Association of Tumor-Infiltrating Lymphocytes and Inflammation Status with Survival Outcome in Patients with High-Grade Serous Ovarian Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
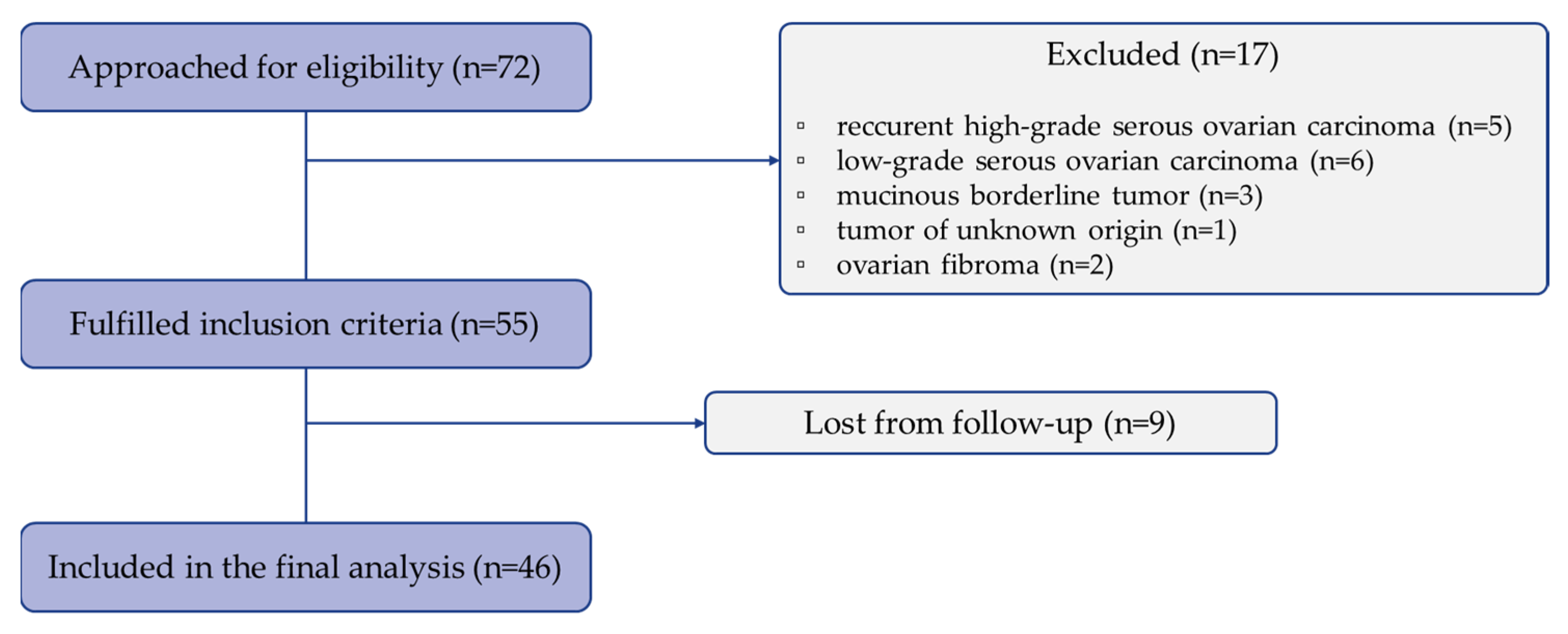
2.1. Patients
2.2. Study Design
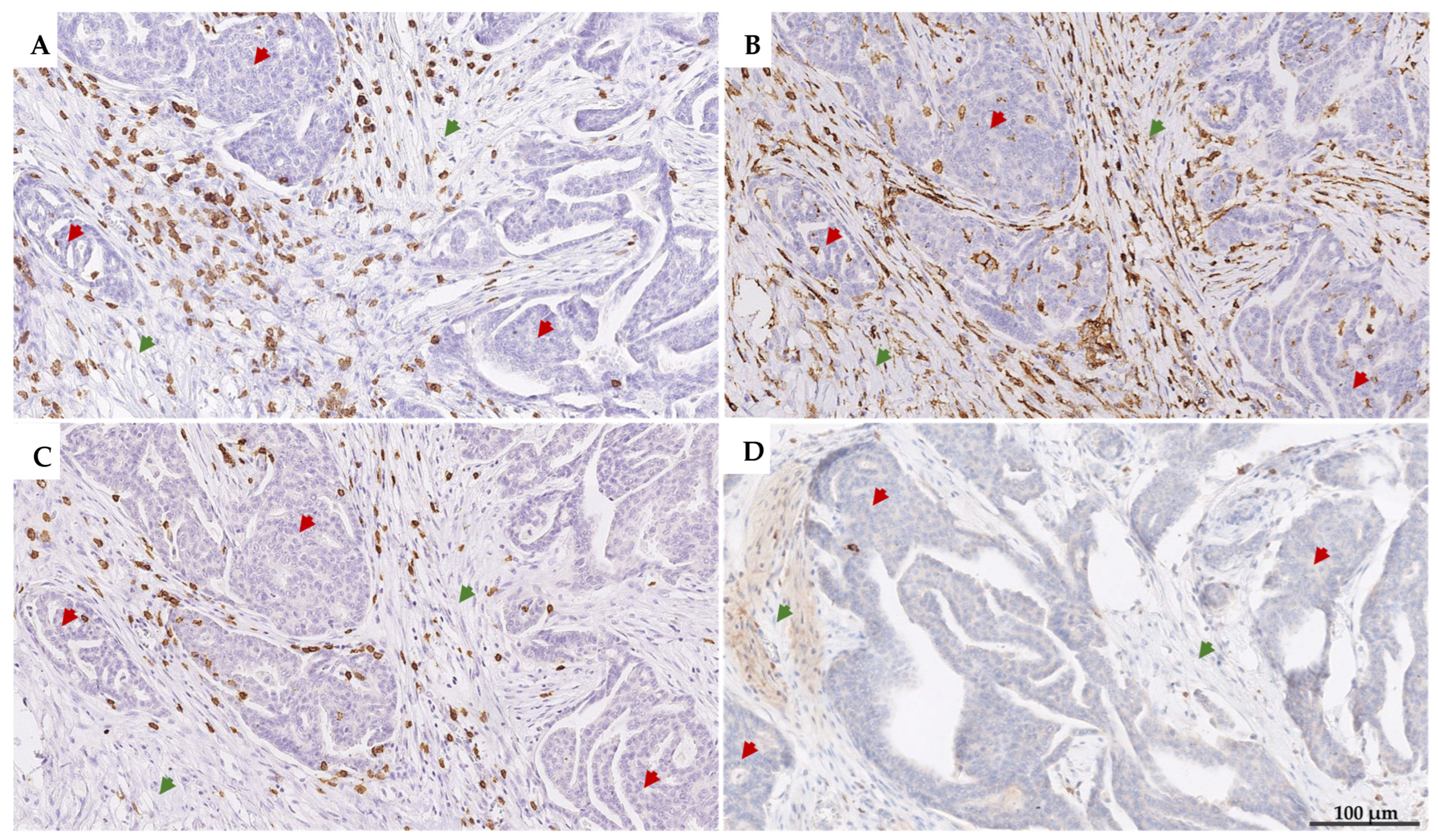
2.3. Immunohistochemistry
2.4. Manual Scoring of iTILs and sTILs
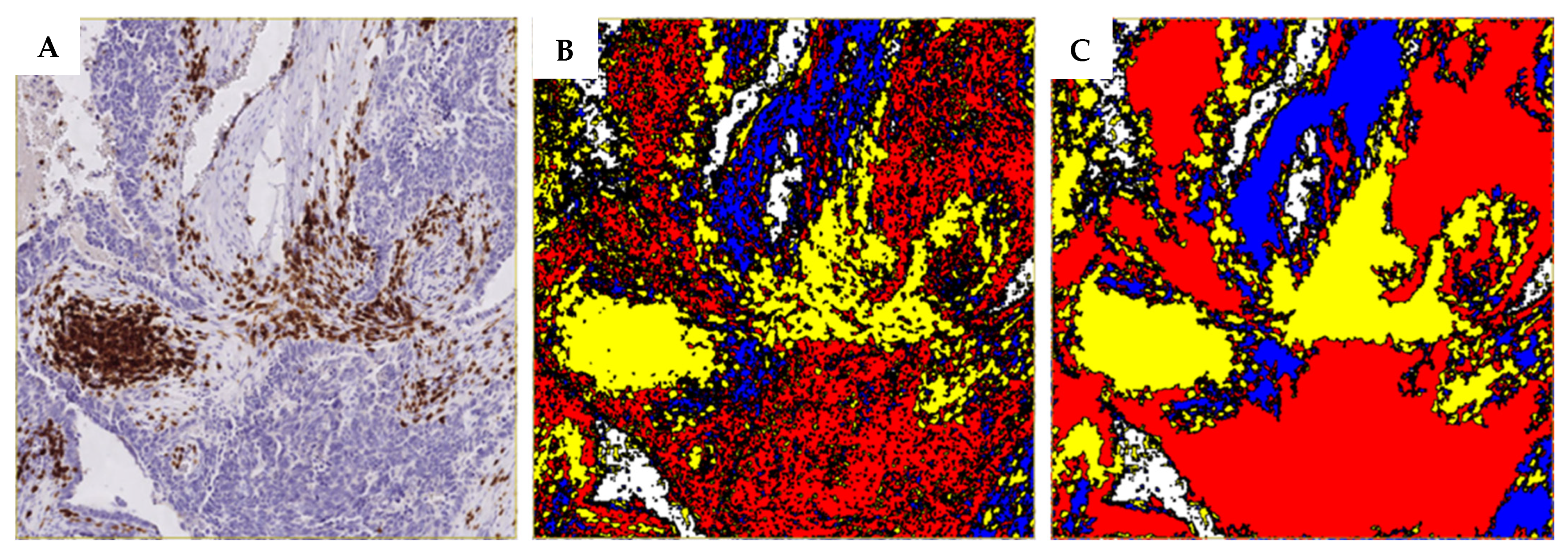
2.5. Digital Image Analysis
2.6. Inflammation Status Calculation
2.7. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
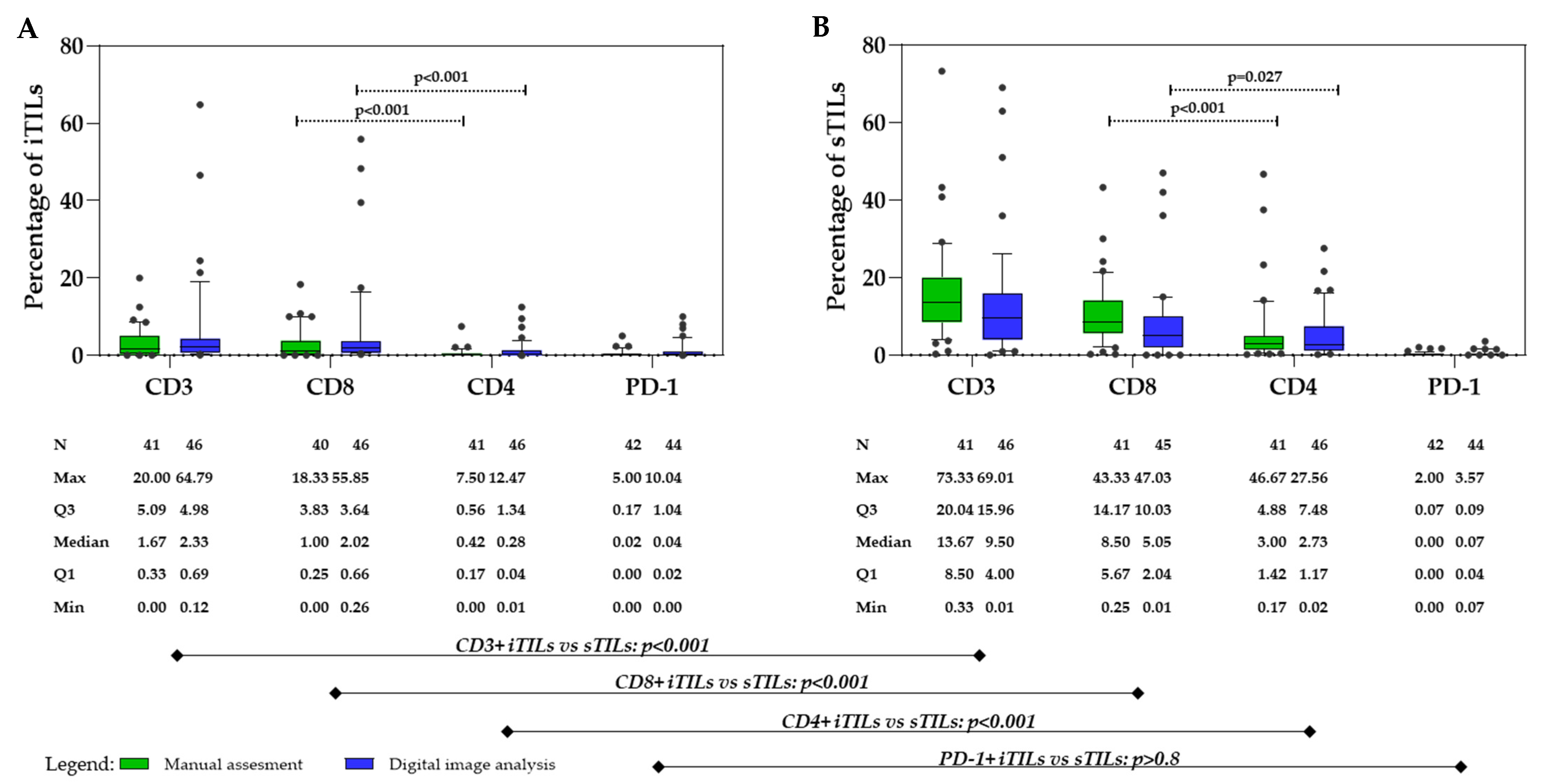
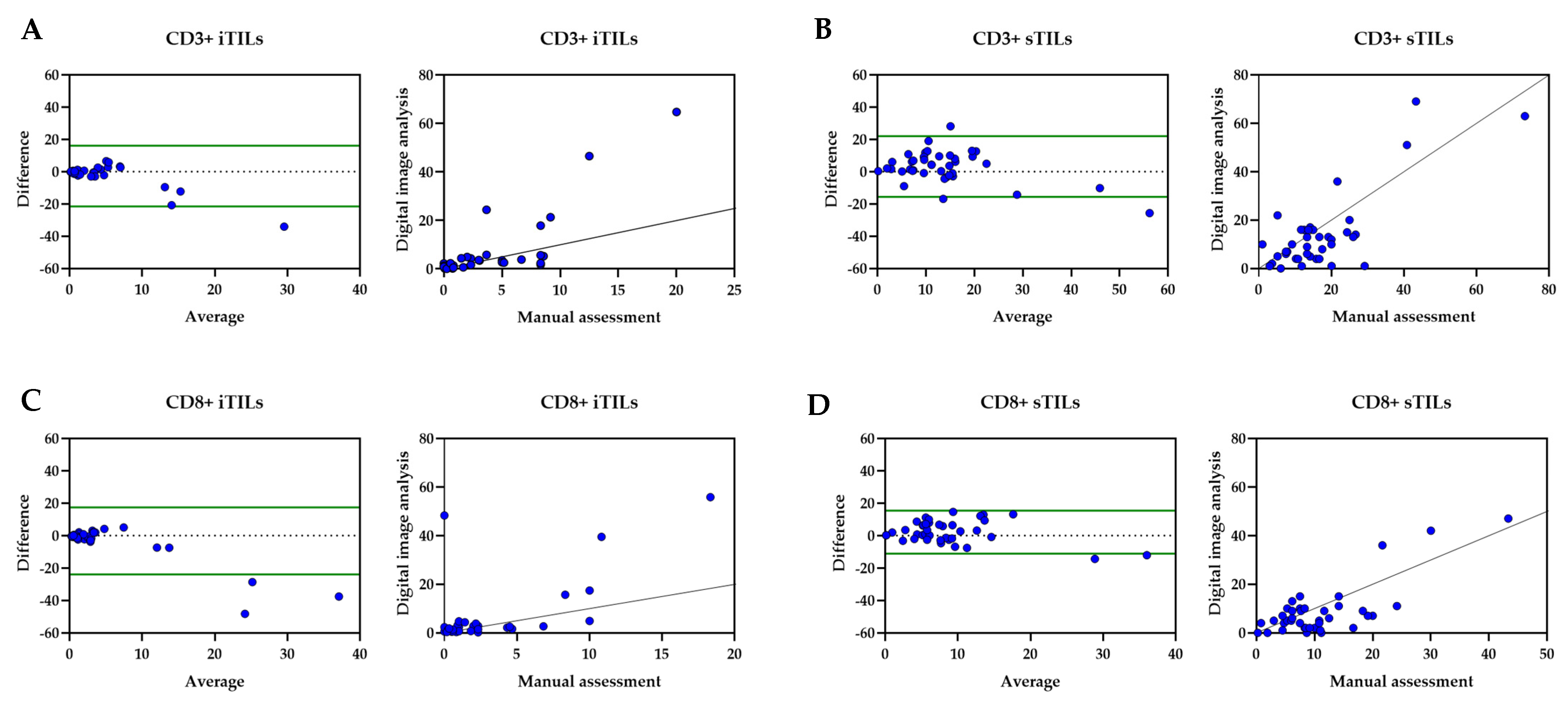
3.2. Tumor-Infiltrating Lymphocytes
3.3. Inflammation Status
3.4. ROC Curve Analysis
3.5. Univariate Analysis
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Jayde, V.; White, K.; Blomfield, P. Symptoms and diagnostic delay in ovarian cancer: A summary of the literature. Contemp. Nurse 2009, 34, 55–65. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Bekos, C.; Pils, D.; Dekan, S.; Hofstetter, G.; Horak, P.; Reinthaller, A.; Polterauer, S.; Schwameis, R.; Aust, S. PD-1 and PD-L1 expression on TILs in peritoneal metastases compared to ovarian tumor tissues and its associations with clinical outcome. Sci. Rep. 2021, 11, 6400. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Lee, S.J.; Lee, J.H.; Kim, K.H.; Suh, D.S.; Kwon, B.S.; Choi, K.U. Stromal tumor-infiltrating lymphocytes evaluated on H&E-stained slides are an independent prognostic factor in epithelial ovarian cancer and ovarian serous carcinoma. Oncol. Lett. 2019, 17, 4557–4565. [Google Scholar]
- James, F.R.; Jiminez-Linan, M.; Alsop, J.; Mack, M.; Song, H.; Brenton, J.D.; Pharoah, P.D.P.; Ali, H.R. Association between tumour infiltrating lymphocytes, histotype and clinical outcome in epithelial ovarian cancer. BMC Cancer 2017, 17, 657. [Google Scholar] [CrossRef]
- Ovarian Tumor Tissue Analysis (OTTA) Consortium; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Tołoczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar]
- Stanske, M.; Wienert, S.; Castillo-Tong, D.C.; Kreuzinger, C.; Vergote, I.; Lambrechts, S.; Gabra, H.; Gourley, C.; Ganapathi, R.N.; Kolaschinski, I.; et al. Dynamics of the Intratumoral Immune Response during Progression of High-Grade Serous Ovarian Cancer. Neoplasia 2018, 20, 280–288. [Google Scholar] [CrossRef]
- Fanale, D.; Dimino, A.; Pedone, E.; Brando, C.; Corsini, L.R.; Filorizzo, C.; Fiorino, A.; Lisanti, M.C.; Magrin, L.; Randazzo, U.; et al. Prognostic and Predictive Role of Tumor-Infiltrating Lymphocytes (TILs) in Ovarian Cancer. Cancers 2022, 14, 4344. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; Van De Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar]
- Laury, A.R.; Blom, S.; Ropponen, T.; Virtanen, A.; Carpén, O.M. Artificial intelligence-based image analysis can predict outcome in high-grade serous carcinoma via histology alone. Sci. Rep. 2021, 11, 19165. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Boschman, J.; Farnell, D.; Darbandsari, A.; Zhang, A.; Ahmadvand, P.; Jones, S.J.M.; Huntsman, D.; Köbel, M.; Gilks, C.B.; et al. Deep learning-based histotype diagnosis of ovarian carcinoma whole-slide pathology images. Mod. Pathol. Off. J. United States Can. Acad. Pathol. 2022, 35, 1983–1990. [Google Scholar] [CrossRef]
- Machuca-Aguado, J.; Conde-Martín, A.F.; Alvarez-Muñoz, A.; Rodríguez-Zarco, E.; Polo-Velasco, A.; Rueda-Ramos, A.; Rendón-García, R.; Ríos-Martin, J.J.; Idoate, M.A. Machine Learning Quantification of Intraepithelial Tumor-Infiltrating Lymphocytes as a Significant Prognostic Factor in High-Grade Serous Ovarian Carcinomas. Int. J. Mol. Sci. 2023, 24, 16060. [Google Scholar] [CrossRef]
- Song, Q.; Xu, S.X.; Wu, J.Z.; Ling, L.; Wang, S.; Shu, X.H.; Ying, D.N.; Pei, W.W.; Wu, Y.C.; Sun, S.F.; et al. The preoperative platelet to neutrophil ratio and lymphocyte to monocyte ratio are superior prognostic indicators compared with other inflammatory biomarkers in ovarian cancer. Front. Immunol. 2023, 14, 1177403. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; D’Indinosante, M.; Marchetti, C.; Tudisco, R.; Turchiano, F.; Scambia, G.; Fagotti, A. The prognostic role of systemic inflammatory markers in apparent early-stage ovarian cancer. Int. J. Clin. Oncol. 2023, 28, 314–320. [Google Scholar] [CrossRef]
- Liao, W.; Li, J.; Feng, W.; Kong, W.; Shen, Y.; Chen, Z.; Yang, H. Pan-immune-inflammation value: A new prognostic index in epithelial ovarian cancer. BMC Cancer 2024, 24, 1052. [Google Scholar] [CrossRef]
- Deeba, F.; Khatun, S.; Alam, M.M.; Shahida, S.M. Serum LDH and CA-125: Markers for Diagnosis of Ovarian Malignancy. Mymensingh Med. J. MMJ 2015, 24, 334–340. [Google Scholar]
- Hefler, L.A.; Concin, N.; Hofstetter, G.; Marth, C.; Mustea, A.; Sehouli, J.; Zeillinger, R.; Leipold, H.; Lass, H.; Grimm, C.; et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 710–714. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C.; ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv259. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Han, X.; Zha, H.; Tao, H.; Li, X.; Yuan, F.; Chen, G.; Wang, L.; Ma, J.; Hu, Y. Systemic Immune-Inflammation Index and Changes of Neutrophil-Lymphocyte Ratio as Prognostic Biomarkers for PATIENTSWITH Pancreatic Cancer Treated with Immune Checkpoint Blockade. Front. Oncol. 2021, 11, 585271. [Google Scholar] [CrossRef]
- Efil, S.C.; Guner, G.; Guven, D.C.; Celikten, B.; Celebiyev, E.; Taban, H.; Akyol, A.; Isik, A.; Kilickap, S.; Yalcin, S.; et al. Prognostic and predictive value of tumor infiltrating lymphocytes in combination with systemic inflammatory markers in colon cancer. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102171. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Le Guellec, S.; Meignan, S.; Bécourt, S.; Pasquesoone, C.; El Hajj, H.; Martínez-Gómez, C.; Leblanc, É.; Narducci, F.; Ladoire, S. Tumor-Infiltrating Lymphocytes (TILs) in Epithelial Ovarian Cancer: Heterogeneity, Prognostic Impact, and Relationship with Immune Checkpoints. Cancers 2022, 14, 5332. [Google Scholar] [CrossRef] [PubMed]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Masoumeh, G.; et al. The tale of TILs in breast cancer: A report from the International Immuno-Oncology Biomarker Working Group. npj Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef]
- Miceska, S.; Skof, E.; Bucek, S.; Kuhar, C.G.; Gasljevic, G.; Smrkolj, S.; Prevodnik, V.K. The prognostic significance of tumor-immune microenvironment in ascites of patients with high-grade serous carcinoma. Radiol. Oncol. 2023, 57, 493–506. [Google Scholar] [CrossRef]
- Miceska, S.; Škof, E.; Gašljević, G.; Kloboves-Prevodnik, V. Morphological and Immunocytochemical Characterization of Tumor Spheroids in Ascites from High-Grade Serous Carcinoma. Cells 2023, 12, 2390. [Google Scholar] [CrossRef]
- Drakes, M.L.; Mehrotra, S.; Aldulescu, M.; Potkul, R.K.; Liu, Y.; Grisoli, A.; Joyce, C.; O’Brien, T.E.; Stack, M.S.; Stiff, P.J. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J. Ovarian Res. 2018, 11, 43. [Google Scholar] [CrossRef]
- Darb-Esfahani, S.; Kunze, C.A.; Kulbe, H.; Sehouli, J.; Wienert, S.; Lindner, J.; Budczies, J.; Bockmayr, M.; Dietel, M.; Denkert, C.; et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016, 7, 1486–1499. [Google Scholar] [CrossRef]
- Martin de la Fuente, L.; Westbom-Fremer, S.; Arildsen, N.S.; Hartman, L.; Malander, S.; Kannisto, P.; Måsbäck, A.; Hedenfalk, I. PD-1/PD-L1 expression and tumor-infiltrating lymphocytes are prognostically favorable in advanced high-grade serous ovarian carcinoma. Virchows Arch. Int. J. Pathol. 2020, 477, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Thagaard, J.; Stovgaard, E.S.; Vognsen, L.G.; Hauberg, S.; Dahl, A.; Ebstrup, T.; Doré, J.; Vincentz, R.E.; Jepsen, R.K.; Roslind, A.; et al. Automated Quantification of sTIL Density with H&E-Based Digital Image Analysis Has Prognostic Potential in Triple-Negative Breast Cancers. Cancers 2021, 13, 3050. [Google Scholar]
- Li, J.; Wang, J.; Chen, R.; Bai, Y.; Lu, X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017, 8, 15621–15631. [Google Scholar] [CrossRef]
- Tubridy, E.A.; Eiva, M.A.; Liu, F.; Omran, D.K.; Gysler, S.; Brown, E.G.; Roy, A.G.; Zeng, Y.; Oh, J.; Cao, Q.; et al. CD137+ tumor infiltrating lymphocytes predicts ovarian cancer survival. Gynecol. Oncol. 2024, 184, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Liu, L.; Huang, H.; Chen, S.; Chen, B.; Cao, J.; Luo, X.; Wang, F.; Luo, R.; Liu, J. Nomograms to Predict the Density of Tumor-Infiltrating Lymphocytes in Patients with High-Grade Serous Ovarian Cancer. Front. Oncol. 2021, 11, 590414. [Google Scholar] [CrossRef]
- Pizarro, D.; Romero, I.; Pérez-Mies, B.; Redondo, A.; Caniego-Casas, T.; Carretero-Barrio, I.; Cristóbal, E.; Gutiérrez-Pecharromán, A.; Santaballa, A.; D’Angelo, E.; et al. The Prognostic Significance of Tumor-Infiltrating Lymphocytes, PD-L1, BRCA Mutation Status and Tumor Mutational Burden in Early-Stage High-Grade Serous Ovarian Carcinoma-A Study by the Spanish Group for Ovarian Cancer Research (GEICO). Int. J. Mol. Sci. 2023, 24, 11183. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Kumar, S.; Konwar, R.; Makker, A.; Negi, M.P.; Goel, M.M. CD3+, CD4+ & CD8+ tumour-infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J. Med. Res. 2014, 140, 361–369. [Google Scholar]
- Gomez-Macias, G.S.; Molinar-Flores, G.; Lopez-Garcia, C.A.; Santuario-Facio, S.; Decanini-Arcaute, H.; Valero-Elizondo, J.; Treviño-Alvarado, V.; Ortiz-Lopez, R.; Dono, A.; Esteban-Zubero, E.; et al. Immunotyping of tumor-infiltrating lymphocytes in triple-negative breast cancer and genetic characterization. Oncol. Lett. 2020, 20, 140. [Google Scholar] [CrossRef]
- Aliyeva, T.; Aktas, B.Y.; Gundogdu, F.; Chalabiyev, E.; Arik, Z.; Usubutun, A. The predictive role of PD-L1 expression and CD8+ TIL levels in determining the neoadjuvant chemotherapy response in advanced ovarian cancer. J. Ovarian Res. 2024, 17, 234. [Google Scholar] [CrossRef]
- Karakaya, A.; Atıgan, Y.; Güler, Ö.T.; Demiray, A.G.; Bir, F. The relation of CD3, CD4, CD8 and PD-1 expression with tumor type and prognosis in epithelial ovarian cancers. Ginekol. Pol. 2021, 92, 344–351. [Google Scholar] [CrossRef]
- Buderath, P.; Mairinger, F.; Mairinger, E.; Böhm, K.; Mach, P.; Schmid, K.W.; Kimmig, R.; Kasimir-Bauer, S.; Bankfalvi, A.; Westerwick, D.; et al. Prognostic significance of PD-1 and PD-L1 positive tumor-infiltrating immune cells in ovarian carcinoma. Inter. J. Gynecol. Cancer 2019, 29, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Baş, Y.; Koç, N.; Helvacı, K.; Koçak, C.; Akdeniz, R.; Şahin, H.H.K. Clinical and pathological significance of programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) expression in high grade serous ovarian cancer. Transl. Oncol. 2021, 14, 100994. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Goto, K.; Morimoto-Okazawa, A.; Haruna, M.; Yamamoto, K.; Yamamoto, Y.; Nakagawa, S.; Hiramatsu, K.; Matsuzaki, S.; Kobayashi, E.; et al. PD-1+ Tim3+ tumor-infiltrating CD8 T cells sustain the potential for IFN-γ production, but lose cytotoxic activity in ovarian cancer. Int. Immunol. 2020, 32, 397–405. [Google Scholar] [CrossRef]
- Koh, C.H.; Lee, S.; Kwak, M.; Kim, B.S.; Chung, Y. CD8 T-cell subsets: Heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.P.; Balmaceda, C.; Bravo, M.L.; Kato, S.; Villarroel, A.; Owen, G.I.; Roa, J.C.; Cuello, M.A.; Ibañez, C. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 151, 10–17. [Google Scholar] [CrossRef]
- Barna, A.J.; Herold, Z.; Acs, M.; Bazsa, S.; Gajdacsi, J.; Garay, T.M.; Herold, M.; Madaras, L.; Muhl, D.; Nagy, A.; et al. High Tumor-Infiltrating Lymphocyte Count Is Associated with Distinct Gene Expression Profile and Longer Patient Survival in Advanced Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 13684. [Google Scholar] [CrossRef]
- Preston, C.C.; Maurer, M.J.; Oberg, A.L.; Visscher, D.W.; Kalli, K.R.; Hartmann, L.C.; Goode, E.L.; Knutson, K.L. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS ONE 2013, 8, e80063. [Google Scholar] [CrossRef]
- Farolfi, A.; Scarpi, E.; Greco, F.; Bergamini, A.; Longo, L.; Pignata, S.; Casanova, C.; Cormio, G.; Bologna, A.; Orditura, M.; et al. Inflammatory indexes as predictive factors for platinum sensitivity and as prognostic factors in recurrent epithelial ovarian cancer patients: A MITO24 retrospective study. Sci. Rep. 2020, 10, 18190. [Google Scholar] [CrossRef]
- Mao, H.; Yang, F. Prognostic significance of systemic immune-inflammation index in patients with ovarian cancer: A meta-analysis. Front. Oncol. 2023, 13, 1193962. [Google Scholar] [CrossRef]
- Chu, B.; Chen, Y.; Pan, J. Prognostic significance of systemic immune inflammation index for ovarian cancer: An updated systematic review and meta-analysis. J. Ovarian Res. 2025, 18, 41. [Google Scholar] [CrossRef]
- Nie, D.; Gong, H.; Mao, X.; Li, Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: A retrospective study. Gynecol. Oncol. 2019, 152, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kuang, T.; Qiu, Z.; Wang, K.; Zhang, L.; Dong, K.; Wang, W. Pan-immune inflammation value as a prognostic biomarker for cancer patients treated with immune checkpoint inhibitors. Front. Immunol. 2024, 15, 1326083. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Venkatraman, E.S.; Masson, V.; Hoskins, W.J. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecol. Oncol. 2000, 77, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, R.; Brucker, S.; Stäbler, A.; Krämer, B.; Ladurner, R.; Königsrainer, A.; Wallwiener, D.; Bachmann, C. Erratum: Prognostic relevance of high pretreatment CA125 levels in primary serous ovarian cancer. Mol. Clin. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Ay, S.; Ozyukseler, D.T.; Dulgar, O.; Basak, M.; Yildirim, M.E.; Gumus, M. Initial CA125 value as a predictive marker for high-grade serous ovarian cancer. J. Coll. Physicians Surg. Pak. 2021, 31, 651–656. [Google Scholar] [PubMed]
- Asali, A.; Haj-Yehia, N.; Zehavi, T.; Perry, T.; Beiner, M.; Fishman, A.; Kadan, Y. High grade, advanced, serous ovarian cancer with low serum CA125 levels. J. Obstet. Gynaecol. 2021, 41, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Yamaguchi, K.; Yamakage, H.; Abiko, K.; Satoh-Asahara, N.; Takakura, K.; Konishi, I. Serum lactate dehydrogenase is a possible predictor of platinum resistance in ovarian cancer. Obstet. Gynecol. Sci. 2020, 63, 709–718. [Google Scholar] [CrossRef]
- Bastani, A.; Asghary, A.; Heidari, M.H.; Karimi-Busheri, F. Evaluation of the sensitivity and specificity of serum level of prostasin, CA125, LDH, AFP, and hCG+β in epithelial ovarian cancer patients. Eur. J. Gynaecol. Oncol. 2017, 38, 418–424. [Google Scholar]
- Yang, D.; Li, H.; Sun, X.; Yang, S.; Wang, K.; Liu, Z. Clinical usefulness of high levels of C-reactive protein for diagnosing epithelial ovarian cancer. Sci. Rep. 2020, 10, 20056. [Google Scholar] [CrossRef]

| Clinical Characteristics | |
|---|---|
| Age at diagnosis, years | |
| Mean (range) | 64 (44–86) |
| FIGO stage, No. (%) | |
| II | 2 (4.3) |
| IIIA | 1 (2.2) |
| IIIB | 1 (2.2) |
| IIIC | 32 (69.6) |
| IVA | 4 (8.7) |
| IVB | 6 (13.0) |
| Positive family history, No. (%) | |
| breast, ovary, prostate, pancreas | 17 (36.9) |
| Chemotherapy, No. (%) | |
| neoadjuvant | 31 (67.4) |
| adjuvant | 15 (32.6) |
| Surgery, No. (%) | |
| Primary | 15 (32.6) |
| Interval | 25 (54.4) |
| Inoperable | 6 (13.0) |
| Patient status, No. (%) | |
| Progression | 39 (84.8) |
| Death | 31 (61.4) |
| Ascites presence, No. (%) | |
| yes | 46 (100%) |
| Parameter at Diagnosis | Cut-Off Value by ROC Curve | AUC | CI 95% | p-Value | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| CD3+ sTILs manual assessment, low vs. high | 12.9% | 0.645 | 0.474–0.816 | 0.261 | 0.657 | 0.667 |
| CD8+ sTILs manual assessment, low vs. high | 5.66% | 0.731 | 0.508–0.954 | 0.074 | 0.829 | 0.667 |
| CD4+ sTILs manual assessment, low vs. high | 1.83% | 0.764 | 0.615–0.913 | 0.410 | 0.714 | 0.667s |
| CD3+ sTILs digital image analysis, low vs. high | 4.49% | 0.736 | 0.525–0.948 | 0.049 | 0.821 | 0.714 |
| CD8+ sTILs digital image analysis, low vs. high | 2.03% | 0.748 | 0.565–0.931 | 0.039 | 0.816 | 0.714 |
| SII low vs. high | 912.45 × 109/L | 0.715 | 0.464–0.966 | 0.094 | 0.763 | 0.667 |
| PIV low vs. high | 423.92 × 109/L | 0.811 | 0.575–1.000 | 0.015 | 0.868 | 0.667 |
| CA 125 low vs. high | 64.5 U/mL | 0.768 | 0.560–0.975 | 0.370 | 0.737 | 0.833 |
| LDH low vs. high | 3.01 µkat/L | 0.761 | 0.495–1.000 | 0.042 | 0.658 | 0.833 |
| CRP low vs. high | 6.55 mg/L | 0.724 | 0.495–0.952 | 0.081 | 0.579 | 0.833 |
| Parameter at Diagnosis | Hazard Ratio | CI 95% | p-Value |
|---|---|---|---|
| FIGO stage II+III vs. stage IV | 0.66 | 0.32–1.37 | 0.266 |
| Residual disease after surgery no vs. yes | 0.34 | 0.16–0.72 | 0.005 |
| CD3+ sTILs manual score low vs. high | 0.50 | 0.24–1.04 | 0.064 |
| CD8+ sTILs manual score low vs. high | 0.30 | 0.12–0.79 | 0.015 |
| CD3+ sTILs digital score low vs. high | 0.31 | 1.15–0.67 | 0.003 |
| CD8+ sTILs digital score low vs. high | 0.53 | 0.27–1.04 | 0.066 |
| PIV low vs. high | 0.32 | 0.12–0.82 | 0.018 |
| SII low vs. high | 0.51 | 0.24–1.09 | 0.080 |
| CA 125 low vs. high | 0.35 | 0.16–0.75 | 0.007 |
| LDH low vs. high | 0.44 | 0.22–0.89 | 0.022 |
| CRP low vs. high | 0.56 | 0.29–1.09 | 0.090 |
| Model | Variable | Hazard Ratio | CI 95% | p-Value |
|---|---|---|---|---|
| Model 1: Manual assessment of sTILs | ||||
| PIV low vs. high | 0.32 | 0.11–0.91 | 0.032 | |
| Residual disease after surgery no vs. yes | 0.81 | 0.33–2.00 | 0.649 | |
| CD8+ sTILs (manual score) low vs. high | 0.30 | 0.11–0.84 | 0.021 | |
| Model 2: Digital image analysis of sTILs | ||||
| PIV low vs. high | 0.35 | 0.13–0.96 | 0.04 | |
| Residual disease after surgery no vs. yes | 0.21 | 0.08–0.53 | 0.001 | |
| CD3+ sTILs (digital score) low vs. high | 0.16 | 0.06–0.42 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceska, S.; Grašič Kuhar, C.; Frković Grazio, S.; Škof, E.; Krishnamoorthy, P.; Khabele, D.; Kloboves Prevodnik, V. Association of Tumor-Infiltrating Lymphocytes and Inflammation Status with Survival Outcome in Patients with High-Grade Serous Ovarian Carcinoma. Cancers 2025, 17, 2269. https://doi.org/10.3390/cancers17142269
Miceska S, Grašič Kuhar C, Frković Grazio S, Škof E, Krishnamoorthy P, Khabele D, Kloboves Prevodnik V. Association of Tumor-Infiltrating Lymphocytes and Inflammation Status with Survival Outcome in Patients with High-Grade Serous Ovarian Carcinoma. Cancers. 2025; 17(14):2269. https://doi.org/10.3390/cancers17142269
Chicago/Turabian StyleMiceska, Simona, Cvetka Grašič Kuhar, Snježana Frković Grazio, Erik Škof, Praveen Krishnamoorthy, Dineo Khabele, and Veronika Kloboves Prevodnik. 2025. "Association of Tumor-Infiltrating Lymphocytes and Inflammation Status with Survival Outcome in Patients with High-Grade Serous Ovarian Carcinoma" Cancers 17, no. 14: 2269. https://doi.org/10.3390/cancers17142269
APA StyleMiceska, S., Grašič Kuhar, C., Frković Grazio, S., Škof, E., Krishnamoorthy, P., Khabele, D., & Kloboves Prevodnik, V. (2025). Association of Tumor-Infiltrating Lymphocytes and Inflammation Status with Survival Outcome in Patients with High-Grade Serous Ovarian Carcinoma. Cancers, 17(14), 2269. https://doi.org/10.3390/cancers17142269







