Cardiotoxicity in Elderly Breast Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Treatment Options in the Elderly Breast Cancer Patients
3. Cardiotoxicity in Elderly Breast Cancer Patients
3.1. Cancer Therapy Related Cardiac Dysfunction (CTRCD)
3.2. Arrhythmias
3.3. Myocardial Ischemia
3.4. Hypertension
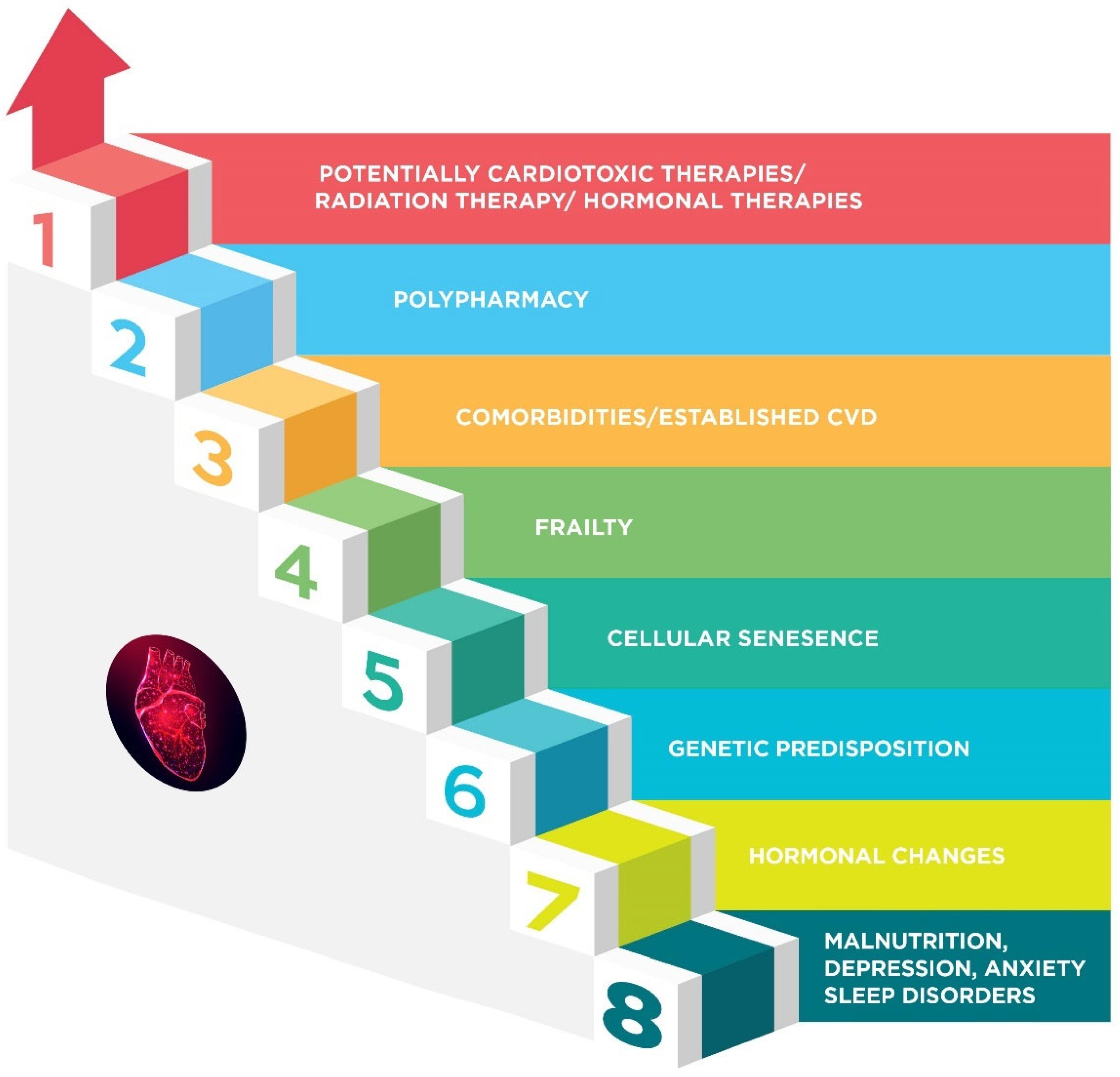
4. Cardiotoxicity Risk Factors in the Elderly
4.1. Frailty
4.2. Comorbidities and Established Cardiovascular Disease
4.3. Hormonal Changes
4.4. Genetics
4.5. Malnutrition, Psychological, and Sleep Disorders
5. Preventive Strategies
5.1. Cardio-Oncological Strategies
5.2. Oncological Strategies
- ✓
- Anthracyclines can be avoided in high- and very high-cardiotoxicity risk patients. Only carefully selected, fit, older patients with high-risk disease (large, node-positive, triple-negative) can be considered for a sequential combination of anthracyclines and taxanes [9].
- ✓
- Limitation of the cumulative anthracycline dose as the risk of CTRCD is dose-dependent [111].
- ✓
- Selection of epirubicin instead of doxorubicin, as it is less cardiotoxic than doxorubicin [112].
- ✓
- ✓
- ✓
- Liposomal doxorubicin that has been approved for metastatic BC can be used instead of unencapsulated anthracycline formulations with a significantly lower cardiotoxicity risk [116].
- ✓
- Dexrazoxane is a cardioprotective agent that has a proven cardioprotective effect [106,117] and is formally approved in adult patients with advanced or metastatic BC who have already received a minimum cumulative anthracycline dose of 300 mg/m2 of doxorubicin or 600 mg/m2 of epirubicin or equivalent [118,119].
- ✓
- Weekly paclitaxel (for 12 weeks) can be an option in patients unfit for polychemotherapy [9].
- ✓
- Shorter courses of chemotherapy or HER2-targeted therapies can be applied in high-risk older patients [9].
5.3. Patient Education
6. Evolution of Cardiotoxicity Prevention in Elderly Breast Cancer Patients and Clinical Trials
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BC | breast cancer |
| CV | cardiovascular |
| HER | human epidermal growth factor receptor |
| HF | heart failure |
| EUSOMA | European Society of Breast Cancer Specialists |
| SIOG | International Society of Geriatric Oncology |
| QoL | quality of life |
| CTRCD | Cancer therapy related cardiac dysfunction |
| ESC | European Society of Cardiology |
Appendix A
References
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- WebMD Breast Cancer Survival Rates. Available online: https://www.webmd.com/breast-cancer/breast-cancer-survival-rates (accessed on 13 February 2024).
- Koric, A.; Chang, C.P.; Mark, B.; Rowe, K.; Snyder, J.; Dodson, M.; Deshmukh, V.G.; Newman, M.G.; Fraser, A.M.; Smith, K.R.; et al. Cardiovascular disease risk in long-term breast cancer survivors: A population-based cohort study. Cancer 2022, 128, 2826–2835. [Google Scholar] [CrossRef]
- Vo, J.B.; Ramin, C.; Barac, A.; Berrington de Gonzalez, A.; Veiga, L. Trends in heart disease mortality among breast cancer survivors in the US, 1975–2017. Breast Cancer Res. Treat. 2022, 192, 611–622. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; DiGuiseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef]
- Park, N.J.; Chang, Y.; Bender, C.; Conley, Y.; Chlebowski, R.T.; van Londen, G.J.; Foraker, R.; Wassertheil-Smoller, S.; Stefanick, M.L.; Kuller, L.H. Cardiovascular disease and mortality after breast cancer in postmenopausal women: Results from the Women’s Health Initiative. PLoS ONE 2017, 12, e0184174. [Google Scholar] [CrossRef]
- Mislang, A.R.; Cheung, K.L.; Hamaker, M.E.; Kunkler, I.; Markopoulos, C.; Orecchia, R.; Brain, E.; Biganzoli, L. Controversial issues in the management of older adults with early breast cancer. J. Geriatr. Oncol. 2017, 8, 397–402. [Google Scholar] [CrossRef]
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCartney, A.; Colloca, G.; Kunkler, I.H.; Cardoso, M.J.; Cheung, K.L.; de Glas, N.A.; Trimboli, R.M.; et al. Updated recommendations regarding the management of older patients with breast cancer: A joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021, 22, e327–e340. [Google Scholar] [CrossRef]
- Williams, G.R.; Mackenzie, A.; Magnuson, A.; Olin, R.; Chapman, A.; Mohile, S.; Allore, H.; Somerfield, M.R.; Targia, V.; Extermann, M.; et al. Comorbidity in older adults with cancer. J. Geriatr. Oncol. 2016, 7, 249–257. [Google Scholar] [CrossRef]
- Hamelinck, V.C.; Stiggelbout, A.M.; van de Velde, C.J.H.; Liefers, G.J.; Bastiaannet, E. Treatment recommendations for older women with breast cancer: A survey among surgical, radiation and medical oncologists. Eur. J. Surg. Oncol. 2017, 43, 1288–1296. [Google Scholar] [CrossRef]
- Keramida, K.; Filippatos, G. Heart failure guidelines implementation: Lifting barriers using registries and networks. Anatol. J. Cardiol. 2020, 24, 41–42. [Google Scholar] [CrossRef]
- Poorvu, P.D.; Vaz-Luis, I.; Freedman, R.A.; Lin, N.U.; Barry, W.T.; Winer, E.P.; Hassett, M.J. Variation in guideline-concordant care for elderly patients with metastatic breast cancer in the United States. Breast Cancer Res. Treat. 2018, 168, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Tamirisa, N.; Lin, H.; Shen, Y.; Shaitelman, S.F.; Sri Karuturi, M.; Giordano, S.H.; Babiera, G.; Bedrosian, I. Association of Chemotherapy With Survival in Elderly Patients With Multiple Comorbidities and Estrogen Receptor-Positive, Node-Positive Breast Cancer. JAMA Oncol. 2020, 6, 1548–1554. [Google Scholar] [CrossRef]
- Wilbers, A.; Quinn, K.R.; Okut, H.; Helmer, S.D.; Tenofsky, P.L. Systemic Therapy in Elderly Patients With Her2/Neu-Positive Breast Cancer: A SEER Database Study. Am. Surg. 2023, 89, 5690–5696. [Google Scholar] [CrossRef]
- Jolly, T.A.; Deal, A.M.; Nyrop, K.A.; Williams, G.R.; Pergolotti, M.; Wood, W.A.; Alston, S.M.; Gordon, B.B.; Dixon, S.A.; Moore, S.G.; et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 2015, 20, 379–385. [Google Scholar] [CrossRef]
- Martelli, G.; Miceli, R.; Daidone, M.G.; Vetrella, G.; Cerrotta, A.M.; Piromalli, D.; Agresti, R. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: Results after 15 years of follow-up. Ann. Surg. Oncol. 2011, 18, 125–133. [Google Scholar] [CrossRef]
- Laface, C.; Giuliani, F.; Melaccio, A.; Pappagallo, M.N.; Santoro, A.N.; Perrone, M.; De Santis, P.; Guarini, C.; Carrozzo, D.; Fedele, P. The Treatment Landscape of Elderly Patients with Hormone Receptor-Positive Her2 Negative Advanced Breast Cancer: Current Perspectives and Future Directions. J. Clin. Med. 2023, 12, 6012. [Google Scholar] [CrossRef]
- Okwuosa, T.M.; Morgans, A.; Rhee, J.W.; Reding, K.W.; Maliski, S.; Plana, J.C.; Volgman, A.S.; Moseley, K.F.; Porter, C.B.; Ismail-Khan, R. Impact of Hormonal Therapies for Treatment of Hormone-Dependent Cancers (Breast and Prostate) on the Cardiovascular System: Effects and Modifications: A Scientific Statement From the American Heart Association. Circ. Genom. Precis. Med. 2021, 14, e000082. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Khouri, M.; Mackey, J.R.; Douglas, P.S.; Jones, L.W. Running on empty: Cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4458–4461. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Pinder, M.C.; Duan, Z.; Goodwin, J.S.; Hortobagyi, G.N.; Giordano, S.H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3808–3815. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.T.; Isaacs, C.; Fu, A.Z.; Warren, J.L.; Freedman, A.N.; Barac, A.; Huang, C.Y.; Potosky, A.L. Risk of cardiovascular adverse events from trastuzumab (Herceptin(®)) in elderly persons with breast cancer: A population-based study. Breast Cancer Res. Treat. 2014, 144, 163–170. [Google Scholar] [CrossRef]
- López-Sendón, J.; Álvarez-Ortega, C.; Zamora Auñon, P.; Buño Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodríguez Rodríguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: The CARDIOTOX registry. Eur. Heart J. 2020, 41, 1720–1729. [Google Scholar] [CrossRef]
- Camilli, M.; Cipolla, C.M.; Dent, S.; Minotti, G.; Cardinale, D.M. Anthracycline Cardiotoxicity in Adult Cancer Patients: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 655–677. [Google Scholar] [CrossRef]
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337. [Google Scholar] [CrossRef]
- Hu, W.; Lv, K.; Teng, R.; Chen, J.; Xu, C.; Jin, L.; Chen, Y.; Zhao, W. Pegylated Liposomal Doxorubicin Versus Epirubicin as Adjuvant Therapy for Stage I-III Breast Cancer. Front. Genet. 2021, 12, 746114. [Google Scholar] [CrossRef]
- Gil-Gil, M.J.; Bellet, M.; Bergamino, M.; Morales, S.; Barnadas, A.; Manso, L.; Saura, C.; Fernández-Ortega, A.; Garcia-Martinez, E.; Martinez-Jañez, N.; et al. Long-Term Cardiac Safety and Survival Outcomes of Neoadjuvant Pegylated Liposomal Doxorubicin in Elderly Patients or Prone to Cardiotoxicity and Triple Negative Breast Cancer. Final Results of the Multicentre Phase II CAPRICE Study. Front. Oncol. 2021, 11, 645026. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Doyle, J.J.; Neugut, A.I.; Jacobson, J.S.; Grann, V.R.; Hershman, D.L. Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 8597–8605. [Google Scholar] [CrossRef]
- Neuendorff, N.R.; Loh, K.P.; Mims, A.S.; Christofyllakis, K.; Soo, W.K.; Bölükbasi, B.; Oñoro-Algar, C.; Hundley, W.G.; Klepin, H.D. Anthracycline-related cardiotoxicity in older patients with acute myeloid leukemia: A Young SIOG review paper. Blood Adv. 2020, 4, 762–775. [Google Scholar] [CrossRef]
- Screever, E.M.; Meijers, W.C.; Moslehi, J.J. Age-Related Considerations in Cardio-Oncology. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 103–113. [Google Scholar] [CrossRef]
- Cusack, B.J.; Musser, B.; Gambliel, H.; Hadjokas, N.E.; Olson, R.D. Effect of dexrazoxane on doxorubicin pharmacokinetics in young and old rats. Cancer Chemother. Pharmacol. 2003, 51, 139–146. [Google Scholar] [CrossRef]
- Li, J.; Gwilt, P.R. The effect of age on the early disposition of doxorubicin. Cancer Chemother. Pharmacol. 2003, 51, 395–402. [Google Scholar] [CrossRef]
- Aladwani, A.; Mullen, A.; Alrashidi, M.; Alfarisi, O.; Alterkait, F.; Aladwani, A.; Kumar, A.; Boyd, M.; Eldosouky, M.E. Comparing trastuzumab-related cardiotoxicity between elderly and younger patients with breast cancer: A prospective cohort study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7643–7653. [Google Scholar] [CrossRef]
- Leung, H.W.; Chan, A.L. Trastuzumab-induced cardiotoxicity in elderly women with HER-2-positive breast cancer: A meta-analysis of real-world data. Expert Opin. Drug Saf. 2015, 14, 1661–1671. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.H.; Davidson, N.E.; Geyer, C.E., Jr.; Martino, S.; Mamounas, E.P.; Kaufman, P.A.; Wolmark, N. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3366–3373. [Google Scholar] [CrossRef]
- Chen, J.; Long, J.B.; Hurria, A.; Owusu, C.; Steingart, R.M.; Gross, C.P. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J. Am. Coll. Cardiol. 2012, 60, 2504–2512. [Google Scholar] [CrossRef]
- Reding, K.W.; Cheng, R.K.; Vasbinder, A.; Ray, R.M.; Barac, A.; Eaton, C.B.; Saquib, N.; Shadyab, A.H.; Simon, M.S.; Langford, D.; et al. Lifestyle and Cardiovascular Risk Factors Associated With Heart Failure Subtypes in Postmenopausal Breast Cancer Survivors. JACC CardioOncol. 2022, 4, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Saiki, H.; Petersen, I.A.; Scott, C.G.; Bailey, K.R.; Dunlay, S.M.; Finley, R.R.; Ruddy, K.J.; Yan, E.; Redfield, M.M. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017, 135, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Calleja, A.; Poulin, F.; Khorolsky, C.; Shariat, M.; Bedard, P.L.; Amir, E.; Rakowski, H.; McDonald, M.; Delgado, D.; Thavendiranathan, P. Right Ventricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. J. Oncol. 2015, 2015, 609194. [Google Scholar] [CrossRef]
- Faggiano, A.; Gherbesi, E.; Giordano, C.; Gamberini, G.; Vicenzi, M.; Cuspidi, C.; Carugo, S.; Cipolla, C.M.; Cardinale, D.M. Anthracycline-Induced Subclinical Right Ventricular Dysfunction in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 3883. [Google Scholar] [CrossRef]
- Jain, H.; Ahmed, M.; Tariq, M.D.; Jain, J.; Goyal, A.; Odat, R.M.; Jha, M.; Shashikumar, S.; Scott, I.; Ahmed, R. Right ventricular strain as a predictor of trastuzumab-induced chemotherapy-related cardiac dysfunction: A meta-analysis. Curr. Probl. Cardiol. 2025, 50, 102919. [Google Scholar] [CrossRef]
- Gorgiladze, N.; Shavdia, M.; Gaprindashvili, T.; Gogua, E.; Gachechiladze, L.; Gujabidze, M.; Pagava, Z. Detection of Cardiotoxicity Using Right Ventricular Free Wall Longitudinal Strain in Low Cardiovascular Risk Breast Cancer Patients Receiving Low-Dose Anthracycline Treatment. Cureus 2024, 16, e63138. [Google Scholar] [CrossRef]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D.; et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur. J. Heart Fail. 2019, 21, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Theetha Kariyanna, P.; Kumar, A.; Jayarangaiah, A.; Shetty, M.; Chowdhury, Y.; Das, S.; Jayarangaiah, A. Chemotherapy induced right ventricular cardiomyopathy; a systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1103941. [Google Scholar] [CrossRef]
- El-Sherbeny, W.S.; Sabry, N.M.; El-Saied, S.B.; Elnagar, B. Detection of right ventricular dysfunction by three-dimensional echocardiography and two-dimensional speckle tracking in breast cancer patients receiving anthracycline-based chemotherapy. Cardiooncology 2023, 9, 20. [Google Scholar] [CrossRef]
- Keramida, K.; Farmakis, D.; Rakisheva, A.; Tocchetti, C.G.; Ameri, P.; Asteggiano, R.; Barac, A.; Bax, J.; Bayes-Genis, A.; Bergler Klein, J.; et al. The right heart in patients with cancer. A scientific statement of the Heart Failure Association (HFA) of the ESC and the ESC Council of Cardio-Oncology. Eur. J. Heart Fail. 2024, 26, 2077–2093. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bhoo-Pathy, N.; Brand, J.S.; Hedayati, E.; Grassmann, F.; Zeng, E.; Bergh, J.; Bian, W.; Ludvigsson, J.F.; Hall, P.; et al. Risk of heart disease following treatment for breast cancer—Results from a population-based cohort study. Elife 2022, 11, e71562. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.; Freitas, L.; Dias, L.; Vasconcelos, L.; Blackman, A. Mini Review Arrhythmias caused by chemotherapy agents used on the treatment of triple-negative Breast cancer: A mini review of literature. Biomed. J. Sci. Tech. Res. 2020, 27, 20943–20947. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Cohen, A.J.; Wasserman, A.G.; Cohen, P.; Ross, A.M. Acute arrhythmogenicity of doxorubicin administration. Cancer 1987, 60, 1213–1218. [Google Scholar] [CrossRef]
- Keramida, K.; Filippatos, G.; Farmakis, D. Cancer treatment and atrial fibrillation: Use of pharmacovigilance databases to detect cardiotoxicity. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 321–323. [Google Scholar] [CrossRef]
- Grouthier, V.; Lebrun-Vignes, B.; Glazer, A.M.; Touraine, P.; Funck-Brentano, C.; Pariente, A.; Courtillot, C.; Bachelot, A.; Roden, D.M.; Moslehi, J.J.; et al. Increased long QT and torsade de pointes reporting on tamoxifen compared with aromatase inhibitors. Heart 2018, 104, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Veronese, P.; Hachul, D.T.; Scanavacca, M.I.; Hajjar, L.A.; Wu, T.C.; Sacilotto, L.; Veronese, C.; Darrieux, F. Effects of anthracycline, cyclophosphamide and taxane chemotherapy on QTc measurements in patients with breast cancer. PLoS ONE 2018, 13, e0196763. [Google Scholar] [CrossRef]
- Buza, V.; Rajagopalan, B.; Curtis, A.B. Cancer Treatment-Induced Arrhythmias: Focus on Chemotherapy and Targeted Therapies. Circ. Arrhythm. Electrophysiol. 2017, 10, e005443. [Google Scholar] [CrossRef]
- Tamargo, J.; Caballero, R.; Delpón, E. Cancer chemotherapy and cardiac arrhythmias: A review. Drug Saf. 2015, 38, 129–152. [Google Scholar] [CrossRef]
- Fradley, M.G.; Moslehi, J. QT Prolongation and Oncology Drug Development. Card. Electrophysiol. Clin. 2015, 7, 341–355. [Google Scholar] [CrossRef]
- Wennstig, A.-K.; Wadsten, C.; Garmo, H.; Fredriksson, I.; Blomqvist, C.; Holmberg, L.; Nilsson, G.; Sund, M. Long-term risk of ischemic heart disease after adjuvant radiotherapy in breast cancer: Results from a large population-based cohort. Breast Cancer Res. 2020, 22, 10. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Andrikopoulou, A.; Dimopoulos, M.A.; Zagouri, F. Cardiovascular toxicity of breast cancer treatment: An update. Cancer Chemother. Pharmacol. 2021, 88, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, K.E.; Park, Y.; Ju, Y.W.; Jung, J.G.; Lee, E.S.; Lee, H.B.; Han, W.; Noh, D.Y.; Yoon, H.J.; et al. Effects of tamoxifen and aromatase inhibitors on the risk of acute coronary syndrome in elderly breast cancer patients: An analysis of nationwide data. Breast 2020, 54, 25–30. [Google Scholar] [CrossRef]
- Cohen, J.B.; Brown, N.J.; Brown, S.A.; Dent, S.; van Dorst, D.C.H.; Herrmann, S.M.; Lang, N.N.; Oudit, G.Y.; Touyz, R.M. Cancer Therapy-Related Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e46–e57. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Cheng, R.K.; Iribarren, C.; Neugebauer, R.; Rana, J.S.; Nguyen-Huynh, M.; Shi, Z.; Laurent, C.A.; Lee, V.S.; Roh, J.M.; et al. Risk of Cardiometabolic Risk Factors in Women With and Without a History of Breast Cancer: The Pathways Heart Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.; Singh, M.; Tiu, J.G.; Kim, A.S. Etiology and management of hypertension in patients with cancer. Cardio-oncology 2021, 7, 14. [Google Scholar] [CrossRef]
- Szczepaniak, P.; Siedlinski, M.; Hodorowicz-Zaniewska, D.; Nosalski, R.; Mikolajczyk, T.P.; Dobosz, A.M.; Dikalova, A.; Dikalov, S.; Streb, J.; Gara, K.; et al. Breast cancer chemotherapy induces vascular dysfunction and hypertension through a NOX4-dependent mechanism. J. Clin. Investig. 2022, 132, e149117. [Google Scholar] [CrossRef]
- Bocchi, E.A.; Avila, M.S.; Ayub-Ferreira, S.M. Aging, cardiotoxicity, and chemotherapy. Aging 2019, 11, 295–296. [Google Scholar] [CrossRef]
- Henry, M.L.; Niu, J.; Zhang, N.; Giordano, S.H.; Chavez-MacGregor, M. Cardiotoxicity and Cardiac Monitoring Among Chemotherapy-Treated Breast Cancer Patients. JACC Cardiovasc. Imaging 2018, 11, 1084–1093. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y. Rate of adverse cardiovascular events in breast cancer patients receiving chemotherapy and targeted therapy: Impact of frailty. Am. Heart J. Plus 2024, 38, 100353. [Google Scholar] [CrossRef] [PubMed]
- Ohman, R.E.; Yang, E.H.; Abel, M.L. Inequity in Cardio-Oncology: Identifying Disparities in Cardiotoxicity and Links to Cardiac and Cancer Outcomes. J. Am. Heart Assoc. 2021, 10, e023852. [Google Scholar] [CrossRef]
- Raj, A.; Chakole, S.; Agrawal, S.; Gupta, A.; Khekade, H.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Menopause on Cardiovascular Aging: A Comprehensive Review of Androgen Influences. Cureus 2023, 15, e43569. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, Y.; Li, L.; Ge, H.; Wang, Y.; Zeng, C.; Ma, F. Genetic factors in the pathogenesis of cardio-oncology. J. Transl. Med. 2024, 22, 739. [Google Scholar] [CrossRef]
- Visscher, H.; Rassekh, S.R.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; Carleton, B.C.; et al. Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics 2015, 16, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Aminkeng, F.; Bhavsar, A.P.; Visscher, H.; Rassekh, S.R.; Li, Y.; Lee, J.W.; Brunham, L.R.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat. Genet. 2015, 47, 1079–1084. [Google Scholar] [CrossRef]
- Wang, X.; Sun, C.L.; Quiñones-Lombraña, A.; Singh, P.; Landier, W.; Hageman, L.; Mather, M.; Rotter, J.I.; Taylor, K.D.; Chen, Y.D.; et al. CELF4 Variant and Anthracycline-Related Cardiomyopathy: A Children’s Oncology Group Genome-Wide Association Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 863–870. [Google Scholar] [CrossRef]
- Kim, Y.; Seidman, J.G.; Seidman, C.E. Genetics of cancer therapy-associated cardiotoxicity. J. Mol. Cell. Cardiol. 2022, 167, 85–91. [Google Scholar] [CrossRef]
- Kopeva, K.V.; Grakova, E.V.; Shilov, S.N.; Berezikova, E.N.; Popova, A.A.; Neupokoeva, M.N.; Ratushnyak, E.T.; Teplyakov, A.T. Anthracycline-induced cardiotoxicity in women without cardiovascular diseases: Molecular and genetic predictors. Acta cardiologica 2022, 77, 805–814. [Google Scholar] [CrossRef]
- Gómez Peña, C.; Dávila-Fajardo, C.L.; Martínez-González, L.J.; Carmona-Sáez, P.; Soto Pino, M.J.; Sánchez Ramos, J.; Moreno Escobar, E.; Blancas, I.; Fernández, J.J.; Fernández, D.; et al. Influence of the HER2 Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients: A meta-analysis. Pharmacogenet. Genom. 2015, 25, 388–393. [Google Scholar] [CrossRef]
- Tan, L.; Su, X.; Li, X.; Li, H.; Hu, B. Correlation of HER2 codon 655 polymorphism with cardiotoxicity risk in Chinese HER2-positive breast cancer patients undergoing epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab adjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2020, 13, 286–294. [Google Scholar] [PubMed]
- Stanton, S.E.; Ward, M.M.; Christos, P.; Sanford, R.; Lam, C.; Cobham, M.V.; Donovan, D.; Scheff, R.J.; Cigler, T.; Moore, A.; et al. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC cancer 2015, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.H.; Udagawa, C.; Shimo, A.; Kojima, Y.; Yoshie, R.; Zaha, H.; Abe, N.; Motonari, T.; Unesoko, M.; Tamura, K.; et al. A Genome-Wide Association Study Identifies Five Novel Genetic Markers for Trastuzumab-Induced Cardiotoxicity in Japanese Population. Biol. Pharm. Bull. 2019, 42, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.; McLaughlin, M.; Bray, J.W.; Saxton, J.M.; Vince, R.V. Nutrition Modulation of Cardiotoxicity in Breast Cancer: A Scoping Review. Nutrients 2024, 16, 3777. [Google Scholar] [CrossRef]
- Zhang, X.; Edwards, B.J. Malnutrition in Older Adults with Cancer. Curr. Oncol. Rep. 2019, 21, 80. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; Knifton, L.; Robb, K.A.; Katikireddi, S.V.; Smith, D.J. Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 2019, 19, 943. [Google Scholar] [CrossRef]
- Valtorta, N.K.; Kanaan, M.; Gilbody, S.; Ronzi, S.; Hanratty, B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: Systematic review and meta-analysis of longitudinal observational studies. Heart 2016, 102, 1009–1016. [Google Scholar] [CrossRef]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef]
- Schoormans, D.; van de Poll-Franse, L.; Vissers, P.; van Herk-Sukel, M.P.P.; Pedersen, S.S.; Rottmann, N.; Horsbøl, T.; Dalton, S.; Denollet, J. Pharmaceutically treated anxiety but not depression prior to cancer diagnosis predicts the onset of cardiovascular disease among breast cancer survivors. Breast Cancer Res. Treat. 2017, 166, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J.; Dunietz, G.L.; Chervin, R.D.; Lisabeth, L.D.; Skolarus, L.E.; Burke, J.F. Recognition and Diagnosis of Obstructive Sleep Apnea in Older Americans. J. Am. Geriatr. Soc. 2018, 66, 1296–1302. [Google Scholar] [CrossRef]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep. Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- Nissen, E.R.; Neumann, H.; Knutzen, S.M.; Henriksen, E.N.; Amidi, A.; Johansen, C.; von Heymann, A.; Christiansen, P.; Zachariae, R. Interventions for insomnia in cancer patients and survivors-a comprehensive systematic review and meta-analysis. JNCI Cancer Spectr. 2024, 8. [Google Scholar] [CrossRef]
- Ravichandran, R.; Gupta, L.; Singh, M.; Nag, A.; Thomas, J.; Panjiyar, B.K. The Interplay Between Sleep Disorders and Cardiovascular Diseases: A Systematic Review. Cureus 2023, 15, e45898. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef] [PubMed]
- ACC. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2024/02/09/18/10/sleep-apnea-a-risk-factor-for-heart-failure-from-cancer-therapy#resources-for-article (accessed on 18 December 2024).
- Aneni, E.; Ahmed, O.; Otite, F. Abstract 4145554: Obstructive Sleep Apnea Increases the Risk of Cardiovascular Disease and Stroke Among Persons with Cancer: Analyses from a Multi-center Electronic Healthcare Records-Based Database. Circulation 2024, 150, A4145554. [Google Scholar] [CrossRef]
- Keramida, K.; Yang, E.H.; Deswal, A. Moving theory and reality closer together in cardio-oncology training. Eur. J. Heart Fail. 2024, 26, 772–775. [Google Scholar] [CrossRef]
- Farmakis, D.; Keramida, K.; Filippatos, G. How to build a cardio-oncology service? Eur. J. Heart Fail. 2018, 20, 1732–1734. [Google Scholar] [CrossRef]
- Pareek, N.; Cevallos, J.; Moliner, P.; Shah, M.; Tan, L.L.; Chambers, V.; Baksi, A.J.; Khattar, R.S.; Sharma, R.; Rosen, S.D.; et al. Activity and outcomes of a cardio-oncology service in the United Kingdom-a five-year experience. Eur. J. Heart Fail. 2018, 20, 1721–1731. [Google Scholar] [CrossRef]
- Caspani, F.; Tralongo, A.C.; Campiotti, L.; Asteggiano, R.; Guasti, L.; Squizzato, A. Prevention of anthracycline-induced cardiotoxicity: A systematic review and meta-analysis. Intern. Emerg. Med. 2021, 16, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhao, Q.; Yang, Z.G.; Diao, K.Y.; He, Y.; Shi, K.; Shen, M.T.; Fu, H.; Guo, Y.K. Protective role of beta-blockers in chemotherapy-induced cardiotoxicity-a systematic review and meta-analysis of carvedilol. Heart Fail. Rev. 2019, 24, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Hirji, S.A.; Qamar, A.; Bajaj, N.; Gupta, A.; Zaha, V.; Chandra, A.; Haykowsky, M.; Ky, B.; Moslehi, J.; et al. Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients with Cancer Undergoing Chemotherapy. JACC CardioOncol. 2019, 1, 54–65. [Google Scholar] [CrossRef]
- Macedo, A.V.S.; Hajjar, L.A.; Lyon, A.R.; Nascimento, B.R.; Putzu, A.; Rossi, L.; Costa, R.B.; Landoni, G.; Nogueira-Rodrigues, A.; Ribeiro, A.L.P. Efficacy of Dexrazoxane in Preventing Anthracycline Cardiotoxicity in Breast Cancer. JACC CardioOncol. 2019, 1, 68–79. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, T.; Xiong, X.; Liu, N.; Pang, B.; Ruan, Y.; Gao, Y.; Shang, H.; Xing, Y. Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104577. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, Y.; Liu, W.; He, C. Effects of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use on cancer therapy-related cardiac dysfunction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2021, 26, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wittayanukorn, S.; Qian, J.; Westrick, S.C.; Billor, N.; Johnson, B.; Hansen, R.A. Prevention of Trastuzumab and Anthracycline-induced Cardiotoxicity Using Angiotensin-converting Enzyme Inhibitors or β-blockers in Older Adults With Breast Cancer. Am. J. Clin. Oncol. 2018, 41, 909–918. [Google Scholar] [CrossRef]
- Torregrosa-Maicas, M.D.; Del Barco-Berrón, S.; Cotes-Sanchís, A.; Lema-Roso, L.; Servitja-Tormo, S.; Gironés-Sarrió, R. Expert consensus to optimize the treatment of elderly patients with luminal metastatic breast cancer. Clin. Transl. Oncol. 2022, 24, 1033–1046. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef]
- Mao, Z.; Shen, K.; Zhu, L.; Xu, M.; Yu, F.; Xue, D.; Li, H.; Xue, C. Comparisons of Cardiotoxicity and Efficacy of Anthracycline-Based Therapies in Breast Cancer: A Network Meta-Analysis of Randomized Clinical Trials. Oncol. Res. Treat. 2019, 42, 405–413. [Google Scholar] [CrossRef]
- Shapira, J.; Gotfried, M.; Lishner, M.; Ravid, M. Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regimen. A prospective randomized evaluation. Cancer 1990, 65, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, M.; Burgess, M.A.; Ewer, M.S.; Mackay, B.; Wallace, S.; Benjamin, R.S.; Ali, M.K.; Bodey, G.P.; Freireich, E.J. Increased therapeutic index of weekly doxorubicin in the therapy of non-small cell lung cancer: A prospective, randomized study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1984, 2, 207–214. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Fujii, T.; Aoi, S.; Kozuch, P.S.; Hortobagyi, G.N.; Blum, R.H. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: A Bayesian network meta-analysis. Eur. J. Cancer 2015, 51, 2314–2320. [Google Scholar] [CrossRef]
- Sotiropoulou, I.M.; Manetas-Stavrakakis, N.; Kourek, C.; Xanthopoulos, A.; Magouliotis, D.; Giamouzis, G.; Skoularigis, J.; Briasoulis, A. Prevention of Anthracyclines and HER2 Inhibitor-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 2419. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, L.R.; Spencer, C.M. Dexrazoxane. A review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy. Drugs 1998, 56, 385–403. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Wilson, R.L.; Christopher, C.N.; Yang, E.H.; Barac, A.; Adams, S.C.; Scott, J.M.; Dieli-Conwright, C.M. Incorporating Exercise Training into Cardio-Oncology Care: Current Evidence and Opportunities: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2023, 5, 553–569. [Google Scholar] [CrossRef]
- Díaz-Balboa, E.; Peña-Gil, C.; Rodríguez-Romero, B.; Cuesta-Vargas, A.I.; Lado-Baleato, O.; Martínez-Monzonís, A.; Pedreira-Pérez, M.; Palacios-Ozores, P.; López-López, R.; González-Juanatey, J.R.; et al. Exercise-based cardio-oncology rehabilitation for cardiotoxicity prevention during breast cancer chemotherapy: The ONCORE randomized controlled trial. Prog. Cardiovasc. Dis. 2024, 85, 74–81. [Google Scholar] [CrossRef]
- Kourek, C.; Touloupaki, M.; Rempakos, A.; Loritis, K.; Tsougkos, E.; Paraskevaidis, I.; Briasoulis, A. Cardioprotective Strategies from Cardiotoxicity in Cancer Patients: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2022, 9, 259. [Google Scholar] [CrossRef]
- ESC Clinical Practice Guidelines on Cardio-Oncology: What the Patient Needs to Know. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.escardio.org/static-file/Escardio/Guidelines/Documents/ESC%2520Patient%2520Guidelines%2520Cardio%2520Oncology%2520-%2520final.pdf&ved=2ahUKEwiU8pTNjJiOAxWL1zQHHU0uMdgQFnoECBkQAw&usg=AOvVaw2Sfg7e36YVpk31bmiqhcBC (accessed on 12 February 2024).
- Serrano, C.; Cortés, J.; De Mattos-Arruda, L.; Bellet, M.; Gómez, P.; Saura, C.; Pérez, J.; Vidal, M.; Muñoz-Couselo, E.; Carreras, M.J.; et al. Trastuzumab-related cardiotoxicity in the elderly: A role for cardiovascular risk factors. Ann. Oncol. 2019, 30, 1178. [Google Scholar] [CrossRef] [PubMed]
- Chavez-MacGregor, M.; Zhang, N.; Buchholz, T.A.; Zhang, Y.; Niu, J.; Elting, L.; Smith, B.D.; Hortobagyi, G.N.; Giordano, S.H. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- Reeder-Hayes, K.E.; Meyer, A.M.; Hinton, S.P.; Meng, K.; Carey, L.A.; Dusetzina, S.B. Comparative Toxicity and Effectiveness of Trastuzumab-Based Chemotherapy Regimens in Older Women With Early-Stage Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3298–3305. [Google Scholar] [CrossRef]
- Tarantini, L.; Gori, S.; Faggiano, P.; Pulignano, G.; Simoncini, E.; Tuccia, F.; Ceccherini, R.; Bovelli, D.; Lestuzzi, C.; Cioffi, G. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: A multicenter cohort analysis. Ann. Oncol. 2012, 23, 3058–3063. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT03711110?cond=cardiotoxicity&term=breast%20cancer,%20elderly&rank=2#publications (accessed on 1 June 2024).
- Alexandraki, A.; Papageorgiou, E.; Zacharia, M.; Keramida, K.; Papakonstantinou, A.; Cipolla, C.M.; Tsekoura, D.; Naka, K.; Mazzocco, K.; Mauri, D.; et al. New Insights in the Era of Clinical Biomarkers as Potential Predictors of Systemic Therapy-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review. Cancers 2023, 15, 3290. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT06334445?cond=cardiotoxicity&term=breast%20cancer,%20elderly&rank=3 (accessed on 1 June 2024).
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; Cameron, D.A.; Dixon, J.M. Breast-Conserving Surgery with or without Irradiation in Early Breast Cancer. N. Engl. J. Med. 2023, 388, 585–594. [Google Scholar] [CrossRef]

| Type of Treatment | 2021 Recommendations by EUSOMA–SIOG |
|---|---|
| Surgery |
|
| Primary endocrine therapy |
|
| Radiotherapy |
|
| Adjuvant chemotherapy in HER2-negative disease |
|
| Adjuvant anti-HER2 therapy |
|
| Adjuvant endocrine therapy |
|
| Chemotherapy (metastatic breast cancer) |
|
| HER2-positive disease (metastatic breast cancer) |
|
| Targeted therapies |
|
| Adjuvant bone modifying agents |
|
| Types of Breast Cancer Treatment | Cardiotoxicities |
|---|---|
| Anthracyclines e.g. doxorubicin | HF or asymptomatic LVD or RVD Arrhythmias, Takotsubo syndrome |
| Taxanes e.g. docetaxel, nab-paclitaxel | Arrhythmias, conduction disorders myocardial ischemia, hypertension |
| Vinca alkaloids e.g. vinorelbine | Myocardial ischemia, AF |
| Antimetabolites Pyrimidine analogues e.g. 5-Fluoruracil, capecitabine | Myocardial ischemia, HF, or asymptomatic LVD |
| Alkylating agents e.g. cyclophosphamide, carboplatin, oxaliplatin | Myocardial ischemia, HF, or asymptomatic LVD, hypertension, pericarditis, myocarditis, arrhythmias |
| HER2 targeted therapies | HF or asymptomatic LVD or RVD |
| Monoclonal antibodies e.g. trastuzumab, pertuzumab | |
| Dual blockade e.g. trastuzumab + pertuzumab | Hypertension |
| Antibody Drug Conjugates (ADCs) Trastuzumab emtansine (TD-M1) Sacituzumab govitecanTrastuzumab deruxtecan (T-DXd) | LVD, QTC prolongation |
| HER2 TKIs Neratinib, tucatinib, lapatinib | LVD, Prinzmetal’s angina |
| Poly (ADP-ribose) polymerase inhibitors e.g. niraparib | Hypertension |
| mTOR inhibitors e.g. everolimus, sirolimus | Hypertension |
| Anti-VEGF e.g. bevacizumab | Hypertension |
| Immune checkpoint inhibitors e.g. pembrolizumab | New onset hypertension, stable angina, acute HF, Myocarditis <1%, arrhythmias |
| Microtubule dynamics inhibitor e.g. Eribulin | QTc prolongation |
| PARP inhibitors Olaparib, Talazoparib | MACEs, hypertension, thromboembolic events |
| CDK4/6 inhibitors abemaciclib, palbociclib, ribociclib. dalbiciclib | Thromboembolic events, QTc prolongation, LVD, HF, AF |
| Endocrine therapy Aromatase inhibitors (e.g. anastrozole, letrozole, exemestane) | Myocardial ischemia, HF, hypertension, dyslipidemia |
| Estrogen Receptor Downregulators (e.g. fulvestrant) | Hypertension |
| Selective Estrogen Receptors Modulators (e.g. tamoxifen) | VTE, ↑ triglycerides, diabetes risk, body fat |
| Radiotherapy | Myocardial ischemia, valvular disease pericarditis, HF, or asymptomatic LVD conduction abnormalities, hypertension |
| Adjuvant bone modifying agents (bisphosphonates: zoledronic acid, clodronate) | AF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keramida, K.; Constantinidou, A.; Tsekoura, D.; Kampouroglou, E.; Aidarinis, C.; Saloustros, E.; Karanasiou, G.; Sacco, G.G.A.; Matos, E.; Papakonstantinou, A.; et al. Cardiotoxicity in Elderly Breast Cancer Patients. Cancers 2025, 17, 2198. https://doi.org/10.3390/cancers17132198
Keramida K, Constantinidou A, Tsekoura D, Kampouroglou E, Aidarinis C, Saloustros E, Karanasiou G, Sacco GGA, Matos E, Papakonstantinou A, et al. Cardiotoxicity in Elderly Breast Cancer Patients. Cancers. 2025; 17(13):2198. https://doi.org/10.3390/cancers17132198
Chicago/Turabian StyleKeramida, Kalliopi, Anastasia Constantinidou, Dorothea Tsekoura, Effrosyni Kampouroglou, Chrissovalantis Aidarinis, Emmanouil Saloustros, Georgia Karanasiou, Gaia Giulia Angela Sacco, Erika Matos, Andri Papakonstantinou, and et al. 2025. "Cardiotoxicity in Elderly Breast Cancer Patients" Cancers 17, no. 13: 2198. https://doi.org/10.3390/cancers17132198
APA StyleKeramida, K., Constantinidou, A., Tsekoura, D., Kampouroglou, E., Aidarinis, C., Saloustros, E., Karanasiou, G., Sacco, G. G. A., Matos, E., Papakonstantinou, A., Tsiknakis, M., Brown, C., Antoniades, A., Cipolla, C., Cardinale, D., Fotiadis, D., Filippatos, G., & Investigators CARDIOCARE Consortium. (2025). Cardiotoxicity in Elderly Breast Cancer Patients. Cancers, 17(13), 2198. https://doi.org/10.3390/cancers17132198








