Oral Microbial Dysbiosis Driven by Periodontitis Facilitates Oral Squamous Cell Carcinoma Progression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Research Methodology
2.2. Establishment of Experimental Periodontitis Model
2.3. Establishment of OSCC Model
2.4. Micro-CT Analysis
2.5. Histopathological Analysis of Maxillae and OSCC Tissues
2.6. Quantitative Real-Time PCR Analysis of Periodontal and OSCC Tissues
2.7. In Vitro Culture and Scratch Assay of SCC-7 Cells
2.8. Microbiome Sampling and 16S rDNA Gene Amplicon Sequencing
2.9. Statistical Analysis and Methods
3. Results
3.1. Murine Periodontitis Model Successfully Established
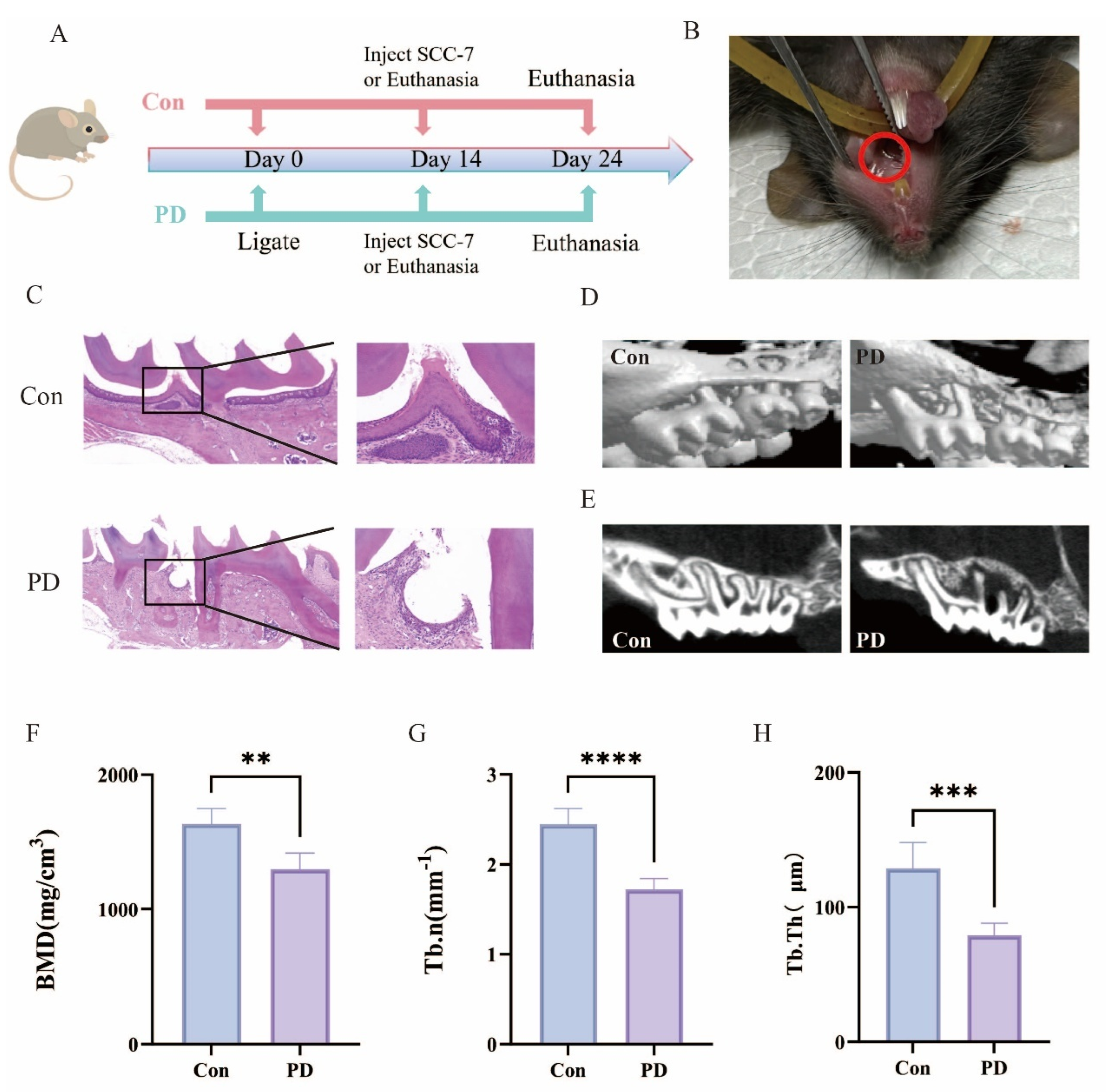
3.2. Periodontitis Exhibits a Significant Positive Association with OSCC Progression
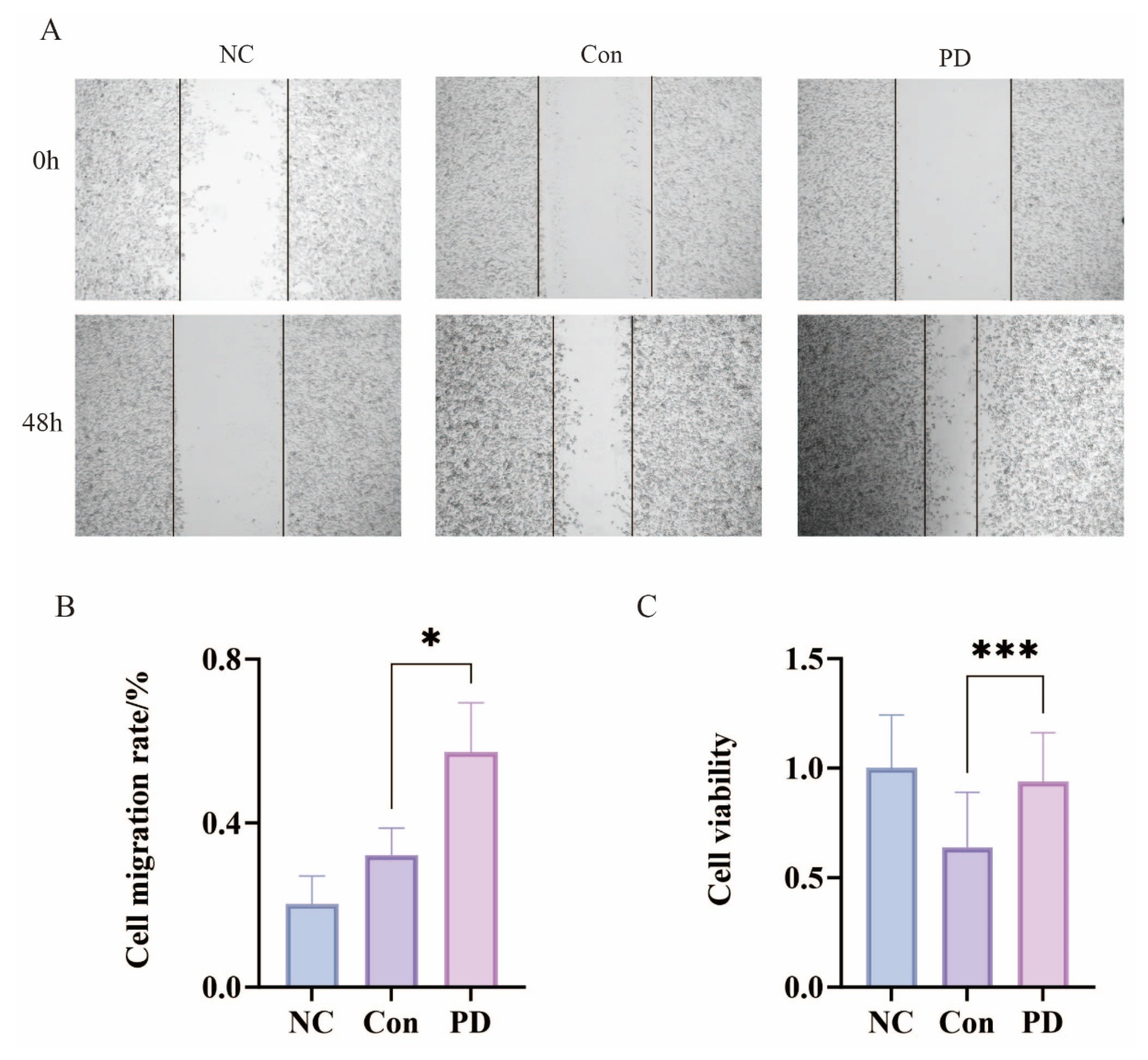
3.3. Periodontitis Enhances Proliferation and Migration of SCC-7 Cells
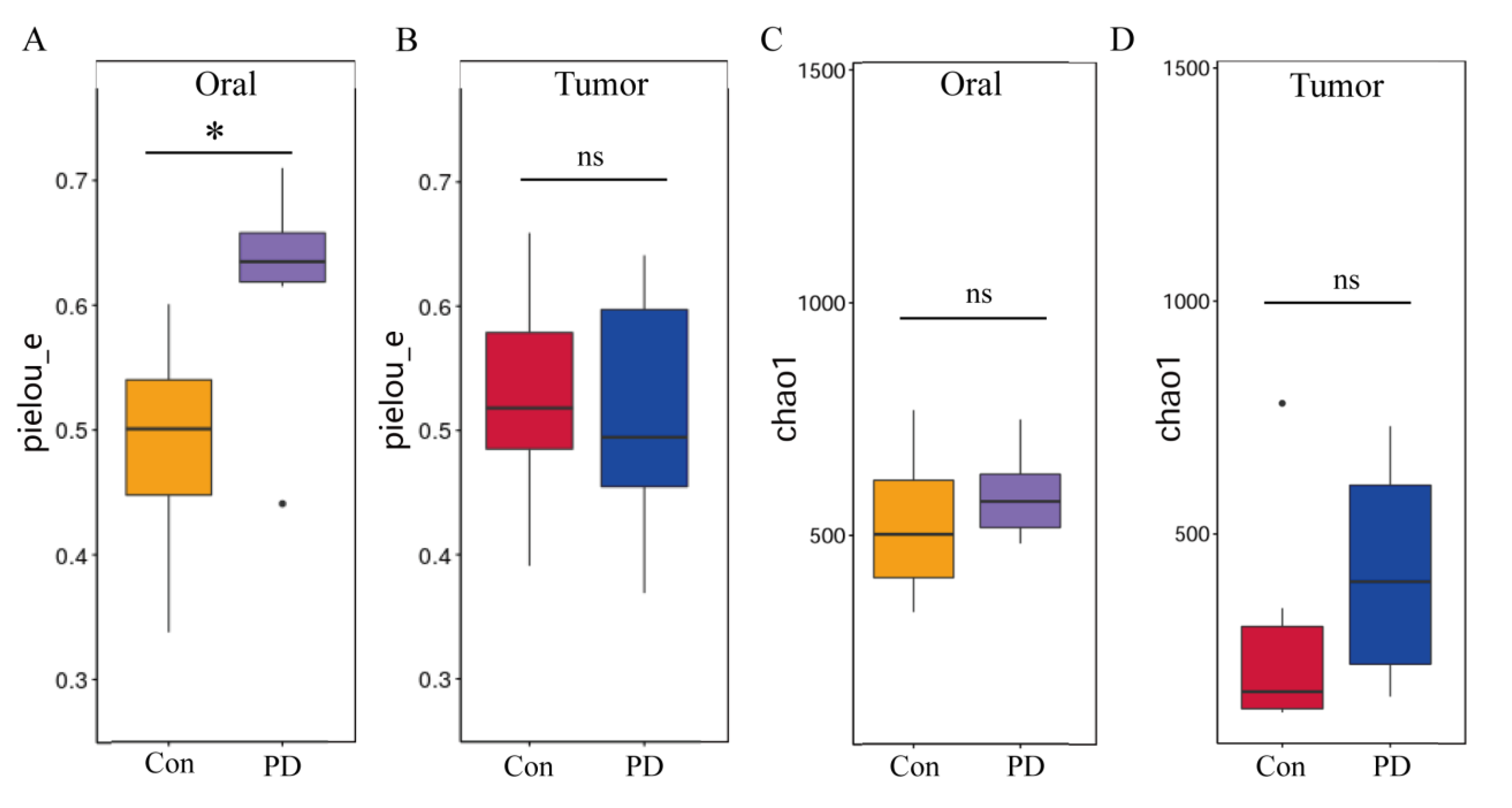
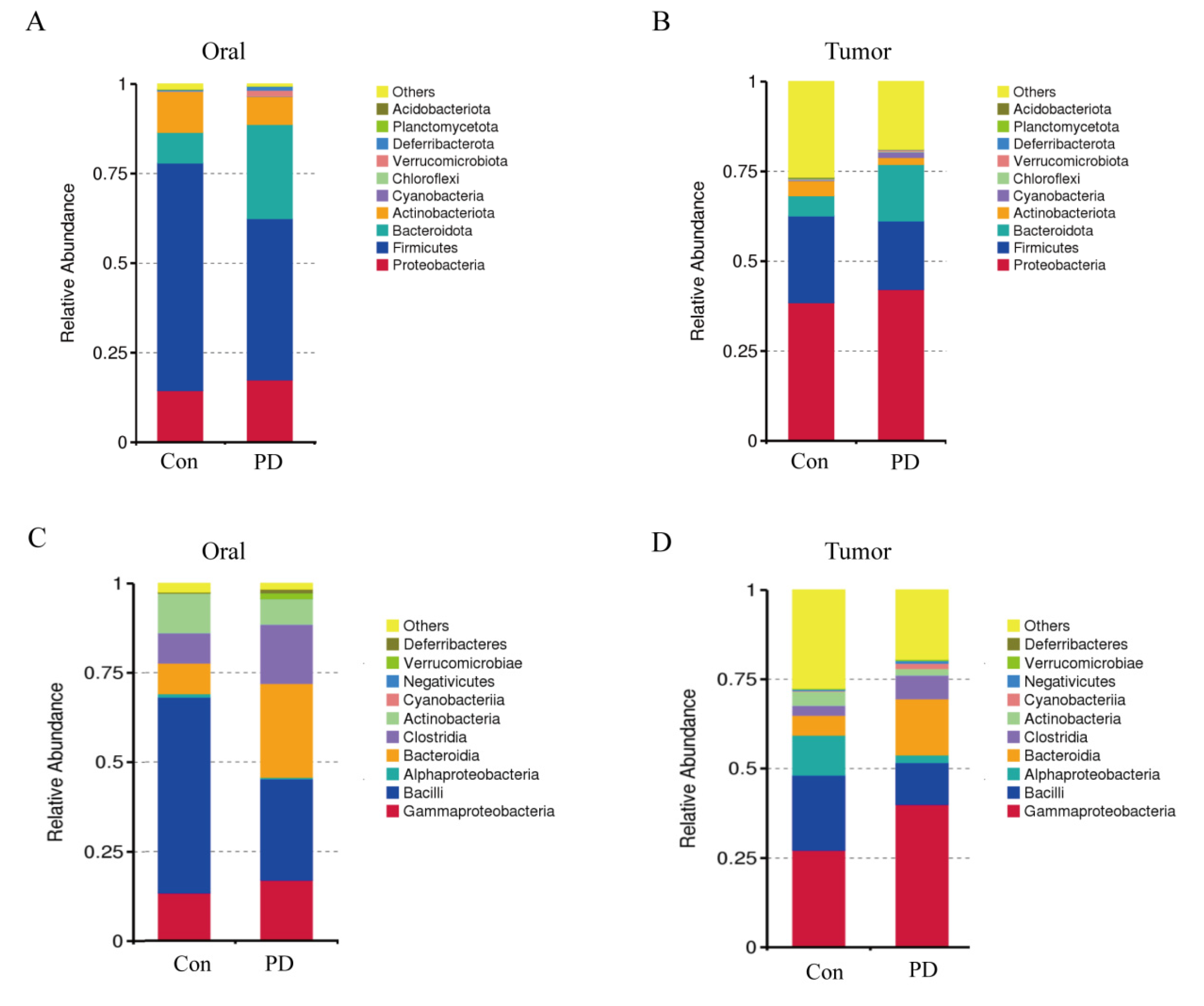
3.4. Periodontitis Alters Microbial Abundance in Oral and OSCC
3.5. Periodontitis Alters Microbial Communities in Oral and OSCC Tissues
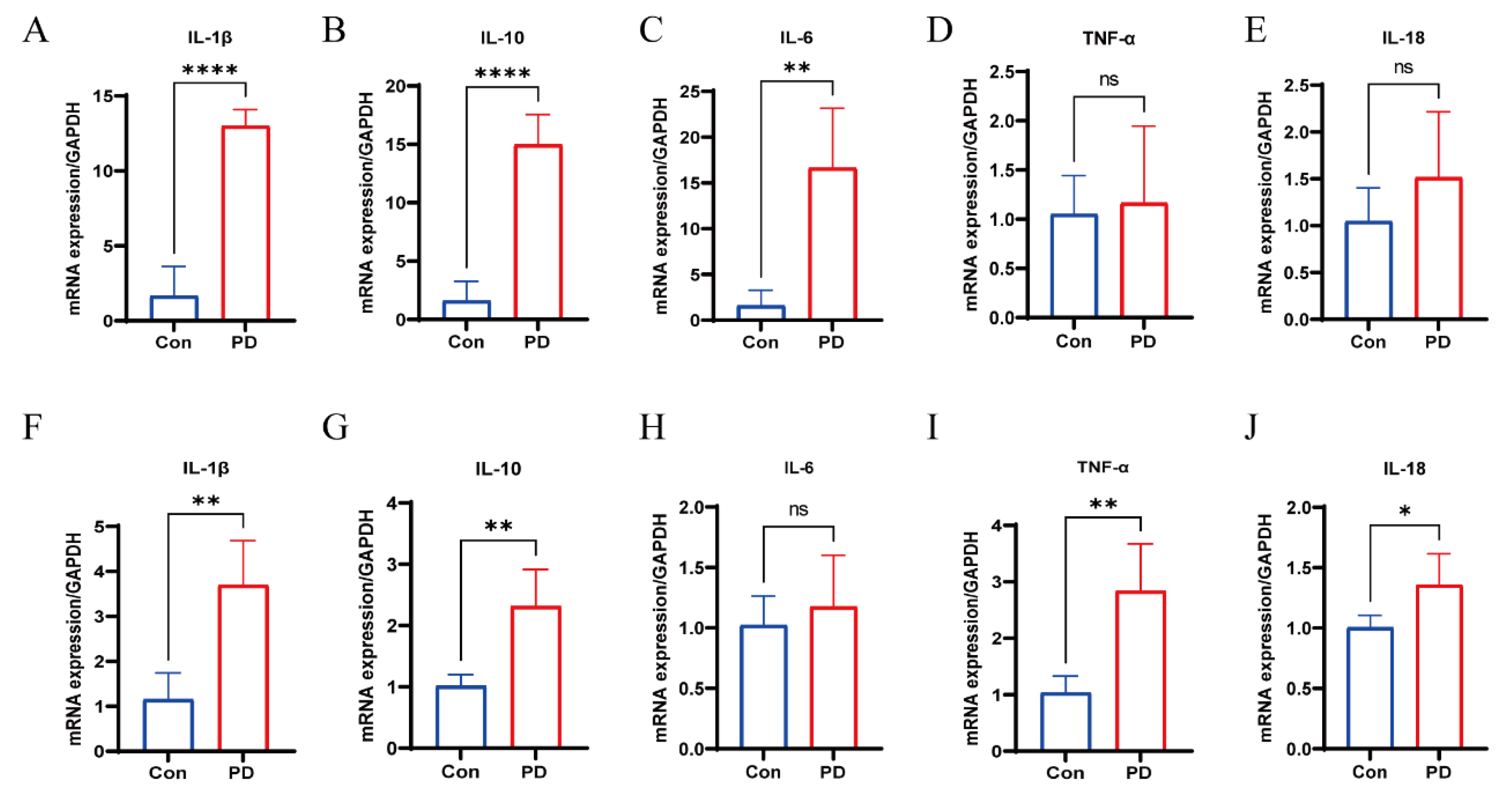
3.6. Upregulation of Cytokines in Periodontal and OSCC Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, X.; Dong, C.; Li, B. Effects of macrophages in OSCC progression. Front. Immunol. 2024, 15, 1517886. [Google Scholar] [CrossRef]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral. Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Li, H.; Vujanovic, L.; Zandberg, D.P.; Ferris, R.L.; Bruno, T.C. Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat. Rev. Cancer 2023, 23, 173–188. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral. Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Bhatia, A.; Burtness, B. Treating Head and Neck Cancer in the Age of Immunotherapy: A 2023 Update. Drugs 2023, 83, 217–248. [Google Scholar] [CrossRef]
- Gili, R.; Gianluca, S.; Paolo, A.; Federica, S.; Paola, L.C.; Simone, C.; Matteo, S.; Almalina, B.; Filippo, M.; Lucia, D.M.; et al. The role of prehabilitation in HNSCC patients treated with chemoradiotherapy. Support. Care Cancer 2024, 32, 638. [Google Scholar] [CrossRef]
- Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jędras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer-Update Review. Cancers 2024, 16, 3156. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Xin, J.; Dai, Y.; Chen, X.; Zou, J.; Wang, R.; Wang, B.; Liu, Z. Development and experimental verification of a prognosis model for disulfidptosis-associated genes in HNSCC. Medicine (Baltimore) 2024, 103, e37308. [Google Scholar] [CrossRef]
- Tran, Q.; Maddineni, S.; Arnaud, E.H.; Divi, V.; Megwalu, U.C.; Topf, M.C.; Sunwoo, J.B. Oral cavity cancer in young, non-smoking, and non-drinking patients: A contemporary review. Crit. Rev. Oncol. Hematol. 2023, 190, 104112. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral. Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 155–175. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yuan, Y.H.; Liu, H.H.; Li, S.S.; Zhang, B.W.; Chen, W.; An, Z.J.; Chen, S.Y.; Wu, Y.Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral. Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Straka, M.; Trapezanlidis, M. Periodontitis and stroke. Neuro Endocrinol. Lett. 2013, 34, 200–206. [Google Scholar]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Glob. Heart 2020, 15, 1. [Google Scholar] [CrossRef]
- Li, R.; Hou, M.; Yu, L.; Luo, W.; Liu, R.; Wang, H. Association between periodontal disease and oral squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Oral. Maxillofac. Surg. 2023, 61, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Komlós, G.; Csurgay, K.; Horváth, F.; Pelyhe, L.; Németh, Z. Periodontitis as a risk for oral cancer: A case-control study. BMC Oral. Health 2021, 21, 640. [Google Scholar] [CrossRef]
- Sung, C.E.; Lin, F.G.; Huang, R.Y.; Fang, W.H.; Cheng, W.C.; Tsai, Y.C.; Chen, W.L. Periodontitis, Helicobacter pylori infection, and gastrointestinal tract cancer mortality. J. Clin. Periodontol. 2022, 49, 210–220. [Google Scholar] [CrossRef]
- Baima, G.; Minoli, M.; Michaud, D.S.; Aimetti, M.; Sanz, M.; Loos, B.G.; Romandini, M. Periodontitis and risk of cancer: Mechanistic evidence. Periodontol 2000 2024, 96, 83–94. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Z.; Chen, Z.; Song, J.; Wang, B.; Jin, F. Association between periodontitis and breast cancer: Two-sample Mendelian randomization study. Clin. Oral. Investig. 2023, 27, 2843–2849. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Amato, A. Periodontitis and Cancer: Beyond the Boundaries of Oral Cavity. Cancers 2023, 15, 1736. [Google Scholar] [CrossRef]
- Mäkinen, A.I.; Pappalardo, V.Y.; Buijs, M.J.; Brandt, B.W.; Mäkitie, A.A.; Meurman, J.H.; Zaura, E. Salivary microbiome profiles of oral cancer patients analyzed before and after treatment. Microbiome 2023, 11, 171. [Google Scholar] [CrossRef]
- Mougeot, J.C.; Stevens, C.B.; Morton, D.S.; Brennan, M.T.; Mougeot, F.B. Oral Microbiome and Cancer Therapy-Induced Oral Mucositis. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz002. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M. Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontol 2000 2018, 78, 7–11. [Google Scholar] [CrossRef]
- Song, X.; Greiner-Tollersrud, O.K.; Zhou, H. Oral Microbiota Variation: A Risk Factor for Development and Poor Prognosis of Esophageal Cancer. Dig. Dis. Sci. 2022, 67, 3543–3556. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S. Extracellular vesicles in oral squamous carcinoma carry oncogenic miRNA profile and reprogram monocytes via NF-κB pathway. Oncotarget 2018, 9, 34838–34854. [Google Scholar] [CrossRef]
- Teles, F.; Collman, R.G.; Mominkhan, D.; Wang, Y. Viruses, periodontitis, and comorbidities. Periodontol 2000 2022, 89, 190–206. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Jun, H.K.; Jung, Y.J.; Choi, B.K. Treponema denticola, Porphyromonas gingivalis, and Tannerella forsythia induce cell death and release of endogenous danger signals. Arch. Oral. Biol. 2017, 73, 72–78. [Google Scholar] [CrossRef]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 2005, 38, 72–122. [Google Scholar] [CrossRef]
- Ursu, R.G.; Iancu, L.S.; Porumb-Andrese, E.; Damian, C.; Cobzaru, R.G.; Nichitean, G.; Ripa, C.; Sandu, D.; Luchian, I. Host mRNA Analysis of Periodontal Disease Patients Positive for Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans and Tannerella forsythia. Int. J. Mol. Sci. 2022, 23, 9915. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Wong-Rolle, A.; Wei, H.K.; Zhao, C.; Jin, C. Unexpected guests in the tumor microenvironment: Microbiome in cancer. Protein Cell 2021, 12, 426–435. [Google Scholar] [CrossRef]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: Roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The Role of Porphyromonas gingivalis Outer Membrane Vesicles in Periodontal Disease and Related Systemic Diseases. Front. Cell Infect. Microbiol. 2020, 10, 585917. [Google Scholar] [CrossRef]
- Lamont, R.J.; Fitzsimonds, Z.R.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontol 2000 2022, 89, 154–165. [Google Scholar] [CrossRef]
- Tan, Q.; Ma, X.; Yang, B.; Liu, Y.; Xie, Y.; Wang, X.; Yuan, W.; Ma, J. Periodontitis pathogen Porphyromonas gingivalis promotes pancreatic tumorigenesis via neutrophil elastase from tumor-associated neutrophils. Gut Microbes 2022, 14, 2073785. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and oral cancer: A critical review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontol 2000 2022, 89, 166–180. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, K.; Long, Z.; Zhou, X.; Zhong, C.; Wang, S.; Lai, H.; Guo, Y.; Lv, D.; Lu, J.; et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome 2022, 10, 94. [Google Scholar] [CrossRef]
- Barot, S.V.; Sangwan, N.; Nair, K.G.; Schmit, S.L.; Xiang, S.; Kamath, S.; Liska, D.; Khorana, A.A. Distinct intratumoral microbiome of young-onset and average-onset colorectal cancer. EBioMedicine 2024, 100, 104980. [Google Scholar] [CrossRef]
- Zhou, H.; Fei, W.; Bai, Y.; Zhu, S.; Luo, E.; Chen, K.; Hu, J. RNA interference-mediated downregulation of hypoxia-inducible factor-1α inhibits angiogenesis and survival of oral squamous cell carcinoma in vitro and in vivo. Eur. J. Cancer Prev. 2012, 21, 289–299. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Greathouse, K.L.; White, J.R.; Vargas, A.J.; Bliskovsky, V.V.; Beck, J.A.; von Muhlinen, N.; Polley, E.C.; Bowman, E.D.; Khan, M.A.; Robles, A.I.; et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018, 19, 123. [Google Scholar] [CrossRef]
- Seeneevassen, L.; Bessède, E.; Mégraud, F.; Lehours, P.; Dubus, P.; Varon, C. Gastric Cancer: Advances in Carcinogenesis Research and New Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 3418. [Google Scholar] [CrossRef]
- Maddineni, G.; Xie, J.J.; Brahmbhatt, B.; Mutha, P. Diet and carcinogenesis of gastric cancer. Curr. Opin. Gastroenterol. 2022, 38, 588–591. [Google Scholar] [CrossRef]
- Megrian, D.; Taib, N.; Witwinowski, J.; Beloin, C.; Gribaldo, S. One or two membranes? Diderm Firmicutes challenge the Gram-positive/Gram-negative divide. Mol. Microbiol. 2020, 113, 659–671. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef]
- Kothe, E. Special Focus: Actinobacteria. J. Basic. Microbiol. 2018, 58, 719. [Google Scholar] [CrossRef]
- Kavazos, C.R.J.; Ricci, F.; Leggat, W.; Casey, J.M.; Choat, J.H.; Ainsworth, T.D. Intestinal Microbiome Richness of Coral Reef Damselfishes (Actinopterygii: Pomacentridae). Integr. Org. Biol. 2022, 4, obac026. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Xiao, Q.; Xiao, Z.; Meng, D.; Yang, Z.; Deng, W.; Yin, H.; Liu, Z. Dissecting the HGT network of carbon metabolic genes in soil-borne microbiota. Front. Microbiol. 2023, 14, 1173748. [Google Scholar] [CrossRef]
- Zhou, C.; Cheng, H.; Wu, Y.; Zhang, J.; Li, D.; Pan, C. Bensulfuron-Methyl, Terbutylazine, and 2,4-D Butylate Disturb Plant Growth and Resistance by Deteriorating Rhizosphere Environment and Plant Secondary Metabolism in Wheat Seedlings. J. Agric. Food Chem. 2022, 70, 12796–12806. [Google Scholar] [CrossRef]
- Facimoto, C.T.; Clements, K.D.; White, W.L.; Handley, K.M. Bacteroidia and Clostridia are equipped to degrade a cascade of polysaccharides along the hindgut of the herbivorous fish Kyphosus sydneyanus. ISME Commun. 2024, 4, ycae102. [Google Scholar] [CrossRef]
- Hui, M.; Wang, A.; Cheng, J.; Sha, Z. Full-length 16S rRNA amplicon sequencing reveals the variation of epibiotic microbiota associated with two shrimp species of Alvinocarididae: Possibly co-determined by environmental heterogeneity and specific recognition of hosts. PeerJ 2022, 10, e13758. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.O.; Lim, M.; Ok, S.H.; Lee, S.K.; Chun, K.S.; Park, K.K.; Hu, Y.; Chung, W.Y.; Song, N.Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef]
- Rakitin, A.L.; Kulichevskaya, I.S.; Beletsky, A.V.; Mardanov, A.V.; Dedysh, S.N.; Ravin, N.V. Verrucomicrobia of the Family Chthoniobacteraceae Participate in Xylan Degradation in Boreal Peat Soils. Microorganisms 2024, 12, 2271. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, H.; Liu, W.; Huang, L.; Huang, J.; Wang, B.; Dong, H.; Chu, R.K.; Tolic, N. Potential utilization of terrestrially derived dissolved organic matter by aquatic microbial communities in saline lakes. Isme J. 2020, 14, 2313–2324. [Google Scholar] [CrossRef]
- Vasconcellos, R.L.F.; Romagnoli, E.M.; Taketani, R.G.; Santos, S.N.; Zucchi, T.D.; Melo, I.S. Impact of Inoculation with Pseudomonas aestus CMAA 1215(T) on the Non-target Resident Bacterial Community in a Saline Rhizosphere Soil. Curr. Microbiol. 2021, 78, 218–228. [Google Scholar] [CrossRef]
- Khijmatgar, S.; Yong, J.; Rübsamen, N.; Lorusso, F.; Rai, P.; Cenzato, N.; Gaffuri, F.; Del Fabbro, M.; Tartaglia, G.M. Salivary biomarkers for early detection of oral squamous cell carcinoma (OSCC) and head/neck squamous cell carcinoma (HNSCC): A systematic review and network meta-analysis. Jpn. Dent. Sci. Rev. 2024, 60, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mahale, A.; Routholla, G.; Lavanya, S.; Sharma, P.; Ghosh, B.; Kulkarni, O.P. Pharmacological blockade of HDAC6 attenuates cancer progression by inhibiting IL-1β and modulating immunosuppressive response in OSCC. Int. Immunopharmacol. 2024, 132, 111921. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Liu, L.; Li, W.; Li, Y.; Li, B. ENO1 Promotes OSCC Migration and Invasion by Orchestrating IL-6 Secretion from Macrophages via a Positive Feedback Loop. Int. J. Mol. Sci. 2023, 24, 737. [Google Scholar] [CrossRef]
- Gayathri, R.; Ramani, P.; Veeraraghavan, V.P. Longitudinal study on salivary IL-6 trajectories in postoperative OSCC patients after chemotherapy and radiotherapy. J. Stomatol. Oral. Maxillofac. Surg. 2024, 125, 101909. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, W.; Liu, Y.; Ding, C.; Zhu, W. The Changes of Phyllosphere Fungal Communities among Three Different Populus spp. Microorganisms 2023, 11, 2479. Microorganisms 2023, 11, 2479. [Google Scholar] [CrossRef]
- Zhou, K.; Peng, M.; Tan, Z.; Xiao, N. Diarrhea Caused by High-Fat and High-Protein Diet Was Associated With Intestinal Lactase-Producing Bacteria. Turk. J. Gastroenterol. 2023, 34, 691–699. [Google Scholar] [CrossRef]
- Zhou, T.; Boettger, M.; Knopp, J.L.; Lange, M.; Heep, A.; Chase, J.G. Model-based subcutaneous insulin for glycemic control of pre-term infants in the neonatal intensive care unit. Comput. Biol. Med. 2023, 160, 106808. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, T.; Burmańczuk, A.; Derlacz, R.; Stefaniak, T.; Rząsa, A.; Borkowski, J. Ustekinumab pharmacokinetics after subcutaneous administration in swine model. J. Vet. Sci. 2021, 22, e47. [Google Scholar] [CrossRef]
- Song, F.; Hou, C.; Huang, Y.; Liang, J.; Cai, H.; Tian, G.; Jiang, Y.; Wang, Z.; Hou, J. Lactylome analyses suggest systematic lysine-lactylated substrates in oral squamous cell carcinoma under normoxia and hypoxia. Cell Signal 2024, 120, 111228. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Q.; Ma, X.; Ma, G.; Liu, C.; Chen, Z.; Yu, L.; Liu, X.; Zhang, Y.; Shao, S.; et al. Establishment of a new OSCC cell line derived from OLK and identification of malignant transformation-related proteins by differential proteomics approach. Sci. Rep. 2015, 5, 12668. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Yan, F.; Zhao, Y.; Zhao, H.; Guo, Y. A Novel Immune-Related Gene Signature to Identify the Tumor Microenvironment and Prognose Disease Among Patients With Oral Squamous Cell Carcinoma Patients Using ssGSEA: A Bioinformatics and Biological Validation Study. Front. Immunol. 2022, 13, 922195. [Google Scholar] [CrossRef] [PubMed]
- Chipashvili, O.; Bor, B. Ligature-induced periodontitis mouse model protocol for studying Saccharibacteria. STAR Protoc. 2022, 3, 101167. [Google Scholar] [CrossRef] [PubMed]

| Primer | 5′→3′ |
|---|---|
| Ki67 | F:GTCCATAAAGACCCTTTTCAGCCA |
| R:ATGTCCTCGTTTCCGATTATAGT | |
| IL-1β | F:CTACAGGCTCCGAGATGAACAAC |
| R:TCCATTGAGGTGGAGAGCTTTC | |
| IL-10 | F:GGAAGACAATAACTGCACCCACTT |
| R:AAGGCAGTCCGCAGCTCTAG | |
| IL-6 | F:AGTTGCCTTCTTGGGACTGATG |
| R:GGGAGTGGTATCCTCTGTGAAGTCT | |
| TNF-α | F:GGTCCCCAAAGGGATGAGAA |
| R:TGAGGGTCTGGGCCATAGAA | |
| IL-18 | F:GACAGCCTGTGTTCGAGGATATG |
| R:TGTTCTTACAGGAGAGGGTAGAC | |
| GAPDH | F:GGTTGTCTCCTGCGACTTCA |
| R:TGGTCCAGGGTTTCTTACTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; Wu, H.; Tan, H.; Wang, X.; Cao, Y.; Chen, G. Oral Microbial Dysbiosis Driven by Periodontitis Facilitates Oral Squamous Cell Carcinoma Progression. Cancers 2025, 17, 2181. https://doi.org/10.3390/cancers17132181
Yuan Q, Wu H, Tan H, Wang X, Cao Y, Chen G. Oral Microbial Dysbiosis Driven by Periodontitis Facilitates Oral Squamous Cell Carcinoma Progression. Cancers. 2025; 17(13):2181. https://doi.org/10.3390/cancers17132181
Chicago/Turabian StyleYuan, Qing, Hao Wu, Hanyue Tan, Xinxing Wang, Yang Cao, and Gang Chen. 2025. "Oral Microbial Dysbiosis Driven by Periodontitis Facilitates Oral Squamous Cell Carcinoma Progression" Cancers 17, no. 13: 2181. https://doi.org/10.3390/cancers17132181
APA StyleYuan, Q., Wu, H., Tan, H., Wang, X., Cao, Y., & Chen, G. (2025). Oral Microbial Dysbiosis Driven by Periodontitis Facilitates Oral Squamous Cell Carcinoma Progression. Cancers, 17(13), 2181. https://doi.org/10.3390/cancers17132181






