Sex Disparities and Female Reproductive and Hormonal Factors Associated with Risk of Pancreatic Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
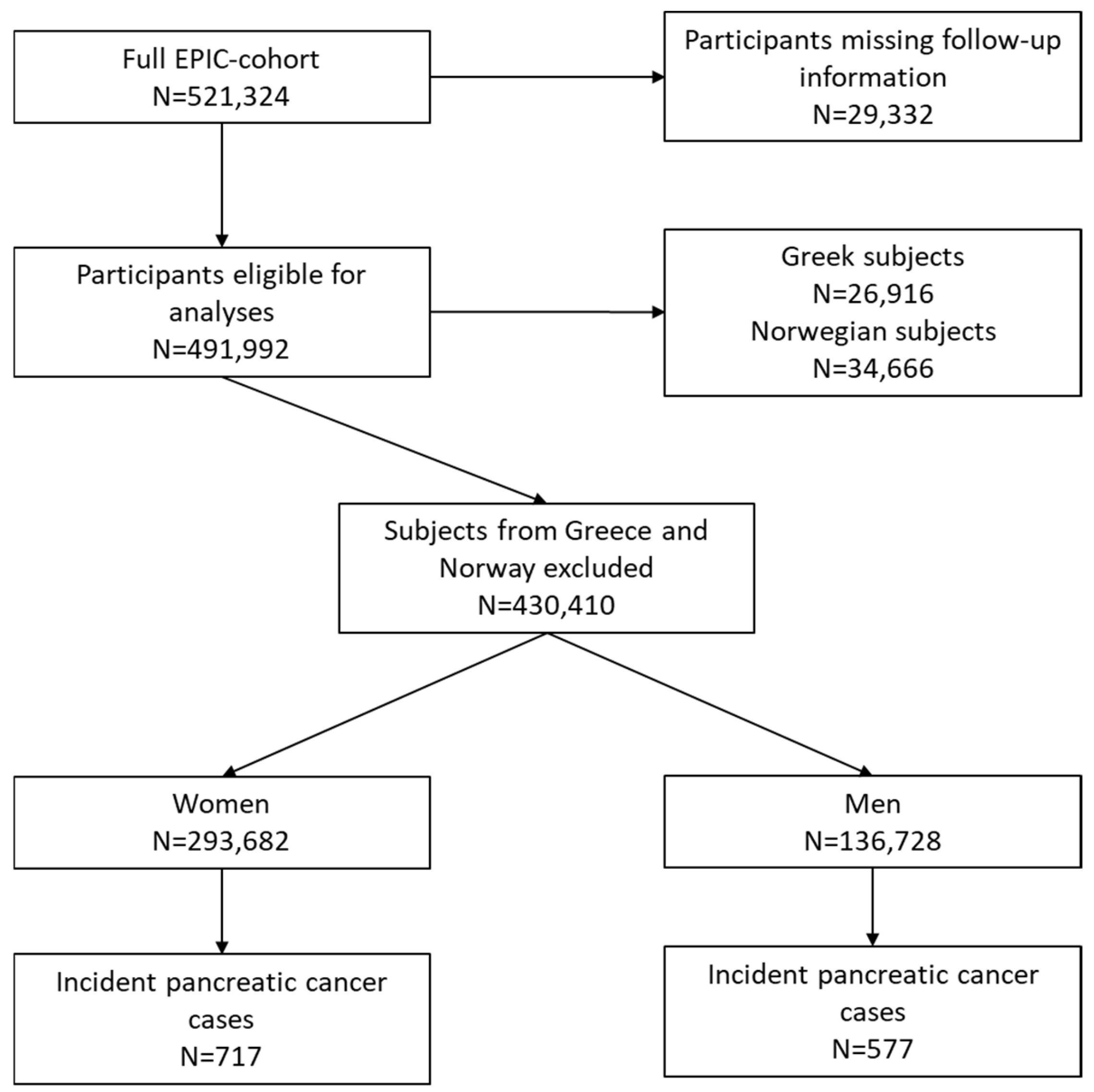
2.1. Study Design
2.2. Follow-Up for Cancer Incidence and Vital Status, PC Characteristics
2.3. Statistical Analyses
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index (kg/m2) |
| CI | Confidence interval |
| Cox PH-model | Cox proportional hazards model |
| EPIC | European Prospective Investigation into Cancer and Nutrition |
| FRP | Full-term pregnancy |
| GDPR | General Data Protection Regulation |
| HRT | Hormone replacement therapy |
| IARC | International Agency for Research on Cancer |
| IQR | Interquartile range |
| OC | Oral contraceptives |
| PanC4 | Pancreatic Cancer Case-Control Consortium |
| PDAC | Pancreatic ductal adenocarcinoma |
| PC | Pancreatic cancer |
| SD | Standard deviation |
| WHO | World Health Organization |
Appendix A
Appendix A.1. Detailed Information on Creation of Reproductive and Hormonal Factors in EPIC
- Cumulative duration of menstrual cycles
- Cumulative duration of menstrual cycles without OC use
- Full-term pregnancy (not available in one center)
- Age at first full-term pregnancy (not available in four centers)
- Cumulative duration of breastfeeding (not available in two centers)
- If she breast-fed for the pregnancies for which we have the information, she breastfed for all her pregnancies. The cumulative duration of breastfeeding is then the number of pregnancies multiplied by the mean of each breastfeeding duration.
- If she did not breastfeed for at least one of the pregnancies for which we have the information, then the cumulative duration of breastfeeding is the sum of the other durations.
- Menopausal status
| FACTORS | ALL WOMEN n = 293,682 717 Cases | PREMENO n = 101,252 68 Cases | POSTMENO n = 130,796 524 Cases |
|---|---|---|---|
| Age | 1.09 (1.08–1.10) | 1.11 (1.06–1.17) | 1.06 (1.04–1.08) |
| Diabetes | 1.74 (1.21–2.51) | 0.96 (0.13–7.14) | 1.75 (1.17–2.63) |
| BMI [kg/m2] | |||
| <25 | Reference | Reference | Reference |
| 25–30 | 0.99 (0.84–1.17) | 0.92 (0.51–1.66) | 0.99 (0.79–1.18) |
| ≥30 | 1.02 (0.81–1.28) | 1.16 (0.54–2.48) | 0.87 (0.66–1.14) |
| continuous/5 | 1.05 (0.96–1.14) | 1.20 (0.93–1.56) | 0.99 (0.89–1.10) |
| Body Height [cm]/5 | 1.13 (1.06–1.20) | 1.03 (0.84–1.26) | 1.13 (1.04–1.21) |
| Highest school level | |||
| None | Reference | Reference | Reference |
| Primary school completed | 1.21 (0.71–2.05) | 1.66 (0.36–7.70) | 0.93 (0.49–1.75) |
| Technical/prof. school | 1.08 (0.62–1.88) | 3.48 (0.69–17.56) | 0.86 (0.44–1.67) |
| Secondary school | 0.97 (0.55–1.72) | 2.32 (0.45–12.03) | 0.68 (0.34–1.34) |
| Longer education | 1.04 (0.59–1.83) | 1.66 (0.32–8.76) | 0.83 (0.42–1.64) |
| Smoking status | |||
| Never | Reference | Reference | Reference |
| Former | 0.94 (0.77–1.15) | 0.72 (0.35–1.48) | 0.89 (0.71–1.14) |
| Current | 1.74 (1.45–2.10) | 2.43 (1.41–4.22) | 1.65 (1.32–2.07) |
| Cigarettes smoked/day | |||
| ≤10 | Reference | Reference | Reference |
| 11–20 | 1.88 (1.49–2.36) | 3.16 (1.65–6.09) | 1.76 (1.33–2.33) |
| 21–30 | 2.13 (1.33–3.39) | 4.88 (1.71–13.90) | 2.17 (1.23–3.82) |
| >30 | 1.81 (0.58–5.68) | - | 1.81 (0.44–7.35) |
| continuous, 10/d | 1.45 (1.32–1.59) | 1.68 (1.31–2.15) | 1.43 (1.27–1.62) |
| Duration of smoking | |||
| ≤10 years | Reference | Reference | Reference |
| 11–20 years | 0.94 (0.69–1.28) | 0.95 (0.42–2.15) | 1.03 (0.71–1.49) |
| 21–30 years | 1.29 (0.99–1.66) | 1.81 (0.98–3.35) | 1.03 (0.73–1.45) |
| 31–40 years | 1.72 (1.38–2.14) | 3.14 (1.23–8.01) | 1.59 (1.24–2.06) |
| 41–50 years | 1.38 (1.02–1.88) | - | 1.37 (0.99–1.88) |
| >50 years | 1.32 (0.49–3.58) | - | 1.32 (0.49–3.59) |
| continuous/10 years | 1.12 (1.07–1.18) | 1.27 (1.03–1.57) | 1.09 (1.04–1.16) |
| Time to quitting smoking | |||
| ≤10 years | Reference | Reference | Reference |
| 11–20 years | 0.55 (0.37–0.80) | 0.47 (0.15–1.49) | 0.55 (0.35–0.87) |
| 21–30 years | 0.77 (0.54–1.09) | 0.82 (0.25–2.65) | 0.70 (0.46–1.08) |
| 31–40 years | 0.78 (0.48–1.27) | - | 0.88 (0.54–1.43) |
| >40 years | 0.67 (0.25–1.81) | - | 0.52 (0.16–1.62) |
| continuous/10 years | 0.88 (0.81–0.97) | 0.69 (0.44–1.11) | 0.89 (0.80–0.98) |
| Alcohol intake at recr. | |||
| non drinker | Reference | Reference | Reference |
| >0–3 | 0.97 (0.76–1.25) | 1.22 (0.54–2.79) | 0.86 (0.64–1.14) |
| >3–12 | 0.98 (0.76–1.26) | 1.24 (0.54–2.83) | 0.91 (0.68–1.21) |
| >12–24 | 1.08 (0.81–1.44) | 1.08 (0.41–2.80) | 1.04 (0.74–1.44) |
| >24 | 1.34 (0.98–1.83) | 1.12 (0.38–3.24) | 1.40 (0.98–1.99) |
| continuous, 15 g/d | 1.11 (1.01–1.21) | 1.01 (0.74–1.39) | 1.15 (1.05–1.27) |
| Premenopausal n= 101,252 PC = 68 | Perimenopausal/Unknown n= 52,634 PC = 102 | Postmenopausal n= 130,796 PC = 524 | ||||
|---|---|---|---|---|---|---|
| Factors | HRs (95% CI) adjusted for age; stratified by study centre | HRs (95% CI) multivariate adjusted * | HRs (95% CI) adjusted for age; stratified by study centre | HRs (95% CI) multivariate adjusted * | HRs (95% CI) adjusted for age; stratified by study centre | HRs (95% CI) multivariate adjusted * |
| Reproductive Factors—menarche | ||||||
| Age at menarche (years) | ||||||
| ≤med (30 pre, 31 peri, 155 post cases) | Reference (≤12) | Reference (≤12) | Reference (≤12) | Reference (≤12) | Reference (≤12) | Reference (≤12) |
| >med (32 pre, 54 peri, 288 post cases) | 0.74 (0.45–1.23) | 0.76 (0.45–1.28) | 1.05 (0.67–1.65) | 1.00 (0.63–1.59) | 0.89 (0.73–1.09) | 0.85 (0.68–1.05) |
| continuous (per age-year) | 0.97 (0.82–1.14) | 0.98 (0.82–1.17) | 0.95 (0.83–1.09) | 0.95 (0.83–1.09) | 1.01 (0.96–1.07) | 1.01 (0.96–1.08) |
| Duration menstrual cycling (years) | ||||||
| med (15 pre, 28 peri, 212 post cases) | Reference (≤29) | Reference (≤29) | Reference (≤35) | Reference (≤35) | Reference (≤35) | Reference (≤35) |
| > med (47 pre, 56 peri, 202 post cases) | 1.32 (0.59–2.93) | 1.17 (0.52–2.65) | 1.47 (0.89–2.42) | 1.42 (0.85–2.38) | 0.99 (0.82–1.21) | 0.99 (0.81–1.22) |
| continuous (per age-year) | 1.02 (0.94–1.10) | 1.00 (0.92–1.09) | 1.06 (0.97–1.14) | 1.04 (0.96–1.13) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) |
| Duration menstrual cycling without OC (years) | ||||||
| ≤med (20 pre, 27 peri, 209 post cases) | Reference (≤24) | Reference (≤24) | Reference (≤32) | Reference (≤32) | Reference (≤33) | Reference (≤33) |
| >med (3 pre, 46 peri, 184 post cases) | 0.83 (0.43–1.58) | 0.72 (0.37–1.39) | 1.3 (0.79–2.14) | 1.18 (0.71–1.96) | 0.86 (0.70–1.05) | 0.87 (0.69–1.08) |
| continuous (per age-year) | 0.99 (0.96–1.03) | 0.98 (0.95–1.02) | 1.01 (0.98–1.04) | 1.00 (0.97–1.03) | 0.99 (0.98–1.00) | 0.99 (0.98–1.01) |
| Reproductive Factors—pregnancy | ||||||
| Full-term pregnancy | ||||||
| no (13 pre, 13 peri, 67 post cases) | Reference | Reference | Reference | Reference | Reference | Reference |
| yes (52 pre, 77 peri, 441 post cases) | 0.61 (0.33–1.15) | 0.61 (0.32–1.18) | 0.69 (0.38–1.25) | 0.67 (0.37–1.21) | 0.97 (0.75–1.26) | 0.93 (0.71–1.23) |
| Number of full-term pregnancies | ||||||
| ≤med (52 pre, 63 peri, 346 post cases) | Reference (≤2) | Reference (≤2) | Reference (≤2) | Reference (≤2) | Reference (≤2) | Reference (≤2) |
| >med (10 pre, 23 peri, 156 post cases) | 0.63 (0.31–1.26) | 0.59 (0.28–1.22) | 1.01 (0.62–1.65) | 0.98 (0.59–1.62) | 0.87 (0.71–1.05) | 0.82 (0.66–1.01) |
| continuous | 0.84 (0.66–1.08) | 0.83 (0.64–1.07) | 0.93 (0.76–1.13) | 0.91 (0.74–1.12) | 0.96 (0.90–1.03) | 0.95 (0.88–1.02) |
| Age at first full-term pregnancy (years) | ||||||
| ≤med (27 pre, 51 peri, 249 post cases) | Reference (≤25) | Reference (≤25) | Reference (≤24) | Reference (≤24) | Reference (≤24) | Reference (≤24) |
| >med (25 pre, 25 peri, 190 post cases) | 1.42 (0.81–2.48) | 1.65 (0.92–2.98) | 0.59 (0.36–0.95) | 0.58 (0.35–0.97) | 1.03 (0.85–1.25) | 1.06 (0.85–1.31) |
| continuous (per age-year) | 1.03 (0.97–1.09) | 1.05 (0.98–1.12) | 0.93 (0.87–0.98) | 0.92 (0.86–0.98) | 1.01 (0.99–1.03) | 1.01 (0.99–1.04) |
| Breastfeeding | ||||||
| no (20 pre, 1 peri, 118 post cases) | Reference | Reference | Reference | Reference | Reference | Reference |
| yes (40 pre, 60 peri, 363 post cases) | 0.78 (0.45–1.35) | 0.88 (0.49–1.58) | 1.22 (0.69–2.13) | 1.28 (0.72–2.28) | 0.96 (0.77–1.18) | 0.96 (0.76–1.20) |
| Cumulative duration breast feeding (months) | ||||||
| ≤med (18 pre, 35 peri, 188 post cases) | Reference (≤6) | Reference (≤6) | Reference (≤6) | Reference (≤6) | Reference (≤6) | Reference (≤6) |
| >med (22 pre, 25 peri, 173 post cases) | 1.21 (0.63–2.32) | 1.13 (0.58–2.18) | 0.63 (0.37–1.07) | 0.63 (0.37–1.08) | 0.80 (0.65–0.99) | 0.81 (0.65–1.02) |
| continuous (months) | 0.98 (0.94–1.03) | 0.98 (0.93–1.03) | 0.97 (0.93–1.01) | 0.97 (0.93–1.02) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) |
| Reproductive Factors—menopause | ||||||
| Age at menopause (years) | ||||||
| ≤med (256 post cases) | Reference (≤50) | Reference (≤50) | ||||
| >med (171 post cases) | 0.98 (0.80–1.19) | 0.95 (0.77–1.17) | ||||
| continuous (per year) | 0.99 (0.98–1.02) | 0.99 (0.97–1.02) | ||||
| Hormonal Factors—OC and HRT | ||||||
| Ever use OC | ||||||
| no (15 pre, 30 peri, 293 post cases) | Reference | Reference | Reference | Reference | Reference | Reference |
| yes (50 pre, 63 peri, 214 post cases) | 1.43 (0.77–2.65) | 1.51 (0.79–2.88) | 0.99 (0.63–1.56) | 0.99 (0.62–1.59) | 1.17 (0.97–1.42) | 1.25 (1.02–1.53) |
| Cumulative duration of OC use (years) | ||||||
| ≤med (31 pre, 27 peri, 82 post cases) | Reference (≤5) | Reference (≤5) | Reference (≤5) | Reference (≤5) | Reference (≤5) | Reference (≤5) |
| >med (16 pre, 23 peri, 110 post cases) | 0.71 (0.37–1.36) | 0.77 (0.40–1.49) | 0.96 (0.54–1.71) | 1.02 (0.57–1.85) | 1.49 (1.11–2.00) | 1.33 (0.98–1.81) |
| continuous (per year) | 1.01 (0.96–1.05) | 1.01 (0.97–1.06) | 1.00 (0.97–1.04) | 1.00 (0.97–1.04) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) |
| Ever use of HRT | ||||||
| no (62 pre, 58 peri, 294 post cases) | Reference | Reference | Reference | Reference | ||
| yes (1 pre, 24 peri, 175 post cases) | 0.91 (0.56–1.49) | 0.96 (0.58–1.58) | 1.12 (0.91–1.37) | 1.08 (0.87–1.35) | ||
| Cumulative duration of HRT use (years) among HRT users | ||||||
| ≤med (0 pre, 9 peri, 99 post cases) | Reference (≤1) | Reference (≤1) | Reference (≤3) | Reference (≤3) | ||
| >med (1 pre, 11 peri, 61 post cases) | 1.39 (0.56–3.48) | 1.42 (0.57–3.53) | 0.66 (0.47–0.91) | 0.66 (0.47–0.93) | ||
| continuous (per year) | 1.00 (0.88–1.15) | 1.01 (0.89–1.15) | 0.98 (0.95–1.02) | 0.98 (0.95–1.02) | ||
| Hormonal Factors—surgery | ||||||
| Hysterectomy | ||||||
| no (62 pre, 56 peri, 375 post cases) | Reference | Reference | Reference | Reference | ||
| yes (1 pre, 17 peri, 60 post cases) | 0.88 (0.50–1.54) | 0.92 (0.53–1.62) | 1.05 (0.79–1.39) | 1.01 (0.73–1.38) | ||
| Ovariectomy | ||||||
| no (62 pre, 66 peri, 392 post cases) | Reference | Reference | ||||
| yes (0 pre, 3 peri, 29 post cases) | 1.06 (0.76–1.48) | 1.08 (0.75, 1.57) | ||||
References
- IARC. GLOBOCAN—Global Cancer Observatory. Cancer Today. 2022. Available online: https://gco.iarc.fr/today/online-analysis-dual-bars-2 (accessed on 2 November 2022).
- ECIS. European Cancer Information System: Estimates of Survival by Country and Cancer Site. 2022. Available online: https://ecis.jrc.ec.europa.eu (accessed on 2 November 2022).
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Lowenfels, A.B. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int. J. Epidemiol. 2015, 44, 186–198. [Google Scholar] [CrossRef] [PubMed]
- WCRF. Pancreatic Cancer. 2018. Available online: https://www.wcrf.org/diet-activity-and-cancer/cancer-types/pancreatic-cancer/ (accessed on 1 June 2025).
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Gentiluomo, M.; Canzian, F.; Nicolini, A.; Gemignani, F.; Landi, S.; Campa, D. Germline genetic variability in pancreatic cancer risk and prognosis. Semin. Cancer Biol. 2022, 79, 105–131. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef]
- Bourhis, J.; Lacaine, F.; Augusti, M.; Huguier, M. Protective effect of oestrogen in pancreatic cancer. Lancet 1987, 2, 977. [Google Scholar] [CrossRef]
- Pozios, I.; Seel, N.N.; Hering, N.H.; Hartmann, L.; Liu, V.; Camaj, P.; Müller, M.H.; Lee, L.D.; Bruns, C.J.; Kreis, M.E.; et al. Raloxifene inhibits pancreatic adenocarcinoma growth by interfering with ERbeta and IL-6/gp130/STAT3 signaling. Cell. Oncol. 2021, 44, 167–177. [Google Scholar] [CrossRef]
- Sumi, C.; Longnecker, D.S.; Roebuck, B.D.; Brinck-Johnsen, T. Inhibitory effects of estrogen and castration on the early stage of pancreatic carcinogenesis in Fischer rats treated with azaserine. Cancer Res. 1989, 49, 2332–2336. [Google Scholar]
- Xue, J.; Yao, Y.; Yao, Q.; Tian, X.; Feng, Y.; Su, H.; Kong, D.; Cui, C.; Yan, L.; Hao, C.; et al. Important roles of estrogen receptor alpha in tumor progression and anti-estrogen therapy of pancreatic ductal adenocarcinoma. Life Sci. 2020, 260, 118302. [Google Scholar] [CrossRef]
- Alvarez, A.; Benjaminsen Borch, K.; Rylander, C. Reproductive Factors, Use of Exogenous Hormones, and Pancreatic Cancer Incidence: The Norwegian Women and Cancer Study. Clin. Epidemiol. 2021, 13, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Borgquist, S.; Jirstrom, K. Hormonal factors and pancreatic cancer risk in women: The Malmo Diet and Cancer Study. Int. J. Cancer 2018, 143, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Sadr-Azodi, O.; Konings, P.; Brusselaers, N. Menopausal hormone therapy and pancreatic cancer risk in women: A population-based matched cohort study. United Eur. Gastroenterol. J. 2017, 5, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Archibugi, L.; Graglia, B.; Valente, R.; Stigliano, S.; Roberto, M.; Capalbo, C.; Marchetti, P.; Nigri, G.; Capurso, G. Gynecological and reproductive factors and the risk of pancreatic cancer: A case-control study. Pancreatology 2020, 20, 1149–1154. [Google Scholar] [CrossRef]
- Lujan-Barroso, L.; Zhang, W.; Olson, S.H.; Gao, Y.T.; Yu, H.; Baghurst, P.A.; Bracci, P.M.; Bueno-de-Mesquita, H.B.; Foretová, L.; Gallinger, S.; et al. enstrual and Reproductive Factors, Hormone Use, and Risk of Pancreatic Cancer: Analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4). Pancreas 2016, 45, 1401–1410. [Google Scholar] [CrossRef]
- Guan, H.B.; Wu, L.; Wu, Q.K.; Zhu, J.; Gong, T. Parity and pancreatic cancer risk: A dose-response meta-analysis of epidemiologic studies. PLoS ONE 2014, 9, e92738. [Google Scholar] [CrossRef]
- Ilic, M.; Milicic, B.; Ilic, I. Association between oral contraceptive use and pancreatic cancer risk: A systematic review and meta-analysis. World J. Gastroenterol. 2021, 27, 2643–2656. [Google Scholar] [CrossRef]
- Luo, A.J.; Feng, R.H.; Wang, X.W.; Wang, F.Z. Older age at first birth is a risk factor for pancreatic cancer: A meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 125–130. [Google Scholar] [CrossRef]
- Tang, B.; Lv, J.; Li, Y.; Yuan, S.; Wang, Z.; He, S. Relationship between female hormonal and menstrual factors and pancreatic cancer: A meta-analysis of observational studies. Medicine 2015, 94, e177. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, L.; Han, J.; Chen, W.; Shen, N.; Zhong, R.; Li, J.; Chen, X.; Liu, C.; Shi, Y.; et al. Parity and pancreatic cancer risk: Evidence from a meta-analysis of twenty epidemiologic studies. Sci. Rep. 2014, 4, 5313. [Google Scholar] [CrossRef]
- Duell, E.J.; Travier, N.; Lujan-Barroso, L.; Dossus, L.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Tumino, R.; Masala, G.; Krogh, V.; Panico, S.; et al. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int. J. Cancer 2013, 132, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.R.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public. Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E.; Kaaks, R. The EPIC Project: Rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S6–S14. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P. Epidemiological Impact of Pancreatic Cancer. In Clinical Pancreatology for Practising Gastroenterologists and Surgeons; Domingues-Munoz, E., Ed.; John Wiley & Sons Ltd: New Jersey, NJ, USA, 2021; pp. 389–403. [Google Scholar]
- Peduzzi, G.; Archibugi, L.; Farinella, R.; de Leon Pisani, R.P.; Vodickova, L.; Vodicka, P.; Kraja, B.; Sainz, J.; Bars-Cortina, D.; Daniel, N.; et al. The exposome and pancreatic cancer, lifestyle and environmental risk factors for PDAC. Semin. Cancer Biol. 2025, 113, 100–129. [Google Scholar] [CrossRef]
- Gallus, S.; Lugo, A.; Liu, X.; Behrakis, P.; Boffi, R.; Bosetti, C.; Carreras, G.; Chatenoud, L.; Clancy, L.; Continente, X.; et al. Who Smokes in Europe? Data From 12 European Countries in the TackSHS Survey (2017–2018). J. Epidemiol. 2021, 31, 145–151. [Google Scholar] [CrossRef]
- Eurostat. Tobacco Consumption Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Tobacco_consumption_statistics#Data_sources (accessed on 20 April 2025).
- Gram, I.T.; Park, S.Y.; Wilkens, L.R.; Le Marchand, L.; Setiawan, V.W. Smoking and pancreatic cancer: A sex-specific analysis in the Multiethnic Cohort study. Cancer Causes Control 2023, 34, 89–100. [Google Scholar] [CrossRef]
- Arriaga, M.E.; Vajdic, C.M.; MacInnis, R.J.; Canfell, K.; Magliano, D.J.; Shaw, J.E.; Byles, J.E.; Giles, G.G.; Taylor, A.W.; Gill, T.K.; et al. The burden of pancreatic cancer in Australia attributable to smoking. Med. J. Aust. 2019, 210, 213–220. [Google Scholar] [CrossRef]
- Larsson, S.C.; Permert, J.; Håkansson, N.; Näslund, I.; Bergkvist, L.; Wolk, A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br. J. Cancer 2005, 93, 1310–1315. [Google Scholar] [CrossRef]
- Nilsen, T.I.; Vatten, L.J. A prospective study of lifestyle factors and the risk of pancreatic cancer in Nord-Trondelag, Norway. Cancer Causes Control 2000, 11, 645–652. [Google Scholar] [CrossRef]
- Aune, D.; Vieira, A.R.; Chan, D.S.M.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Cade, J.E.; Burley, V.J.; Norat, T. Height and pancreatic cancer risk: A systematic review and meta-analysis of cohort studies. Cancer Causes Control 2012, 23, 1213–1222. [Google Scholar] [CrossRef]
- Albanes, D.; Winick, M. Are cell number and cell proliferation risk factors for cancer? J. Natl. Cancer Inst. 1988, 80, 772–774. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Roberts, C.T., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, B.; Baniasadi, M.M.; Jalalzadeh, M.; Esmaillzadeh, A. Total, unprocessed, and processed red meat intake in relation to the risk of pancreatic cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Clin. Nutr. ESPEN 2025, 67, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Heuch, I.; Jacobsen, B.K.; Albrektsen, G.; Kvåle, G. Reproductive factors and pancreatic cancer risk: A Norwegian cohort study. Br. J. Cancer 2008, 98, 189–193. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kamensky, V.; Rohan, T.E. Reproductive factors, exogenous hormone use, and risk of pancreatic cancer in postmenopausal women. Cancer Epidemiol. 2017, 49, 1–7. [Google Scholar] [CrossRef]
- Lee, E.; Horn-Ross, P.L.; Rull, R.P.; Neuhausen, S.L.; Anton-Culver, H.; Ursin, G.; Henderson, K.D.; Bernstein, L. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am. J. Epidemiol. 2013, 178, 1403–1413. [Google Scholar] [CrossRef]
- Skinner, H.G.; Michaud, D.S.; Colditz, G.A.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol. Biomark. Prev. 2003, 12, 433–438. [Google Scholar]
- Stevens, R.J.; Roddam, A.W.; Green, J.; Pirie, K.; Bull, D.; Reeves, G.K.; Beral, V.; Million Women Study Collaborators. Reproductive history and pancreatic cancer incidence and mortality in a cohort of postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1457–1460. [Google Scholar] [CrossRef]
- Jang, Y.C.; Leung, C.Y.; Huang, H.L. Association of Menopausal Hormone Therapy with Risk of Pancreatic Cancer: A Systematic Review and Meta-analysis of Cohort Studies. Cancer Epidemiol. Biomark. Prev. 2023, 32, 114–122. [Google Scholar] [CrossRef]
- Jolleys, J.V.; Olesen, F. A comparative study of prescribing of hormone replacement therapy in USA and Europe. Maturitas 1996, 23, 47–53. [Google Scholar] [CrossRef]
- Salagame, U.; Kliewer, E.V.; Demers, A.; Banks, E.; Velentzis, L.S.; Goldsbury, D.; Egger, S.; Leslie, W.D.; Canfell, K. Trends in Prescribing Menopausal Hormone Therapy and Bisphosphonates in Australia and Manitoba, Canada and Adherence to Recommendations. J. Women’s Health 2020, 29, 177–186. [Google Scholar] [CrossRef]
- Sprague, B.L.; Trentham-Dietz, A.; Cronin, K.A. A sustained decline in postmenopausal hormone use: Results from the National Health and Nutrition Examination Survey, 1999–2010. Obstet. Gynecol. 2012, 120, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Wahi, M.M.; Shah, N.; Schrock, C.E.; Rosemurgy, A.S., 2nd; Goldin, S.B. Reproductive factors and risk of pancreatic cancer in women: A review of the literature. Ann. Epidemiol. 2009, 19, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Huang, H.L.; Leung, C.Y. Association of hormone replacement therapy with mortality in colorectal cancer survivor: A systematic review and meta-analysis. BMC Cancer 2019, 19, 1199. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Liu, T.P.; Yang, P.M. CDKN2A-Inactivated Pancreatic Ductal Adenocarcinoma Exhibits Therapeutic Sensitivity to Paclitaxel: A Bioinformatics Study. J. Clin. Med. 2020, 9, 4019. [Google Scholar] [CrossRef]
- Troisi, R.; Bjørge, T.; Gissler, M.; Grotmol, T.; Kitahara, C.M.; Myrtveit Saether, S.M.; Ording, A.G.; Sköld, C.; Sørensen, H.T.; Trabert, B.; et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: A review of the evidence. J. Intern. Med. 2018, 283, 430–445. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K. The Women’s Health Initiative randomized trials of menopausal hormone therapy and breast cancer: Findings in context. Menopause 2023, 30, 454–461. [Google Scholar] [CrossRef]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; Masushige, S.; Gotoh, Y.; Nishida, E.; Kawashima, H.; et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270, 1491–1494. [Google Scholar] [CrossRef]
- Liu, S.-L.; Wu, X.-S.; Li, F.-N.; Yao, W.-Y.; Wu, Z.-Y.; Dong, P.; Wang, X.-F.; Gong, W. ERRalpha promotes pancreatic cancer progression by enhancing the transcription of PAI1 and activating the MEK/ERK pathway. Am. J. Cancer Res. 2020, 10, 3622–3643. [Google Scholar]
- Robles-Diaz, G.; Duarte-Rojo, A. Pancreas: A sex steroid-dependent tissue. Isr. Med. Assoc. J. 2001, 3, 364–368. [Google Scholar]
- Choi, J.; Oh, T.G.; Jung, H.-W.; Park, K.-Y.; Shin, H.; Jo, T.; Kang, D.-S.; Chanda, D.; Hong, S.; Kim, J.; et al. Estrogen-Related Receptor gamma Maintains Pancreatic Acinar Cell Function and Identity by Regulating Cellular Metabolism. Gastroenterology 2022, 163, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Jacobs, S.; Kröger, J.; Fritsche, A.; Schienkiewitz, A.; Rubin, D.; Boeing, H.; Schulze, M.B. Breast-feeding and maternal risk of type 2 diabetes: A prospective study and meta-analysis. Diabetologia 2014, 57, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
| FEMALE | MALE | |||
|---|---|---|---|---|
| Characteristics | Incident PC n = 717 | Non-cases n = 292,965 | Incident PC n = 577 | Non-cases n = 136,151 |
| length of follow up (years) | 10 (6, 13) | 15 (14, 16) | 10 (6, 13) | 16 (12, 17) |
| age at diagnosis (years) | 68 (62, 73) | - | 67 (62, 72) | - |
| age at recruitment (years) | 58 (52, 62) | 51 (45, 57) | 58 (52, 62) | 53 (46, 59) |
| BMI (kg/m2) | 25.0 (23, 28) | 24.0 (22, 27) | 26.3 (24, 29) | 26.0 (24, 28) |
| alcohol intake at recruitment | Missing 2% | Missing 1% | Missing 2% | Missing 3% |
| non drinker | 112 (16) | 43,513 (15) | 38 (7) | 8417 (6) |
| >0–3 w/>0–6 m | 208 (30) | 86,742 (30) | 114 (20) | 33,753 (25) |
| >3–12 w/>6–12 m | 198 (28) | 87,585 (30) | 86 (15) | 22,342 (16) |
| >12–24 | 103 (15) | 43,230 (15) | 126 (22) | 27,329 (20) |
| >24–60 | 71 (10) | 26,429 (9) | 138 (24) | 32,730 (24) |
| ≥60 w/>60–96 m | 10 (1) | 2274 (1) | 47(8) | 7238 (5) |
| ≥96 m | - | - | 13 (2) | 1729 (1) |
| continuous (g/d) (0.01% miss.) | 3.6 (0.6, 11.9) | 4.1 (0.6, 12) | 15.8 (5.2, 34.5) | 12.5 (3.9, 29.5) |
| meat intake (g/d) | 88 (59, 126) | 86 (53. 122) | 133 (90, 176) | 121 (80, 167) |
| type 2 diabetes | Missing 13% | Missing 8% | Missing 10% | Missing 12% |
| no | 599 (83) | 264,153 (89) | 472 (82) | 115,572 (85) |
| yes | 32 (4) | 6208 (2) | 36 (6) | 4486 (3) |
| unknown | 3 | 1097 (1) | 12(2) | 2 |
| smoking status | ||||
| never | 361 (50) | 167,470 (57) | 136 (24) | 45,457 (33) |
| former | 151 (21) | 66,229 (23) | 213 (37) | 49,891 (37) |
| current | 193 (27) | 53,257 (18) | 220 (38) | 38,737 (28) |
| unknown | 12 (2) | 6009 (2) | 8 (1) | 2066 (2) |
| smoking history | ||||
| never | 361 (50) | 167,470 (57) | 136 (24) | 45,457 (33) |
| current, 1–15 cig/day | 120 (17) | 35,915 (12) | 137 (24) | 24,282 (18) |
| current, 16–25 cig/day | 58 (8) | 14,538 (5) | 59 (10) | 10,708 (8) |
| current, 26+ cig/day | 15 (2) | 2804 (1) | 24 (4) | 3747 (3) |
| former, quit ≤ 10 years | 64 (10) | 26,168 (9) | 90 (16) | 19,006 (14) |
| former, quit 11–20 years | 28 (4) | 20,901 (7) | 50 (9) | 15,385 (11) |
| former, quit 20+ years | 59 (8) | 19,160 (6) | 73 (13) | 15,500 (11) |
| unknown | 12 (2) | 6009 (2) | 8 (1) | 2066 (2) |
| FEMALE Characteristics | Incident PC n = 717 | Non-cases n = 292,965 | ||
| Reproductive Factors—menarche | ||||
| age at menarche (years), (4% missing) | ||||
| never | - | 43 (0.01) | ||
| ≤9 | 2 (0.3) | 1647 (0.5) | ||
| 10–12 | 243 (31) | 113,605 (35) | ||
| 13–15 | 418 (54) | 181,664 (55) | ||
| ≥16 | 74 (10) | 18,818 (6) | ||
| duration menstrual cycles (years) (15% excluding participants with 0 and missing age at Menarche) | 35 (31,38) | 33 (28, 36) | ||
| duration menstrual cycles without OC (years) (15% missing excluding participants with 0 and missing age at Menarche and among pill non-users) | 32 (26, 36) | 29 (22, 34) | ||
| Reproductive Factors—pregnancy | ||||
| full term pregnancy (5% missing) | ||||
| no | 88 (12) | 45,148 (15) | ||
| yes | 602 (84) | 232,795 (81) | ||
| number of full-term pregnancies, (7% missing) | 2 (1, 3) | 2 (1, 3) | ||
| age at first full-term pregnancy (years) (0.4% missing among child bearing women) | 24 (22, 28) | 24 (22, 27) | ||
| breastfeeding (11% missing) | ||||
| no | 160 (22) | 77,799 (25) | ||
| yes | 480 (70) | 178,626 (63) | ||
| duration breast feeding among ever fed (months), (1% missing) | 6 (3, 12) | 6 (3, 11) | ||
| Reproductive Factors—menopause | ||||
| menopausal status | ||||
| premenopausal | 68 (10) | 101,184 (34) | ||
| postmenopausal | 524 (73) | 130,272 (43) | ||
| perimenopausal/unknown | 102 (14) | 52,532 (20) | ||
| surgical menopause | 23 (3) | 8977 (3) | ||
| age at menopause (years) (excluding premenopausal women, 47% missing) | 50 (47, 52) | 50 (46, 52) | ||
| Hormonal Factors—OC and HRT | ||||
| use of OC (3% missing) | ||||
| no | 352 (49) | 112,097 (38) | ||
| yes | 336 (47) | 171,435 (59) | ||
| duration of OC use (years), (47% missing) (10% missing among ever Pill users) | 6 (2, 11) | 6 (2,10) | ||
| use of HRT (6% missing: 11% cases, 7% non-cases) | ||||
| no | 424 (59) | 198,837 (68) | ||
| yes | 212 (30) | 71,400 (25) | ||
| use of HRT among postmenopausal women (8% missing: 11% cases, 6% non-cases) | ||||
| no | 294 (63) | 70,626 (58) | ||
| yes | 175 (37) | 51,674 (36) | ||
| duration of HRT use (years), (77% missing, 19% missing among ever hormone users) | 2.0 (1, 6) | 2.4 (1, 5) | ||
| duration of HRT use among all hormone users (years), (10% missing among ever hormone users) | 2.5 (1, 7) | 2.5 (1, 5) | ||
| duration of HRT use among postmenopausal women (years), (61% missing) | 2 (1, 7) | 3 (1, 6) | ||
| Hormonal Factors—surgery | ||||
| hysterectomy (11% missing) | ||||
| no | 495 (69) | 231,889 (78) | ||
| yes | 99 (14) | 32,734 (11) | ||
| Ovariectomy (20% missing) | ||||
| no | 520 (67) | 238,934 (73) | ||
| yes | 57 (8) | 20,266 (7) | ||
| FACTORS | All Subjects n = 430,410 1294 Cases | FEMALE n = 293,682 717 Cases | MALE n = 136,728 577 Cases |
|---|---|---|---|
| Age | 1.08 (1.07–1.09) | 1.09 (1.08–1.10) | 1.08 (1.06–1.09) |
| Sex age and center adjusted | - | Reference | 1.39 (1.24–1.57) |
| Sex fully adjusted | - | Reference | 1.31 (1.15–1.49) |
| Diabetes | 1.74 (1.35–2.23) | 1.74 (1.21–2.51) | 1.72 (1.21–2.43) |
| BMI [kg/m2] | |||
| <25 | Reference | Reference | Reference |
| 25–30 | 0.99 (0.87–1.12) | 0.99 (0.84–1.17) | 0.97 (0.81–1.18) |
| ≥30 | 1.09 (0.92–1.29) | 0.98 (0.78–1.24) | 1.22 (0.95–1.57) |
| per 5 unit increment | 1.07 (0.99–1.14) | 1.05 (0.96–1.14) | 1.10 (0.98–1.24) |
| Body height [cm]/per 5 unit incr. | 1.07 (1.03–1.12) | 1.12 (1.06–1.20) | 1.03 (0.96–1.09) |
| Highest school level | |||
| None | Reference | Reference | Reference |
| Primary school completed | 1.12 (0.77–1.63) | 1.21 (0.71–2.05) | 0.98 (0.57–1.68) |
| Technical/prof. school | 1.02 (0.69–1.51) | 1.08 (0.62–1.88) | 0.93 (0.53–1.63) |
| Secondary school | 1.00 (0.67–1.51) | 0.97 (0.55–1.72) | 1.04 (0.58–1.87) |
| Longer education | 1.02 (0.68–1.51) | 1.04 (0.59–1.83) | 0.92 (0.52–1.61) |
| Smoking status | |||
| Never | Reference | Reference | Reference |
| Former | 1.05 (0.91–1.21) | 0.94 (0.77–1.15) | 1.21 (0.97–1.51) |
| Current | 1.75 (1.52–2.01) | 1.74 (1.45–2.10) | 1.83 (1.46–2.29) |
| Cigarettes smoked/day | |||
| 1–10 | Reference | Reference | Reference |
| 11–20 | 1.59 (1.33–1.91) | 1.74 (1.35–2.24) | 1.49 (1.16–1.91) |
| 21–30 | 1.95 (1.45–2.61) | 1.91 (1.18–3.10) | 1.91 (1.32–2.76) |
| >30 | 1.17 (0.60–2.27) | 1.52 (0.48–4.79) | 1.01 (0.44–2.29) |
| continuous, 10/d | 1.28 (1.07–1.49) | 1.42 (1.27–1.59) | 1.21 (1.10–1.32) |
| Duration of smoking | |||
| 0.08–10 years | Reference | Reference | Reference |
| 11–20 years | 1.04 (0.78–1.37) | 0.94 (0.62–1.40) | 1.13 (0.77–1.67) |
| 21–30 years | 1.32 (1.02–1.70) | 1.26 (0.88–1.81) | 1.37 (0.96–1.97) |
| 31–40 years | 1.65 (1.29–2.09) | 1.80 (1.29–2.53) | 1.56 (1.11–2.22) |
| 41–50 years | 1.55 (1.18–2.03) | 1.59 (1.06–2.40) | 1.55 (1.06–2.25) |
| >50 years | 1.57 (0.85–2.91) | 1.68 (0.59–4.76) | 1.55 (0.71–3.35) |
| continuous/10 years | 1.16 (1.09–1.23) | 1.20 (1.10–1.31) | 1.13 (1.05–1.22) |
| Time since quitting smoking | |||
| 0.08–10 years | Reference | Reference | Reference |
| 11–20 years | 0.51 (0.40–0.65) | 0.53 (0.34–0.84) | 0.59 (0.43–0.79) |
| 21–30 years | 0.63 (0.49–0.80) | 0.72 (0.46–1.11) | 0.65 (0.47–0.89) |
| 31–40 years | 0.65 (0.47–0.91) | 0.72 (0.41–1.27) | 0.69 (0.44–1.08) |
| >40 years | 0.61 (0.32–1.17) | 0.55 (0.19–1.61) | 0.72 (0.31–1.69) |
| continuous/10 years | 0.82 (0.77–0.88) | 0.89 (0.76–1.04) | 0.85 (0.78–0.93) |
| Alcohol intake at recr. | |||
| non drinker | Reference | Reference | Reference |
| >0–6 (m)/>0–3 (w) | 0.89 (0.72–1.09) | 0.97 (0.76–1.25) | 0.75 (0.51–1.09) |
| >6–12 (m)/>3–12 (w) | 0.91 (0.73–1.12) | 0.98 (0.76–1.26) | 0.76 (0.52–1.13) |
| >12–24 | 1.02 (0.82–1.28) | 1.08 (0.81–1.44) | 0.87 (0.59–1.27) |
| >24 | 1.14 (0.91–1.42) | 1.34 (0.98–1.83) | 0.89 (0.62–1.28) |
| continuous, 15 g/d | 1.08 (1.03–1.13) | 1.11 (1.01–1.21) | 1.06 (1.01–1.12) |
| Meat intake at recr. | |||
| ≤65 | Reference | Reference | Reference |
| 66–102 | 1.14 (0.96–1.36) | 1.07 (0.87–1.32) | 1.33 (0.95–1.87) |
| 103–144 | 1.04 (0.87–1.26) | 1.05 (0.84–1.32) | 1.05 (0.74–1.48) |
| ≥145 | 1.35 (1.10–1.65) | 1.44 (1.11–1.87) | 1.27 (0.89–1.79) |
| continuous, 100 g/d | 1.15 (1.03–1.27) | 1.18 (1.02–1.36) | 1.07 (0.92–1.25) |
| Factors | HRs (95% CI) Adjusted for Age, Stratified by Study Centre | HRs (95% CI) Adjusted for Age, Education, Body Height, BMI, Smoking, Alcohol Consumption, Type 2 Diabetes Status; Stratified by Study Centre |
|---|---|---|
| Reproductive Factors—menarche | ||
| Age at menarche (years) | ||
| <12 (98 cases) | reference | |
| 12 (126 cases) | 0.91 (0.69–1.18) | 0.91 (0.69–1.19) |
| 13 (151 cases) | 0.82 (0.63–1.06) | 0.83 (0.64–1.07) |
| 14 (154 cases) | 0.85 (0.66–1.10) | 0.86 (0.66–1.12) |
| ≥15 (154 cases) | 0.95 (0.73–1.24) | 0.96 (0.74–1.25) |
| continuous (per age-year) | 1.00 (0.96–1.05) | 1.01 (0.96–1.06) |
| ≤12 (224 cases) | reference | |
| >12 (387 cases) | 0.89 (0.76–1.06) | 0.90 (0.76–1.07) |
| Cumulative duration menstrual cycling (years) | ||
| 0–27.10 (55 cases) | reference | |
| 27.11–31.28 (83 cases) | 0.97 (0.68–1.38) | 1.00 (0.71–1.43) |
| 31.29–34.19 (117 cases) | 1.03 (0.73–1.43) | 1.08 (0.77–1.51) |
| 34.20–36.91 (146 cases) | 0.99 (0.72–1.38) | 1.03 (0.74–1.44) |
| 36.92–54.0 (178 cases) | 1.04 (0.76–1.43) | 1.11 (0.81–1.54) |
| continuous (per year) | 1.01 (0.99–1.02) | 1.01 (0.99–1.03) |
| ≤33.04 (198 cases) | reference | |
| >33.04 (381 cases) | 1.09 (0.92–1.31) | 1.13 (0.94–1.35) |
| Cumulative duration menstrual cycling without OC (years) | ||
| ≤20.24 (52 cases) | reference | |
| 20.25–27.05 (82 cases) | 0.99 (0.69–1.42) | 1.03 (0.72–1.47) |
| 27.06–31.50 (107 cases) | 0.98 (0.69–1.38) | 1.02 (0.72–1.45) |
| 31.51–35.49 (137 cases) | 0.98 (0.69–1.37) | 1.01 (0.72–1.42) |
| >35.50 (164 cases) | 0.99 (0.71–1.38) | 1.06 (0.76–1.48) |
| continuous (per year) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) |
| ≤29.48 (202 cases) | reference | |
| >29.48 (342 cases) | 0.91 (0.75–1.09) | 0.95 (0.79–1.14) |
| Reproductive Factors—pregnancy | ||
| Full-term pregnancy | ||
| no (97 cases) | reference | |
| yes (589 cases) | 0.89 (0.71–1.10) | 0.87 (0.70–1.09) |
| Number of full-term pregnancies | ||
| 0 (97 cases) | reference | |
| 1 (119 cases) | 0.94 (0.72–1.24) | 0.91 (0.69–1.20) |
| 2 (265 cases) | 0.89 (0.70–1.13) | 0.89 (0.70–1.13) |
| 3 (133 cases) | 0.85 (0.66–1.11) | 0.84 (0.64–1.09) |
| ≥4 (68 cases) | 0.78 (0.57–1.08) | 0.77 (0.56–1.06) |
| continuous | 0.96 (0.90–1.02) | 0.96 (0.90–1.02) |
| ≤2 (472 cases) | reference | |
| >2 (201 cases) | 0.89 (0.76–1.07) | 0.89 (0.75–1.06) |
| Age at first full-term pregnancy (years) among childbearing women | ||
| <22 (183 cases) | reference | |
| 22–24 (113 cases) | 0.92 (0.72–1.16) | 0.94 (0.74–1.19) |
| 25–29 (206 cases) | 0.88 (0.72–1.08) | 0.96 (0.78–1.18) |
| ≥30 (84 cases) | 0.95 (0.73–1.24) | 1.04 (0.79–1.36) |
| continuous (per age-year) | 0.99 (0.98–1.02) | 1.01 (0.98–1.03) |
| ≤24 (296 cases) | reference | |
| >24 (290 cases) | 0.93 (0.79–1.10) | 1.00 (0.84–1.19) |
| Breastfeeding | ||
| no (160 cases) | reference | |
| yes (480 cases) | 0.98 (0.82–1.17) | 0.99 (0.83–1.19) |
| Cumulative duration of breast feeding (months) among breast feeding women | ||
| ≤2.07 (114 cases) | reference | |
| 2.08–5.00 (109 cases) | 0.84 (0.65–1.10) | 0.89 (0.68–1.16) |
| 5.01–8.50 (78 cases) | 0.69 (0.51–0.92) | 0.73 (0.54–0.98) |
| 8.51–15.00 (95 cases) | 0.71 (0.54–0.93) | 0.76 (0.57–1.01) |
| >15.00 (82 cases) | 0.73 (0.54–0.98) | 0.78 (0.58–1.05) |
| continuous (per month) | 0.99 (0.98–1.00) | 0.99 (0.98–1.01) |
| ≤5.74 (237 cases) | reference | |
| >5.74 (241 cases) | 0.74 (0.61–0.89) | 0.77 (0.64–0.93) |
| Reproductive Factors—menopause | ||
| Menopausal status | ||
| Pre (68 cases) | 0.87 (0.61–1.22) | 0.89 (0.63–1.26) |
| Peri/unknown (102 cases) | 1.09 (0.85–1.39) | 1.07 (0.83–1.36) |
| Post (524 cases) | reference | |
| Surgical (23 cases) | 0.74 (0.48–1.12) | 0.68 (0.44–1.05) |
| Age at menopause (years) | ||
| <46 (109 cases) | reference | |
| 46–50 (164 cases) | 1.03 (0.81–1.31) | 1.04 (0.82–1.33) |
| 51–52 (74 cases) | 0.96 (0.71–1.28) | 0.97 (0.72–1.31) |
| ≥53 (100 cases) | 1.02 (0.77–1.34) | 1.06 (0.80–1.39) |
| continuous (per year) | 0.99 (0.98–1.02) | 1.00 (0.98–1.02) |
| ≤50 (273 cases) | reference | |
| >50 (174 cases) | 0.97 (0.80–1.17) | 0.99 (0.82–1.21) |
| Hormonal Factors—OC and HRT | ||
| Ever use OC | ||
| no (352 cases) | reference | |
| yes (336 cases) | 1.16 (0.98–1.36) | 1.13 (0.96–1.34) |
| Cumulative duration of OC use (years) among OC users | ||
| ≤2 (89 cases) | reference | |
| 2–5 (49 cases) | 0.84 (0.59–1.19) | 0.86 (0.61–1.23) |
| 6–10 (75 cases) | 1.04 (0.76–1.42) | 1.04 (0.76–1.43) |
| >10 (85 cases) | 1.05 (0.78–1.43) | 1.04 (0.76–1.41) |
| continuous (per year) | 1.01 (0.99–1.02) | 1.01 (0.99–1.02) |
| ≤6 (147 cases) | reference | |
| >6 (151 cases) | 1.20 (0.95–1.52) | 1.19 (0.93–1.50) |
| Ever use of HRT | ||
| no (424 cases) | reference | |
| yes (212 cases) | 1.04 (0.87–1.23) | 1.01 (0.85–1.21) |
| Cumulative duration of HRT use (years) among HRT users | ||
| ≤1 (74 cases) | reference | |
| 1.1–2.5 (30 cases) | 0.83 (0.54–1.28) | 0.82 (0.53–1.27) |
| 2.6–5.0 (38 cases) | 0.69 (0.46–1.02) | 0.69 (0.47–1.03) |
| >5.0 (50 cases) | 0.63 (0.43–0.914) | 0.64 (0.44–0.93) |
| continuous (per year) | 0.98 (0.96–1.02) | 0.99 (0.96–1.02) |
| ≤2.42 (102 cases) | reference | |
| >2.42 (90 cases) | 0.71 (0.53–0.95) | 0.72 (0.54–0.97) |
| Hormonal Factors—surgery | ||
| Hysterectomy | ||
| no (495 cases) | reference | |
| yes (99 cases) | 0.95 (0.76–1.18) | 0.94 (0.75–1.17) |
| Ovariectomy | ||
| no (520 cases) | reference | |
| yes (57 cases) | 0.89 (0.68–2.17) | 0.87 (0.65–1.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katzke, V.A.; Dutta, S.; Rasokat, A.; Archibugi, L.; Capurso, G.; Peduzzi, G.; Gentiluomo, M.; Canzian, F.; Eriksen, A.K.; Tjønneland, A.; et al. Sex Disparities and Female Reproductive and Hormonal Factors Associated with Risk of Pancreatic Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Cancers 2025, 17, 2275. https://doi.org/10.3390/cancers17142275
Katzke VA, Dutta S, Rasokat A, Archibugi L, Capurso G, Peduzzi G, Gentiluomo M, Canzian F, Eriksen AK, Tjønneland A, et al. Sex Disparities and Female Reproductive and Hormonal Factors Associated with Risk of Pancreatic Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Cancers. 2025; 17(14):2275. https://doi.org/10.3390/cancers17142275
Chicago/Turabian StyleKatzke, Verena A., Srimanti Dutta, Anna Rasokat, Livia Archibugi, Gabriele Capurso, Giulia Peduzzi, Manuel Gentiluomo, Federico Canzian, Anne Kirstine Eriksen, Anne Tjønneland, and et al. 2025. "Sex Disparities and Female Reproductive and Hormonal Factors Associated with Risk of Pancreatic Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort" Cancers 17, no. 14: 2275. https://doi.org/10.3390/cancers17142275
APA StyleKatzke, V. A., Dutta, S., Rasokat, A., Archibugi, L., Capurso, G., Peduzzi, G., Gentiluomo, M., Canzian, F., Eriksen, A. K., Tjønneland, A., Dahm, C. C., Truong, T., Canonico, M., Laouali, N., Schulze, M. B., Tumino, R., Masala, G., Agnoli, C., Dansero, L., ... Kaaks, R. (2025). Sex Disparities and Female Reproductive and Hormonal Factors Associated with Risk of Pancreatic Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Cancers, 17(14), 2275. https://doi.org/10.3390/cancers17142275









