Hydrocodone Rescheduling and Opioid Prescribing Disparities in Breast Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Cohort
2.3. Patient Characteristics
2.4. Prescription Opioids
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peuckmann, V.; Ekholm, O.; Rasmussen, N.K.; Groenvold, M.; Christiansen, P.; Møller, S.; Eriksen, J.; Sjøgren, P. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur. J. Pain 2009, 13, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, D.J.; Looha, M.A.; Akbari, M.E.; Akbari, A. Predictors of postoperative pain six months after breast surgery. Sci. Rep. 2023, 13, 8302. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, R.; Jensen, M.B.; Nielsen, J.; Ewertz, M.; Kroman, N.; Kehlet, H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009, 302, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Bolshakova, M.; Bluthenthal, R.; Sussman, S. Opioid use and misuse: Health impact, prevalence, correlates and interventions. Psychol. Health 2019, 34, 1105–1139. [Google Scholar] [CrossRef]
- International Narcotics Control Board. Comments on the Reported Statistics on Narcotic Drugs; International Narcotics Control Board: Vienna, Austria, 2018. [Google Scholar]
- Puetzler, J.; Feldmann, R.E., Jr.; Brascher, A.-K.; Gerhardt, A.; Benrath, J. Improvements in health-related quality of life by comprehensive cancer pain therapy: A pilot study with breast cancer outpatients under palliative chemotherapy. Oncol. Res. Treat. 2014, 37, 456–462. [Google Scholar] [CrossRef]
- Anderson, K.O.; Green, C.R.; Payne, R. Racial and Ethnic Disparities in Pain: Causes and Consequences of Unequal Care. J. Pain 2009, 10, 1187–1204. [Google Scholar] [CrossRef]
- Green, C.R.; Anderson, K.O.; Baker, T.A.; Campbell, L.C.; Decker, S.; Fillingim, R.B.; Kaloukalani, D.A.; Lasch, K.E.; Myers, C.; Tait, R.C.; et al. The Unequal Burden of Pain: Confronting Racial and Ethnic Disparities in Pain. Pain Med. 2003, 4, 277–294. [Google Scholar] [CrossRef]
- Anderson, K.O.; Richman, S.P.; Hurley, J.; Palos, G.; Valero, V.; Mendoza, T.R.; Gning, I.; Cleeland, C.S. Cancer pain management among underserved minority outpatients. Cancer 2002, 94, 2295–2304. [Google Scholar] [CrossRef]
- Wiznia, D.H.; Zaki, T.; Maisano, J.; Kim, C.-Y.; Halaszynski, T.M.; Leslie, M.P. Influence of medical insurance under the affordable care act on access to pain management of the trauma patient. Reg. Anesth. Pain Med. 2017, 42, 39–44. [Google Scholar] [CrossRef]
- Azhar, A.; Yennurajalingam, S.; Ramu, A.; Zhang, H.; Haider, A.; Williams, J.L.; Dibaj, S.S.; Liu, D.D.; Bruera, E. Timing of Referral and Characteristics of Uninsured, Medicaid, and Insured Patients Referred to the Outpatient Supportive Care Center at a Comprehensive Cancer Center. J. Pain Symptom Manag. 2018, 55, 973–978. [Google Scholar] [CrossRef]
- McNeill, J.; Reynolds, J.; Ney, M.L. Unequal quality of cancer pain management: Disparity in perceived control and proposed solutions. Proc. Oncol. Nurs. Forum 2007, 34, 487. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.J.; Luo, Z.; Given, C.W. Cancer incidence in elderly Medicare and dually eligible beneficiaries. Health Serv. Res. 2008, 43, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Joshu, C.E.; Visvanathan, K.; Connor, A.E. Trends in breast cancer incidence rates by race/ethnicity: Patterns by stage, socioeconomic position, and geography in the United States, 1999–2017. Cancer 2022, 128, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Collin, L.J.; Jiang, R.; Ward, K.C.; Gogineni, K.; Subhedar, P.D.; Sherman, M.E.; Gaudet, M.M.; Breitkopf, C.R.; D’Angelo, O.; Gabram-Mendola, S. Racial disparities in breast cancer outcomes in the metropolitan Atlanta area: New insights and approaches for health equity. JNCI Cancer Spectr. 2019, 3, pkz053. [Google Scholar] [CrossRef]
- Dual-Eligible Beneficiaries. Available online: https://www.macpac.gov/topic/dually-eligible-beneficiaries/ (accessed on 12 June 2025).
- Mojtabai, R.; Amin-Esmaeili, M.; Nejat, E.; Olfson, M. Misuse of prescribed opioids in the U nited S tates. Pharmacoepidemiol. Drug Saf. 2019, 28, 345–353. [Google Scholar] [CrossRef]
- Biancuzzi, H.; Dal Mas, F.; Brescia, V.; Campostrini, S.; Cascella, M.; Cuomo, A.; Cobianchi, L.; Dorken-Gallastegi, A.; Gebran, A.; Kaafarani, H.M. Opioid misuse: A review of the main issues, challenges, and strategies. Int. J. Environ. Res. Public Health 2022, 19, 11754. [Google Scholar] [CrossRef]
- Drug Enforcement Administration; Department of Justice. Schedules of controlled substances: Rescheduling of hydrocodone combination products from schedule III to schedule II. Final rule. Fed. Regist. 2014, 79, 49661–49682. [Google Scholar]
- Gabay, M. Federal controlled substances act: Controlled substances prescriptions. Hosp. Pharm. 2013, 48, 644. [Google Scholar] [CrossRef]
- Shumway, J.W.; McClusky, J.; Peacock, W.F.; Cintron, A.; Ludwig, M.S. Impact of Rescheduling Hydrocodone Combination Products Among Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E527. [Google Scholar] [CrossRef][Green Version]
- Jones, C.M.; Lurie, P.G.; Throckmorton, D.C. Effect of US Drug Enforcement Administration’s rescheduling of hydrocodone combination analgesic products on opioid analgesic prescribing. JAMA Intern. Med. 2016, 176, 399–402. [Google Scholar] [CrossRef]
- Townsend, T.N.; Bohnert, A.S.; Lagisetty, P.; Haffajee, R.L. Did prescribing laws disproportionately affect opioid dispensing to Black patients? Health Serv. Res. 2022, 57, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hendrick, F.; McClair, V. National trends in high-dose chronic opioid utilization among dually eligible and Medicare-only beneficiaries (2006–2015). In Data Analysis Brief: Center for Medicare and Medicaid Services; Centers for Medicare & Medicaid Services: Baltimore, MD, USA, 2018. [Google Scholar]
- Gibson, D.C.; Chou, L.N.; Raji, M.A.; Baillargeon, J.G.; Kuo, Y.F. Opioid Prescribing Trends in Women Following Mastectomy or Breast-Conserving Surgery Before and After the 2014 Federal Reclassification of Hydrocodone. Oncologist 2020, 25, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-F.; Raji, M.A.; Liaw, V.; Baillargeon, J.; Goodwin, J.S. Opioid prescriptions in older Medicare beneficiaries following the 2014 hydrocodone rescheduling. Proc. Pharmacoepidemiol. Drug Saf. 2018, 27, 427. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Prevention for States. Available online: https://www.cdc.gov/overdose-prevention/about/understanding-the-opioid-overdose-epidemic.html (accessed on 1 June 2025).
- Di Lena, É.; Barone, N.; Hopkins, B.; Do, U.; Kaneva, P.; Fiore, J.F., Jr.; Meterissian, S. Opioid prescribing practices in breast oncologic surgery—A retrospective cohort study. World J. Surg. 2024, 48, 642–649. [Google Scholar] [CrossRef]
- Doan, L.V.; Yoon, J.; Chun, J.; Perez, R.; Wang, J. Pain associated with breast cancer: Etiologies and therapies. Front. Pain Res. 2023, 4, 1182488. [Google Scholar] [CrossRef]
- Enewold, L.; Parsons, H.; Zhao, L.; Bott, D.; Rivera, D.R.; Barrett, M.J.; Virnig, B.A.; Warren, J.L. Updated overview of the SEER-Medicare data: Enhanced content and applications. JNCI Monogr. 2020, 2020, 3–13. [Google Scholar]
- Surveillance, Epidemiology, and End Results. Available online: https://seer.cancer.gov/ (accessed on 1 June 2025).
- Comorbidity SAS Macro (2021 Version). Available online: https://healthcaredelivery.cancer.gov/seermedicare/considerations/macro-2021.html (accessed on 9 October 2020).
- Guha, A.; Fradley, M.G.; Dent, S.F.; Weintraub, N.L.; Lustberg, M.B.; Alonso, A.; Addison, D. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: A SEER-Medicare analysis. Eur. Heart J. 2022, 43, 300–312. [Google Scholar] [CrossRef]
- Epstein, R.S.; Nelms, J.; Moran, D.; Girman, C.; Huang, H.; Chioda, M. Treatment patterns and burden of myelosuppression for patients with small cell lung cancer: A SEER-medicare study. Cancer Treat. Res. Commun. 2022, 31, 100555. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, L.; Chen, Q.; Hu, Y.; Zhu, X.; Dai, R.; Chen, X.; Zeng, Y.; Zhu, Y.; Song, D. A retrospective study on the impact of radiotherapy on the survival outcomes of small cell lung cancer patients based on the SEER database. Sci. Rep. 2024, 14, 15552. [Google Scholar] [CrossRef]
- Li, T.; Chen, S.; Zhang, Z.; Lin, L.; Wu, Q.; Li, J.; Lin, Q. Chemotherapy plus radiotherapy versus radiotherapy in patients with small cell carcinoma of the esophagus: A SEER database analysis. Cancer Control 2021, 28, 1073274821989321. [Google Scholar] [CrossRef]
- Wooldridge, J.M. Econometric Analysis of Cross Section and Panel Data; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Nelson, D.B.; Niu, J.; Mitchell, K.G.; Sepesi, B.; Hofstetter, W.L.; Antonoff, M.B.; Giordano, S.H.; Mehran, R.J.; Rice, D.C. Persistent opioid use among the elderly after lung resection: A SEER-Medicare study. Ann. Thorac. Surg. 2020, 109, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Staniorski, C.J.; Yu, M.; Sharbaugh, D.; Stencel, M.G.; Myrga, J.M.; Davies, B.J.; Yabes, J.G.; Jacobs, B. Predictors of persistent opioid use in bladder cancer patients undergoing radical cystectomy: A SEER-Medicare analysis. Proc. Urol. Oncol. Semin. Orig. Investig. 2024, 42, 220.e21–220.e29. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, P.; Safarudin, R.; Varisco, T. The impact of opioid use prior to pancreatic cancer diagnosis on overall survival: Insights from SEER-Medicare data. J. Clin. Oncol. 2025, 43, e16424. [Google Scholar] [CrossRef]
- Liu, Y.; Baker, O.; Schuur, J.D.; Weiner, S.G. Effects of rescheduling hydrocodone on opioid prescribing in Ohio. Pain Med. 2020, 21, 1863–1870. [Google Scholar] [CrossRef]
- McLaughlin, C.G.; Wyszewianski, L. Access to care: Remembering old lessons. Health Serv. Res. 2002, 37, 1441–1443. [Google Scholar] [CrossRef]
- Guadamuz, J.S.; Wilder, J.R.; Mouslim, M.C.; Zenk, S.N.; Alexander, G.C.; Qato, D.M. Fewer Pharmacies In Black And Hispanic/Latino Neighborhoods Compared With White Or Diverse Neighborhoods, 2007–2015: Study examines pharmacy “deserts” in Black and Hispanic/Latino neighborhoods compared with white or diverse neighborhoods. Health Aff. 2021, 40, 802–811. [Google Scholar] [CrossRef]
- Roberts, A.W.; Fergestrom, N.; Neuner, J.M.; Winn, A.N. New-onset persistent opioid use following breast cancer treatment in older adult women. Cancer 2020, 126, 814–822. [Google Scholar] [CrossRef]
- Arabandi, P.R.; Slade, A.N.; Fernandez, E.V.; Carroll, N.V. The relationship between palliative radiotherapy and opioid prescribing patterns among patients with metastatic cancer. Ann. Palliat. Med. 2023, 12, 91218–91918. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Fisk, J.D.; Walld, R.; Bolton, J.M.; Sareen, J.; Patten, S.B.; Singer, A.; Lix, L.M.; Hitchon, C.A.; El-Gabalawy, R. Psychiatric Comorbidity Does Not Enhance Prescription Opioid Use in Inflammatory Bowel Disease as It Does in the General Population. Inflamm. Bowel Dis. 2025, 31, 386–393. [Google Scholar] [CrossRef]
- Raji, M.A.; Kuo, Y.F.; Adhikari, D.; Baillargeon, J.; Goodwin, J.S. Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol. Drug Saf. 2018, 27, 513–519. [Google Scholar] [CrossRef]
- Chua, K.-P.; Nguyen, T.D.; Brummett, C.M.; Bohnert, A.S.; Gunaseelan, V.; Englesbe, M.J.; Waljee, J.F. Changes in surgical opioid prescribing and patient-reported outcomes after implementation of an insurer opioid prescribing limit. Proc. JAMA Health Forum 2023, 4, e233541. [Google Scholar] [CrossRef] [PubMed]
- Hirani, S.; Benkli, B.; Odonkor, C.A.; Hirani, Z.A.; Oso, T.; Bohacek, S.; Wiedrick, J.; Hildebrand, A.; Osuagwu, U.; Orhurhu, V. Racial Disparities in Opioid Prescribing in the United States from 2011 to 2021: A Systematic Review and Meta-Analysis. J. Pain Res. 2024, 17, 3639–3649. [Google Scholar] [CrossRef] [PubMed]
- Rambachan, A.; Fang, M.C.; Prasad, P.; Iverson, N. Racial and Ethnic Disparities in Discharge Opioid Prescribing From a Hospital Medicine Service. J. Hosp. Med. 2021, 16, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Ly, D.P. Association of Patient Race and Ethnicity With Differences in Opioid Prescribing by Primary Care Physicians for Older Adults With New Low Back Pain. JAMA Health Forum 2021, 2, e212333. [Google Scholar] [CrossRef]
- LaForge, K.; Gray, M.; Livingston, C.J.; Leichtling, G.; Choo, E.K. Clinician perspectives on referring medicaid back pain patients to integrative and complementary medicine: A qualitative study. J. Integr. Complement. Med. 2023, 29, 55–60. [Google Scholar] [CrossRef]
- Clark, W.D.; Hulbert, M.M. Research Issues: Dually Eligible Medicare and Medicaid Beneficiaries, Challenges and Opportunities. Health Care Financ. Rev. 1998, 20, 1–10. [Google Scholar]
- Barnett, M.L.; Meara, E.; Lewinson, T.; Hardy, B.; Chyn, D.; Onsando, M.; Huskamp, H.A.; Mehrotra, A.; Morden, N.E. Racial Inequality in Receipt of Medications for Opioid Use Disorder. N. Engl. J. Med. 2023, 388, 1779–1789. [Google Scholar] [CrossRef]
- Advisory, A. A Profile of Medicare-Medicaid Dual Beneficiaries; ATI Asvisory: Washington, DC, USA, 2022. [Google Scholar]
- Jared Sawyer, L.S.; Goetsch, C.; Passero, M.; Linman, S.; Schluterman, N. Access to Care Among Medicare Beneficiaries Aged 65 and Over Living with High-Impact Chronic Pain. Available online: https://www.cms.gov/files/document/access-care-among-medicare-beneficiaries-aged-65-and-over-living-high-impact-chronic-pain.pdf (accessed on 20 February 2025).
- Reyes-Gibby, C.C.; Aday, L.A.; Todd, K.H.; Cleeland, C.S.; Anderson, K.O. Pain in aging community-dwelling adults in the United States: Non-Hispanic whites, non-Hispanic blacks, and Hispanics. J. Pain 2007, 8, 75–84. [Google Scholar] [CrossRef]
- Joyce, G.; Blaylock, B.; Chen, J.; Van Nuys, K. Medicare Part D Plans Greatly Increased Utilization Restrictions On Prescription Drugs, 2011–2020: Study examines Medicare Part D restrictions. Health Aff. 2024, 43, 391–397. [Google Scholar] [CrossRef]
- Green, C.R.; Ndao-Brumblay, S.K.; West, B.; Washington, T. Differences in prescription opioid analgesic availability: Comparing minority and white pharmacies across Michigan. J. Pain 2005, 6, 689–699. [Google Scholar] [CrossRef]
- Seago, S.; Hayek, A.; Pruszynski, J.; Newman, M.G. Change in prescription habits after federal rescheduling of hydrocodone combination products. Proc. Bayl. Univ. Med. Cent. Proc. 2016, 29, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.A.; Hollmann, J.; Goodin, A.; Hincapie-Castillo, J.M.; Adkins, L.E.; Ourhaan, N.; Oueini, R.; Bhagwandass, H.; Easey, T.; Vouri, S.M. Effects of hydrocodone rescheduling on opioid use outcomes: A systematic review. J. Am. Pharm. Assoc. 2021, 61, e20–e44. [Google Scholar] [CrossRef] [PubMed]
- Rao, I.J.; Humphreys, K.; Brandeau, M.L. Effectiveness of policies for addressing the US opioid epidemic: A model-based analysis from the Stanford-Lancet Commission on the North American Opioid Crisis. Lancet Reg. Health Am. 2021, 3, 100031. [Google Scholar]
- H.R.6—SUPPORT for Patients and Communities Act. Available online: https://www.congress.gov/bill/115th-congress/house-bill/6? (accessed on 12 June 2025).
- Medicare, C.f.; Services, M. A Prescriber’s Guide to Medicare Prescription Drug (Part D) Opioid Policies; Centers for Medicare & Medicaid Services: Baltimore, MD, USA, 2023. [Google Scholar]
- Heins, S.E.; Castillo, R.C. Changes in opioid prescribing following the implementation of state policies limiting morphine equivalent daily dose in a commercially insured population. Med. Care 2021, 59, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.Y.; Wong, E.S.; Rosen, J.E.; Gordon, D.B.; Flum, D.R.; Liao, J.M. Association between statewide Medicaid opioid policy and postoperative opioid prescribing among surgeons at a large safety-net hospital. J. Am. Coll. Surg. 2022, 235, 519–528. [Google Scholar] [CrossRef]
- Keister, L.A.; Stecher, C.; Aronson, B.; McConnell, W.; Hustedt, J.; Moody, J.W. Provider bias in prescribing opioid analgesics: A study of electronic medical records at a hospital emergency department. BMC Public Health 2021, 21, 1518. [Google Scholar] [CrossRef]
- Morden, N.E.; Chyn, D.; Wood, A.; Meara, E. Racial inequality in prescription opioid receipt—Role of individual health systems. N. Engl. J. Med. 2021, 385, 342–351. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, P.; Lee, S.; Shen, S.; Dunham, E. Racial disparities in opioid administration and prescribing in the emergency department for pain. Am. J. Emerg. Med. 2022, 55, 167–173. [Google Scholar] [CrossRef]
- Lindner, S.R.; Hart, K.; Manibusan, B.; McCarty, D.; McConnell, K.J. State-and county-level geographic variation in opioid use disorder, medication treatment, and opioid-related overdose among medicaid enrollees. Proc. JAMA Health Forum 2023, 4, e231574. [Google Scholar] [CrossRef]
- Baker, M.B.; Liu, E.C.; Bully, M.A.; Hsieh, A.; Nozari, A.; Tuler, M.; Binda, D.D. Overcoming barriers: A comprehensive review of chronic pain management and accessibility challenges in rural America. Proc. Healthc. 2024, 12, 1765. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, H.; Hartung, D.M.; Witkin, L.R.; Paice, J.A. Medicare part D coverage restrictions and patient cost-sharing for opioids commonly used for cancer pain, 2015–2021. JCO Oncol. Pract. 2022, 18, e1574–e1586. [Google Scholar] [CrossRef] [PubMed]
- Van Den Beuken-Van, M.H.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J. Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e1079. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Thornton, J.D.; Newport, K.; Schaefer, E.; Zhou, S.; Yee, N.S.; Dodge, D.; Leslie, D. Trends and patterns in the use of opioids among metastatic breast cancer patients. Sci. Rep. 2020, 10, 21698. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.C.; Scheffey, C.; Young, H.M.; Vilches, A.O.; Davis, M.S.; Connor, S.R. Opioid prescribing practices before and after initiation of palliative care in outpatients. J. Pain Symptom Manag. 2013, 45, 1107–1111. [Google Scholar] [CrossRef]
- Assessing Data Sources for Measuring Health Care Utilization by Medicare Advantage Enrollees: Encounter Data and Other Sources. Available online: https://www.medpac.gov/wp-content/uploads/2024/06/Jun24_Ch3_MedPAC_Report_To_Congress_SEC.pdf (accessed on 12 June 2025).
- Jung, J.; Carlin, C.; Feldman, R. Measuring resource use in Medicare Advantage using Encounter data. Health Serv. Res. 2022, 57, 172–181. [Google Scholar] [CrossRef]
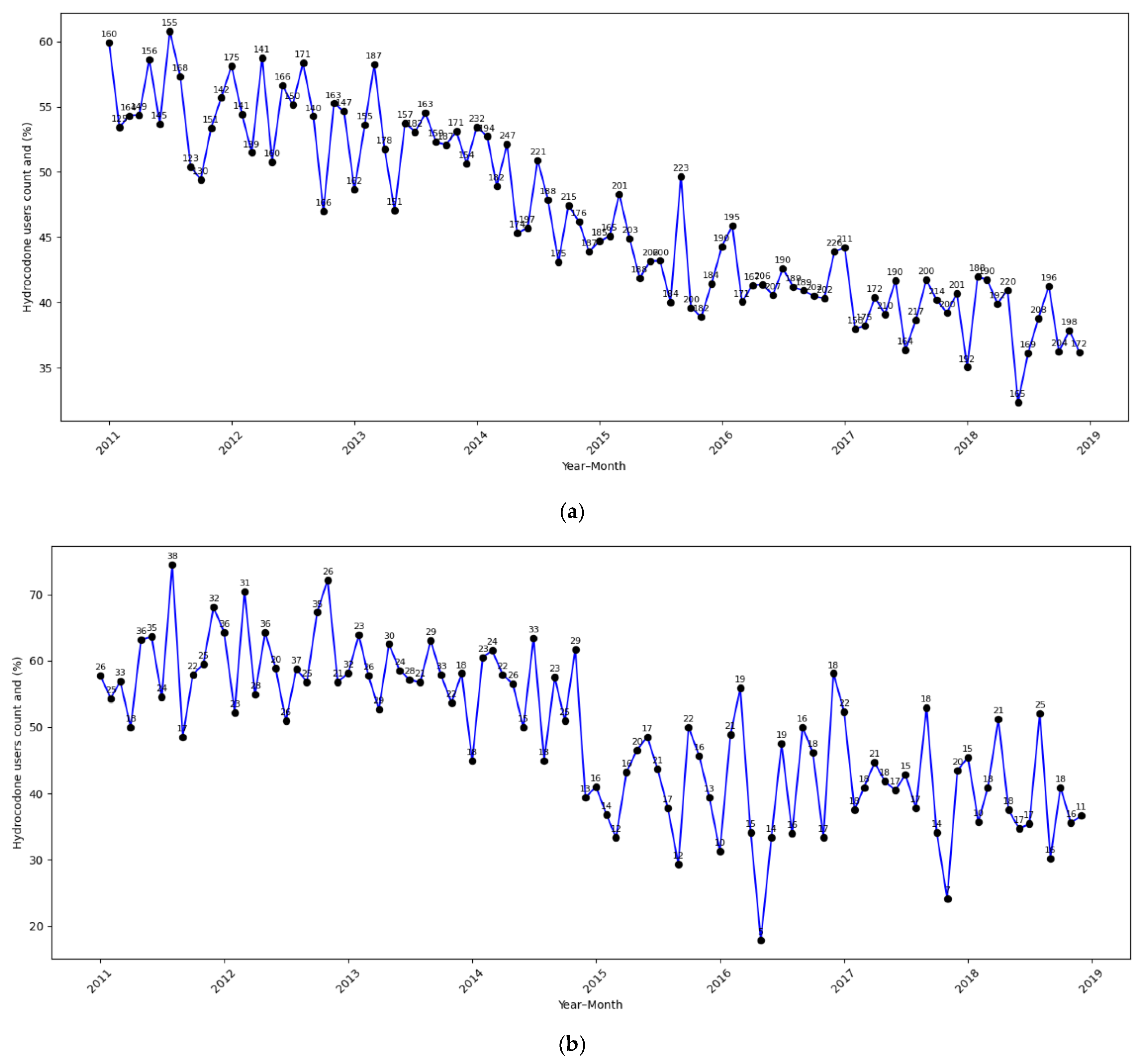
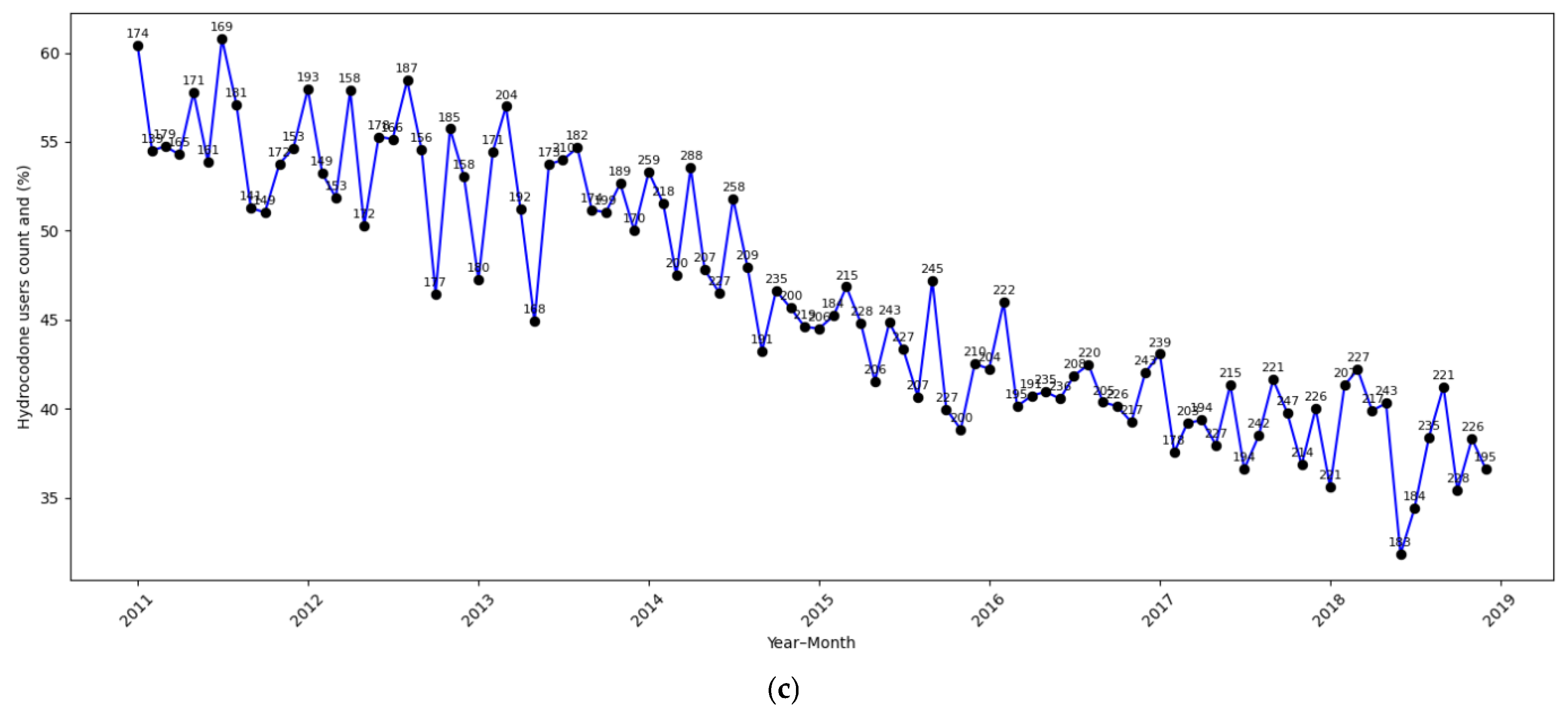
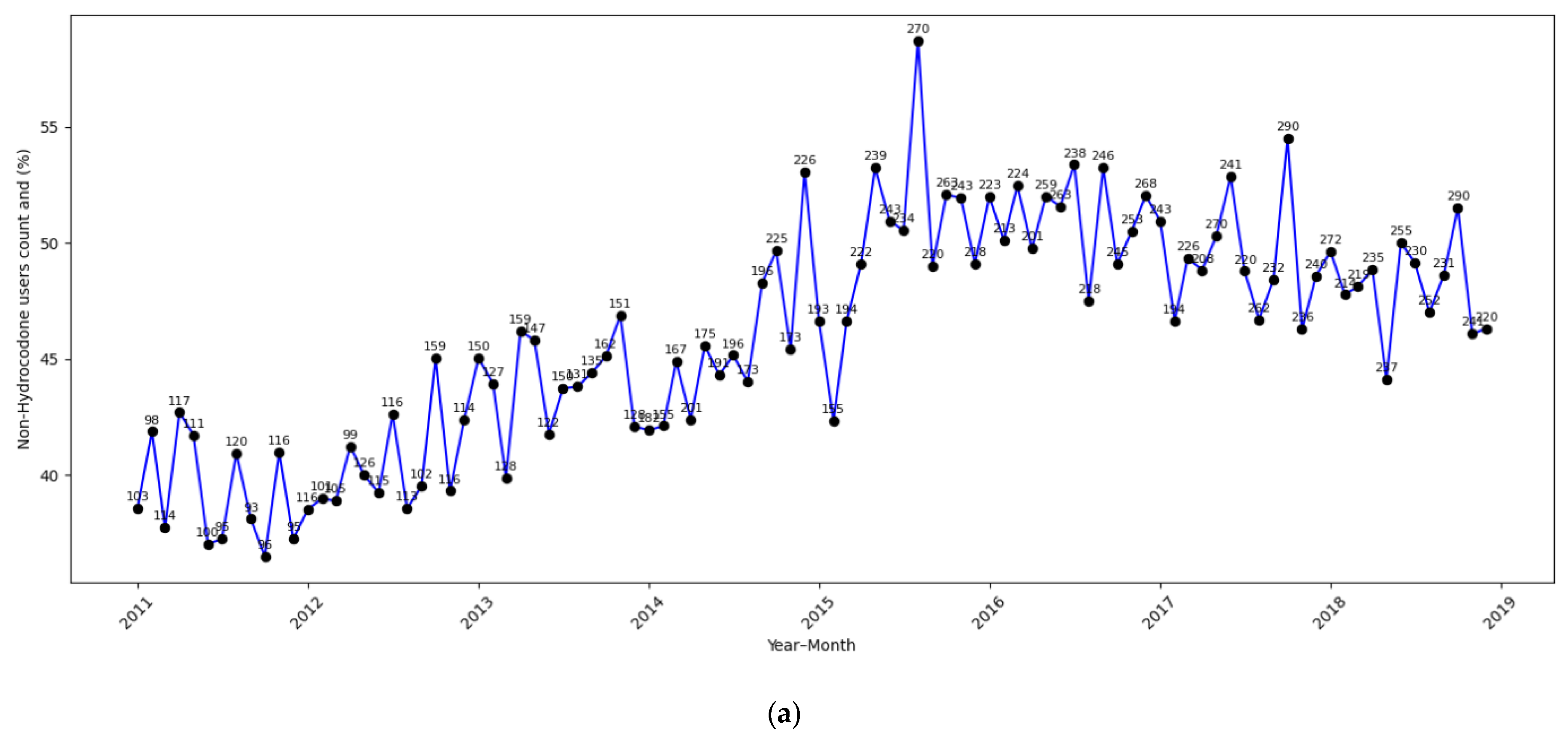
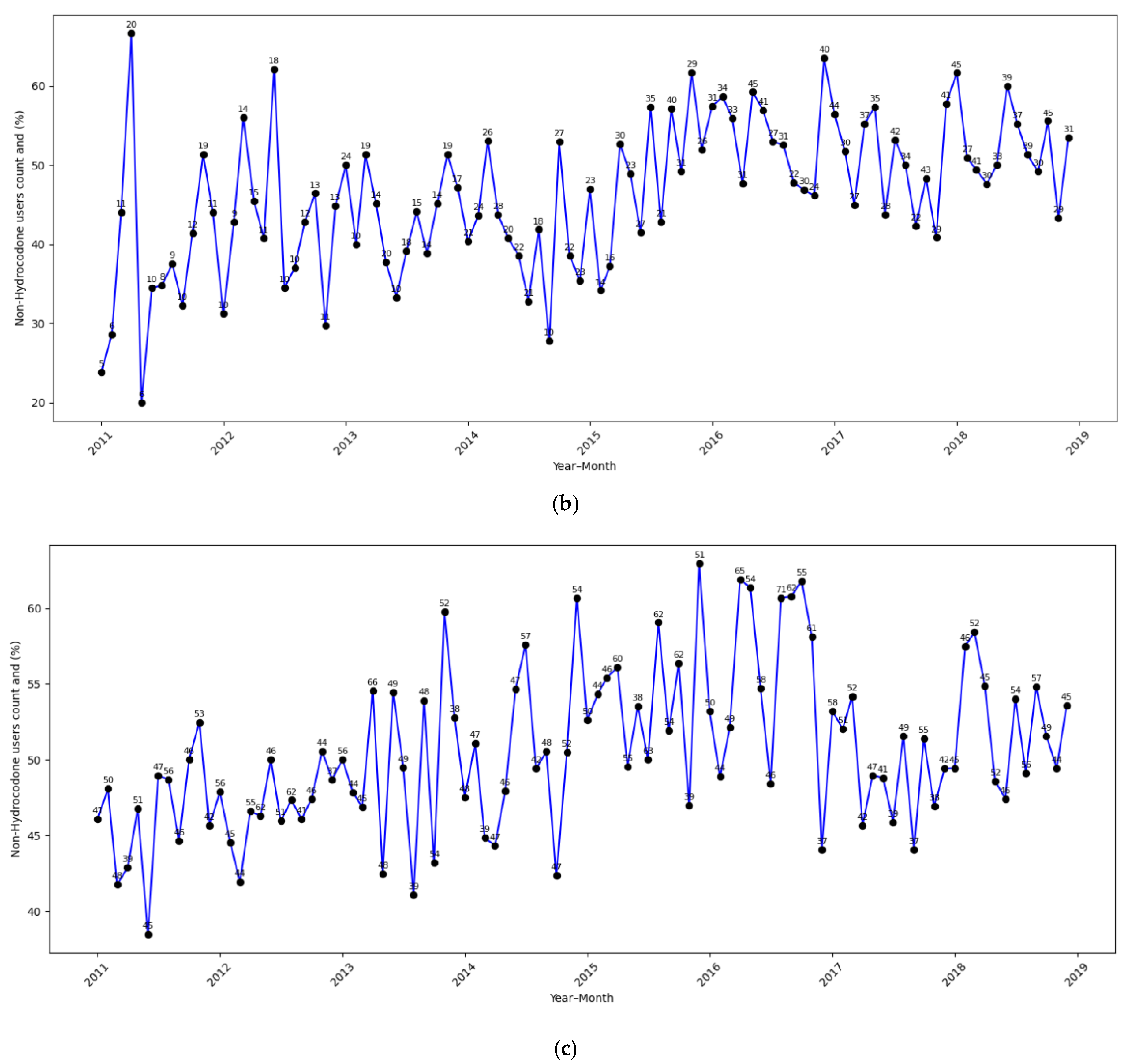
| Total (N = 52,306) | |
|---|---|
| Age | |
| 66–69 | 12,727 (24.3%) |
| 70–74 | 15,277 (29.2%) |
| 75–79 | 11,229 (21.5%) |
| ≥80 | 13,073 (25.0%) |
| Race/Ethnicity | |
| Non-Hispanic White | 43,459 (83.1%) |
| Non-Hispanic Black | 3192 (6.1%) |
| Hispanic/Latino | 2984 (5.7%) |
| Other | 2671 (5.1%) |
| % of Non-high school degree | |
| 0–<5% | 5713 (10.9%) |
| 5% to <10% | 7942 (15.2%) |
| 10% to <20% | 12,690 (24.3%) |
| 20% to 100% | 25,899 (49.6%) |
| % below poverty | |
| 0–<5% | 13,817 (26.4%) |
| 5% to <10% | 11,701 (22.4%) |
| 10% to <20% | 13,875 (26.6%) |
| 20% to 100% | 12,851 (24.6%) |
| Income quartile | |
| First quartile | 13,412 (25.6%) |
| Second quartile | 12,795 (24.5%) |
| Third quartile | 12,727 (24.3%) |
| Fourth quartile | 13,372 (25.6%) |
| Hydrocodone | |
| Yes | 23,979 (45.8%) |
| No | 28,327 (54.2%) |
| Non-Hydrocodone opioids | |
| Yes | 24,897 (47.6%) |
| No | 27,409 (52.4%) |
| Radiation therapy | |
| Yes | 30,455 (58.2%) |
| No | 21,851 (41.8%) |
| Chemotherapy | |
| Yes | 14,905 (28.5%) |
| No | 37,401 (71.5%) |
| Immunotherapy | |
| Yes | 7351 (14.1%) |
| No | 44,955 (85.9%) |
| Hormonal therapy | |
| Yes | 46,880 (89.6%) |
| No | 5426 (10.4%) |
| Dual Eligibility | |
| Yes | 9393 (18.0%) |
| No | 42,913 (82.0%) |
| Depression | |
| Yes | 11,200 (21.4%) |
| No | 41,106 (78.6%) |
| Charlson comorbidity | |
| No | 20,258 (42.3%) |
| 1 | 13,431 (28.1%) |
| 2 | 8110 (16.9%) |
| 3 or more | 6054 (12.7%) |
| Policy Change | |
| Pre-policy | 20,254 (38.7%) |
| Post policy | 32,052 (61.3%) |
| Continuous month | |
| N (Missing) | 52,306 (0) |
| Mean (SD) | 53.4 (26.98) |
| Median (Range) | 56.0 (1.0, 96.0) |
| Hydrocodone Use | ||||
|---|---|---|---|---|
| Variables | AOR | 95% CI | p-Value | |
| Dual-eligible non-Hispanic White | ||||
| Policy Change | ||||
| Post policy change | 0.75 | [0.60, 0.94] | <0.013 | |
| Before policy change (reference) | ||||
| Time Trend | ||||
| in 12 months | 0.90 | [0.86, 0.95] | <0.001 | |
| Dual-eligible racial–ethnic minority | ||||
| Policy Change | ||||
| Post policy change | 0.57 | [0.44, 0.74] | <0.001 | |
| Before policy change (reference) | ||||
| Time Trend | ||||
| in 12 months | 0.95 | [0.90, 1.00] | 0.063 | |
| Non-dual-eligible | ||||
| Policy Change | ||||
| Post policy change | 0.84 | [0.78, 0.90] | <0.001 | |
| Before policy change (reference) | ||||
| Time Trend | ||||
| in 12 months | 0.91 | [0.90, 0.93] | <0.001 | |
| Non-Hydrocodone Use | ||||
|---|---|---|---|---|
| Non-dual-eligible non-Hispanic White | ||||
| Policy Change | ||||
| Post policy change | 1.29 | [1.19, 1.40] | <0.001 | |
| Before policy change (reference) | ||||
| Time Trend | ||||
| in 12 months | 1.00 | [0.98, 1.02] | 0.815 | |
| Non-dual-eligible racial–ethnic minority | ||||
| Policy Change | ||||
| Post policy change | 1.22 | [0.97, 1.53] | 0.087 | |
| Before policy change (reference) | ||||
| Time Trend | ||||
| in 12 months | 1.03 | [0.98, 1.09] | 0.178 | |
| Dual-eligible | ||||
| Policy Change | ||||
| Post policy change | 1.12 | [0.95, 1.32] | 0.178 | |
| Before policy change (reference) | ||||
| Time Trend | ||||
| in 12 months | 1.01 | [0.97, 1.04] | 0.727 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Ikram, M.; Zhou, S.; Klein, R.; Leslie, D.; Thornton, J.D. Hydrocodone Rescheduling and Opioid Prescribing Disparities in Breast Cancer Patients. Cancers 2025, 17, 2146. https://doi.org/10.3390/cancers17132146
Shen C, Ikram M, Zhou S, Klein R, Leslie D, Thornton JD. Hydrocodone Rescheduling and Opioid Prescribing Disparities in Breast Cancer Patients. Cancers. 2025; 17(13):2146. https://doi.org/10.3390/cancers17132146
Chicago/Turabian StyleShen, Chan, Mohammad Ikram, Shouhao Zhou, Roger Klein, Douglas Leslie, and James Douglas Thornton. 2025. "Hydrocodone Rescheduling and Opioid Prescribing Disparities in Breast Cancer Patients" Cancers 17, no. 13: 2146. https://doi.org/10.3390/cancers17132146
APA StyleShen, C., Ikram, M., Zhou, S., Klein, R., Leslie, D., & Thornton, J. D. (2025). Hydrocodone Rescheduling and Opioid Prescribing Disparities in Breast Cancer Patients. Cancers, 17(13), 2146. https://doi.org/10.3390/cancers17132146







