, base of tongue and hypopharynx) and oligometastatic disease with various primary tumors (Total n = 28) | The low-pass, whole-genome sequencing-based detection of copy number alterations and fragment length distribution | Monitoring of treatment response in locally advanced HNSCC and oligometastatic disease | High-dose RT (70 Gy) and cisplatin | ctDNA analysis based on copy number alterations is useful for the detection of progression following RT | [39] |
| HPV-negative locoregionally confined HNSCC (Total n = 30) | The personalized sequencing-based mutation detection and sequencing of immunoprecipitated methylated plasma DNA | Predicting disease recurrence in HPV-negative locoregionally confined nonmetastatic HNSCC | Surgery in all patients, plus RT or CRT in most patients | Higher ctDNA abundance after treatment compared to baseline is indicative of disease recurrence | [40] |
| Oral cavity, larynx (Total n = 28) | Personalized commercial ctDNA test | Detection of minimal residual disease | All patients underwent surgical resection. The majority of high-risk patients received adjuvant CRT | The rate of disease recurrence was higher in patients with high-risk features and correlated with ctDNA | [41] |
| Mostly oropharynx (Total n = 20) | Exosome sequencing of 71 genes frequently mutated in HNC | Predicting recurrence | Surgical resection in all patients; CRT in 11 patients | During follow-up, no ctDNA was detected in 13 recurrence-free cases, but it was detected in 5 of the 7 recurrent cases; relapse-free survival time is significantly shorter in those with ctDNA detection post-treatment | [42] |
| Oropharynx, hypopharynx, oral cavity (Total n = 20) | The FFPE-tumor tissue informed sequencing of 127 driver genes in plasma | Prediction of disease recurrence | Definitive CRT with cumulative radiation dose of 70–77 Gy and concomitant CT with cisplatin weekly or a combination therapy of 5-fluorouracil and mitomycin C | In patients with ctDNA detection at the first follow-up point, disease recurrence occurred. All of the patients with detectable MRD suffered a relapse | [43] |
| Oropharynx, larynx, oral cavity, hypopharynx (Total n = 32) | Tumor-informed RaDaR® assay and a tumor-naive CAPP-seq assay | Impact of ctDNA analysis in MRD detection in locally advanced HNSCC patients | Curative intent (i) surgery followed by adjuvant CRT (ii) definitive RT or (iii) CRT, with CRT being the most frequent (78%) treatment | ctDNA detection during follow-up was associated with shorter relapse-free survival (p < 0.001). RaDaR assay proved to be better in MRD detection than CAPP-seq assay | [44] |
| Oral cavity, oropharynx, hypopharynx, larynx, the external auditory canal (Total n = 26) | The tumor-informed monitoring of an HNSSC-related panel of genes (n = 31) in plasma by digital PCR | Monitoring treatment response and relapse in HNSCC | Of the patients, 1 was treated with RT, 9 with surgery, 5 with CRT, 3 with induction CT and CRT, 2 with induction CT and surgery, 2 with surgery plus CRT and 3 with induction CT, surgery, and CRT | In cases with relapse, the ctDNA reverted to positive or did not become negative after the initial curative treatment. Patients who remained ctDNA negative after initial curative treatment were alive without recurrence and had a significantly better prognosis than those who reverted to ctDNA positivity | [45] |
| Oral cavity, oropharynx, larynx, hypopharynx, and others (paranasal sinus, nasopharyngeal, and salivary gland cancers, unknown primary) (Total n = 116) | The sequencing of a panel of 26 genes, including the most-frequently mutated genes in HNSCC and two HPV-16 genes | Utility of ctDNA to detect MRD in unselected locally advanced HNSSC | Most patients (92.4%) received curative-intent CRT as the primary treatment while the remaining patients (7.6%) were treated with surgery | Median 2-year PFS rate was 23.53% and 86.6% in MRD-positive and MRD-negative patients. Median survival was 28.37 months for MRD-positive patients and was not reached for the MRD-negative cohort (p = 0.011) | [46] |
| Larynx, oropharynx oral cavity, hypopharynx nasopharynx parotid gland, neck (Total n = 38) | The sequencing of a panel of approximately 70 genes at baseline and after the completion of treatment | Feasibility of ctDNA in identifying high-risk patients | CRT | In the patients with complete or partial response to CRT, less mutations (26.6%) were observed than in nonresponders (75%) (p = 0.03). Assessment of mutations before and after CRT is helpful to characterize patients with a high risk of locoregional recurrence or metastatic progression | [47] |
| Oral cavity, oropharynx, hypopharynx, larynx, HNC unknown primary (Total n = 97) | A tumor-agnostic sequencing assay including 37 genes frequently mutated in recurrent/metastatic HNSCC | Predictive value of ctDNA profiling in the efficacy of single-agent PD1 inhibitor in recurrent/metastatic HNSCC | Treatment with non-curative intent anti-PD1 therapy (pembrolizumab or nivolumab) without concomitant CTx | Among the 35 patients with ctDNA positivity at baseline, 17 had a decrease (negative ΔctDNA) in the mean VAF and the majority of them (76.4%) achieved either a complete response, partial response, or stable disease, with 4 cases experiencing disease progression. Among those with an increase (positive ΔctDNA) in the mean VAF, the majority of the cases (72%) had progressive disease | [48] |
| Oral cavity (the tongue, buccal mucosa, gingiva, and other oral locations) (Total n = 12) | The targeted sequencing of 74 genes | ctDNA as real-time biomarker for monitoring response to ICB therapy in HNSCC | Nivolumab was second-line therapy for 3 patients who were refractory to platinum-based CTx, whereas the other 7 patients received pembrolizumab as first-line therapy | Changes in the mean VAF, detected in the early phase of the treatment course, often preceded radiological progression. Among patients with an achieved partial response or stable disease, mean VAF remained relatively stable whereas in the patients with progressive disease, early increases in the mean VAF occurred and accurately predicted disease progression | [49] |
| Oral cavity, oropharynx, larynx, hypopharynx, nasal cavity (Total n = 53) | Sequencing a panel of genes (CAPP-seq) optimized for HNSCC | Monitoring of immune checkpoint blockade therapy in metastatic HNSCC | Patients received one or more cycles of first-line, platinum-based, systemic treatment (n = 16); second-line anti-PD1/PL1 monotherapy (n = 30); or combination immunotherapy with two agents (n = 7) | Baseline ctDNA was not informative for OS or PFS. However, a change in ctDNA VAF after one cycle of treatment, compared to baseline (ΔVAF), was predictive of both PFS (p < 0.01) and OS (p < 0.01). A decrease in ΔVAF identified patients with longer OS despite early radiological progression. | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gezer, U.; Meral, R.; Özgür, E.; Yörüker, E.E.; Bronkhorst, A.; Holdenrieder, S. A Narrative Review of the Role of Non-Viral Circulating Tumor DNA Profiling in Predicting the Treatment Response and Recurrence in Head and Neck Squamous Cell Carcinoma. Cancers 2025, 17, 2279. https://doi.org/10.3390/cancers17142279
Gezer U, Meral R, Özgür E, Yörüker EE, Bronkhorst A, Holdenrieder S. A Narrative Review of the Role of Non-Viral Circulating Tumor DNA Profiling in Predicting the Treatment Response and Recurrence in Head and Neck Squamous Cell Carcinoma. Cancers. 2025; 17(14):2279. https://doi.org/10.3390/cancers17142279
Chicago/Turabian StyleGezer, Ugur, Rasim Meral, Emre Özgür, Ebru. E. Yörüker, Abel Bronkhorst, and Stefan Holdenrieder. 2025. "A Narrative Review of the Role of Non-Viral Circulating Tumor DNA Profiling in Predicting the Treatment Response and Recurrence in Head and Neck Squamous Cell Carcinoma" Cancers 17, no. 14: 2279. https://doi.org/10.3390/cancers17142279
APA StyleGezer, U., Meral, R., Özgür, E., Yörüker, E. E., Bronkhorst, A., & Holdenrieder, S. (2025). A Narrative Review of the Role of Non-Viral Circulating Tumor DNA Profiling in Predicting the Treatment Response and Recurrence in Head and Neck Squamous Cell Carcinoma. Cancers, 17(14), 2279. https://doi.org/10.3390/cancers17142279









