Risk Factors of Multiple Primary Cancers Among Colorectal Cancer Survivors
Simple Summary
Abstract
1. Introduction
2. Methods
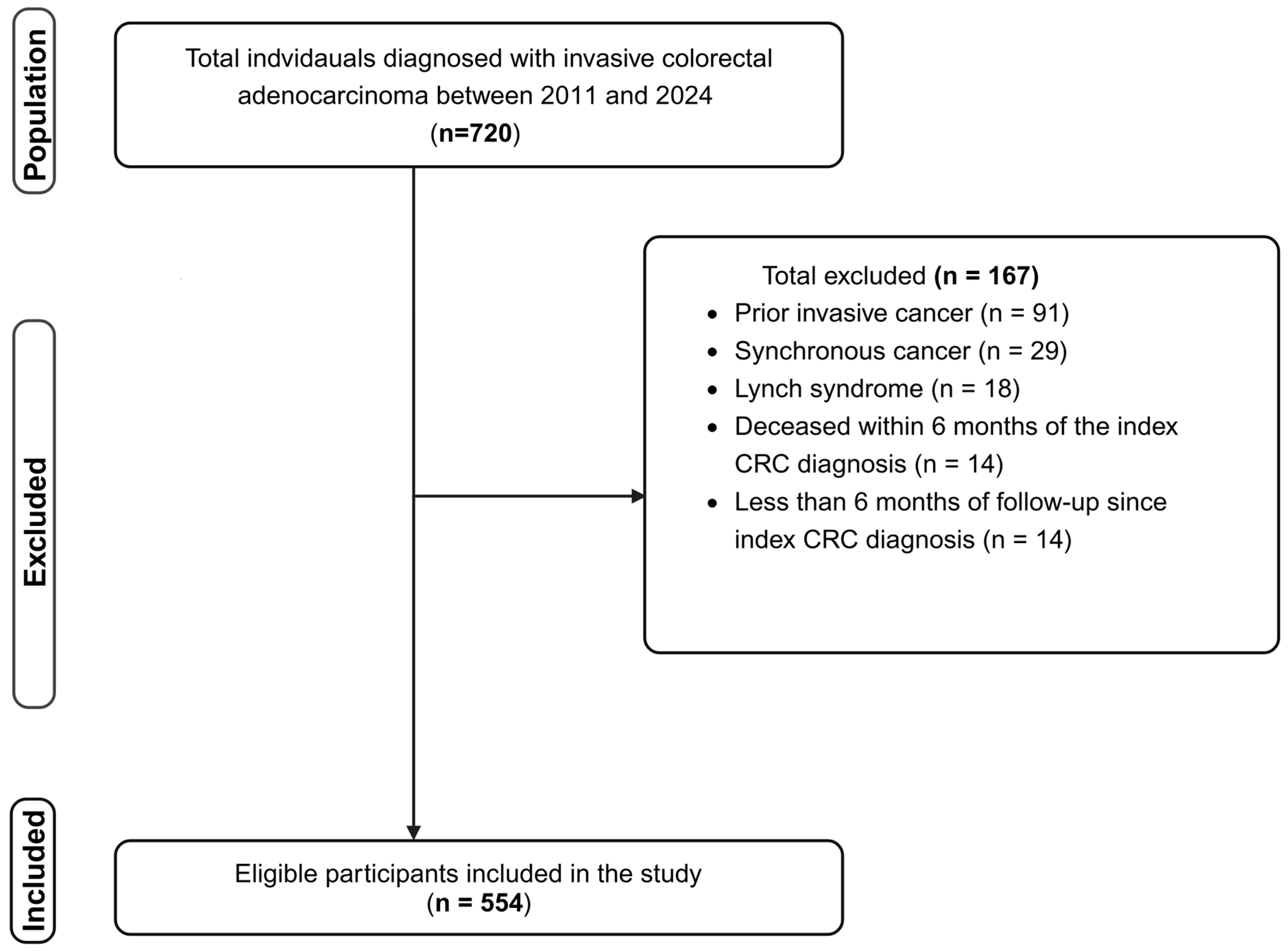
2.1. Study Design, Population, Eligibility, and Outcomes
2.2. Study Variables
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
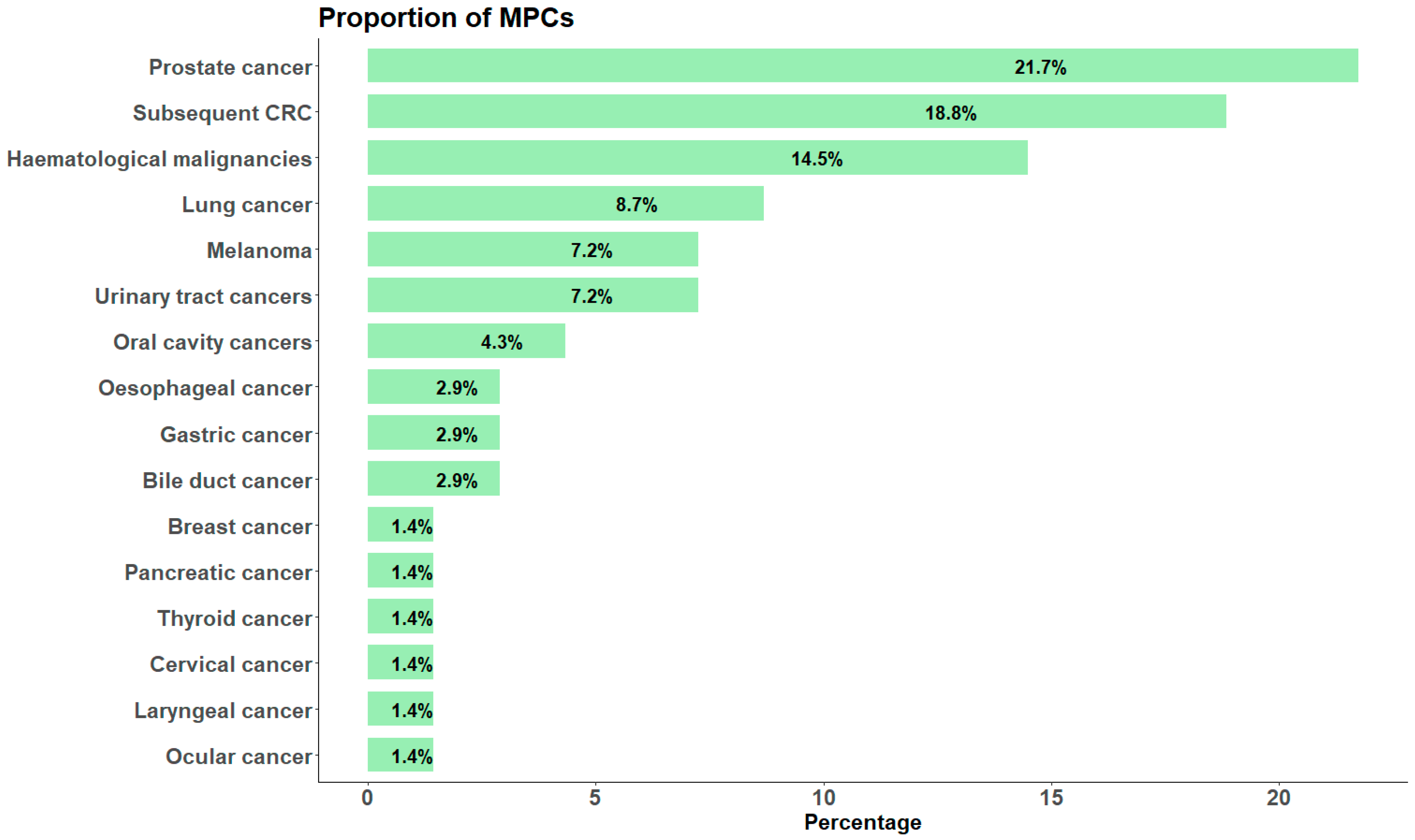
3.2. Incidence, Risk, and Common Types of MPCs
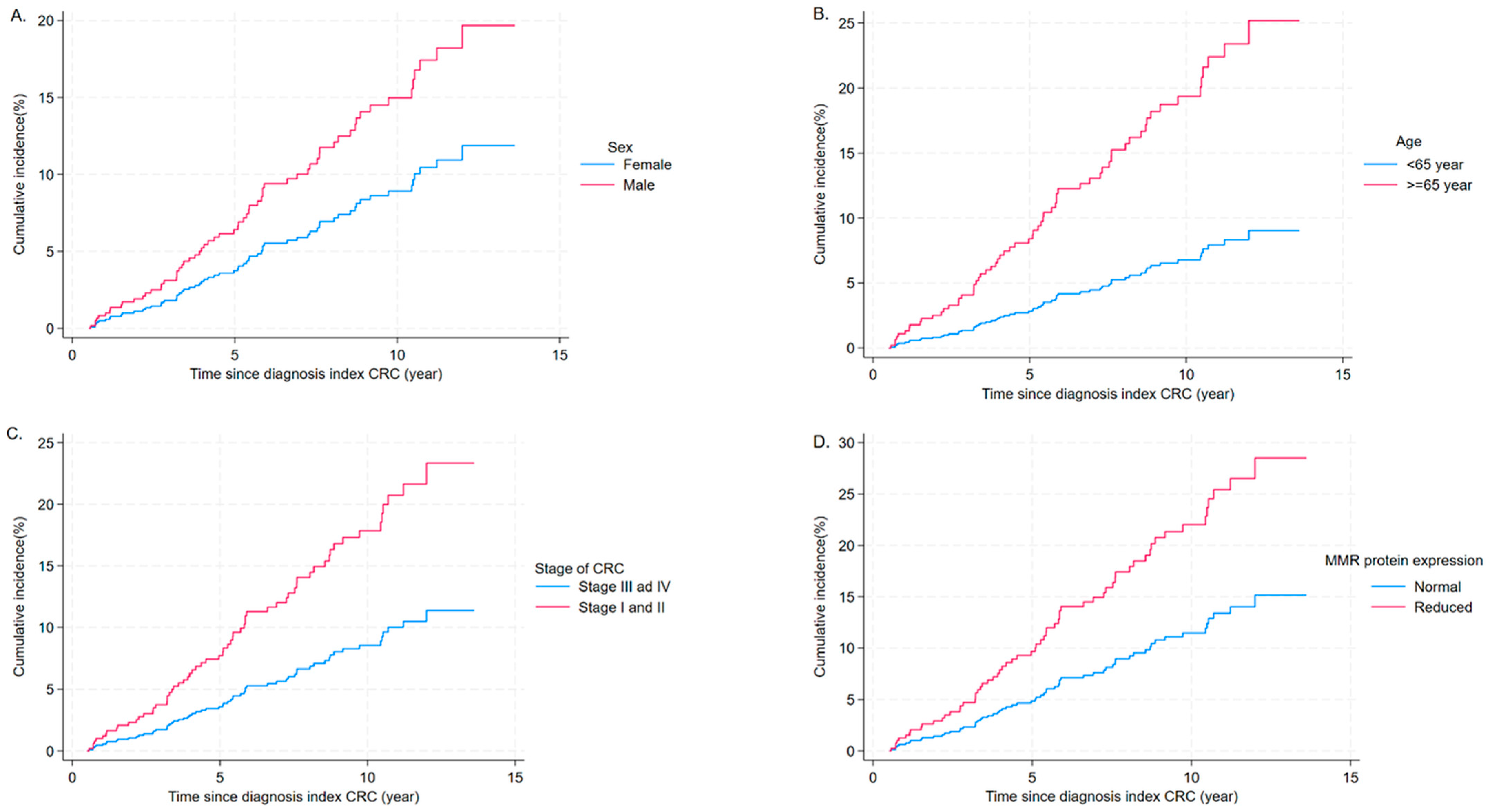
3.3. Risk Factors Associated with MPCs
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- IARC/WHO. Cancer Tomorrow: Colorectal Cancer—Estimated Number of New Cases from 2022 to 2050, Both Sexes, Age [0–85+], World. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/bubbles?types=0&sexes=0&mode=cancer&group_populations=0&multiple_populations=0&multiple_cancers=1&cancers=41&populations=900&apc=cat_ca20v1.5_ca23v-1.5&group_cancers=0&years=2050 (accessed on 29 January 2025).
- IARC/WHO. Cancer Tomorrow: Colorectal Cancer—Estimated Number of New Deaths from 2022 to 2050, Both Sexes, Age [0–85+], World. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/bubbles?types=1&sexes=0&mode=cancer&group_populations=0&multiple_populations=0&multiple_cancers=1&cancers=41&populations=900&apc=cat_ca20v1.5_ca23v-1.5&group_cancers=0&years=2050 (accessed on 29 January 2025).
- Wong, M.C.S.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.J. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966.e1. [Google Scholar] [CrossRef]
- AARC/WHO. Cancer Over Time: Colorectal Cancer. Available online: https://gco.iarc.fr/overtime/en/dataviz/trends?multiple_populations=1&group_populations=1&populations=752_840_32_40_616_124_36_152_170_188_191_203_208_233_246_250_276_372_376_380_392_410_428_440_528_554_578_608_630_724_705_756_8262_8263_8401_8402&years=1990_2019&sexes=1&cancers=106&types=0_1 (accessed on 29 January 2025).
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024, 156, 1336–1346. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, H.; Li, Z.; Ji, X.; Shen, Q.; Tuo, J.; Bi, J.; Li, H.; Xiang, Y. Global pattern and trends of colorectal cancer survival: A systematic review of population-based registration data. Cancer Biol. Med. 2021, 19, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Cancer Summary Data Visualisation: Cancer Data in Australia. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary-dashboard (accessed on 28 January 2025).
- Australian Institute of Health and Welfare. Cancer Mortality by Age Visualisation. Cancer Data in Australia. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-mortality-by-age-visualisation (accessed on 28 January 2025).
- Australian Institute of Health and Welfare. Cancer Incidence and Survival by Histology (Selected Cancers): Cancer Data in Australia. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-incidence-and-survival-by-histology-selecte (accessed on 8 October 2024).
- Robertson, D.; Ng, S.K.; Baade, P.D.; Lam, A.K. Risk of extracolonic second primary cancers following a primary colorectal cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2022, 37, 541–551. [Google Scholar] [CrossRef]
- Working Group Report. International Rules for Multiple Primary Cancers (ICD-0 Third Edition). Eur. J. Cancer Prev. 2005, 14, 307–308. [Google Scholar] [CrossRef]
- Dasgupta, P.; Youlden, D.R.; Baade, P.D. Multiple primary cancers among colorectal cancer survivors in Queensland, Australia, 1996–2007. Cancer Causes Control 2012, 23, 1387–1398. [Google Scholar] [CrossRef]
- Melku, M.; Best, O.G.; Winter, J.M.; Thurgood, L.A.; Ahmed, M.; Kichenadasse, G.; Mittinty, M.; Wassie, M.M.; Symonds, E.L. Incidence, Risk and Trends of Multiple Primary Cancers in Patients with Colorectal Cancer: Evidence From the South Australian Cancer Registry. Cancer Med. 2025, 14, e70984. [Google Scholar] [CrossRef]
- Liang, L.A.; Tseng, Y.J.; Tanaka, L.F.; Klug, S.J. Second primary cancer among 217702 colorectal cancer survivors: An analysis of national German cancer registry data. Int. J. Cancer 2023, 153, 1459–1471. [Google Scholar] [CrossRef]
- Chen, Y.; Han, C.; Huang, Y.; Liu, C.; Sheng, S.; Ji, L.; Zhu, J.; Fu, G.; Mao, X.; Huang, M.; et al. The incidence of second primary cancer in male and female patients with initial colorectal cancer: A SEER population-based study. Eur. J. Cancer Prev. 2022, 31, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Liu, C.J.; Hu, Y.W.; Teng, C.J.; Tzeng, C.H.; Yeh, C.M.; Chen, T.J.; Lin, J.K.; Lin, C.C.; Lan, Y.T.; et al. Incidence of Second Primary Malignancies Following Colorectal Cancer: A Distinct Pattern of Occurrence Between Colon and Rectal Cancers and Association of Co-Morbidity with Second Primary Malignancies in a Population-Based Cohort of 98,876 Patients in Taiwan. Medicine 2015, 94, e1079. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.L.; Pedersen, S.K.; Murray, D.; Byrne, S.E.; Roy, A.; Karapetis, C.; Hollington, P.; Rabbitt, P.; Jones, F.S.; LaPointe, L.; et al. Circulating epigenetic biomarkers for detection of recurrent colorectal cancer. Cancer 2020, 126, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; World Health Organization: Geneva, Switzerland, 2013; Available online: https://iris.who.int/bitstream/handle/10665/96612/9789241548496_eng.pdf?sequence=1 (accessed on 5 February 2025).
- Yang, J.; Wu, F.; An, H.; Gan, H. Incidence and risk outcomes of second primary malignancy of patients with post-operative colorectal cancer. Int. J. Color. Dis. 2023, 38, 88. [Google Scholar] [CrossRef]
- Australian Government National Health Medical Research Council. Australian Guidelines to Reduce Health Risks from Drinking Alcohol. 2020. Available online: https://www.nhmrc.gov.au/file/18327/download?token=RohIfYFA (accessed on 5 February 2025).
- Australian Bureau of Statistics. National, State and Territory Population. Population at 30 June, by Sex and Single Year of Age, SA, from 1971 Onwards; Population—South Australia; 2024. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/latest-release#data-downloads (accessed on 14 April 2024).
- Travis, L.B.; Demark Wahnefried, W.; Allan, J.M.; Wood, M.E.; Ng, A.K. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat. Rev. Clin. Oncol. 2013, 10, 289–301. [Google Scholar] [CrossRef]
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, J.H.; Zhao, Y.Q.; Sui, W.N.; Tian, T.; Han, W.X.; Ni, J. An atlas on risk factors for gastrointestinal cancers: A systematic review of Mendelian randomization studies. Prev. Med. 2024, 189, 108147. [Google Scholar] [CrossRef]
- Li, H.; Liu, K.; Boardman, L.A.; Zhao, Y.; Wang, L.; Sheng, Y.; Oi, N.; Limburg, P.J.; Bode, A.M.; Dong, Z. Circulating Prostaglandin Biosynthesis in Colorectal Cancer and Potential Clinical Significance. eBioMedicine 2015, 2, 165–171. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
- Dubois, R.N. Role of inflammation and inflammatory mediators in colorectal cancer. Trans. Am. Clin. Climatol. Assoc. 2014, 125, 358–372; discussion 372–373. [Google Scholar] [PubMed]
- Zhang, B.; Guo, K.; Zheng, X.; Sun, L.; Shen, M.; Ruan, S. Risk of Second Primary Malignancies in Colon Cancer Patients Treated with Colectomy. Front. Oncol. 2020, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Toomey, S.; Carr, A.; Mezynski, M.J.; Elamin, Y.; Rafee, S.; Cremona, M.; Morgan, C.; Madden, S.; Abdul-Jalil, K.I.; Gately, K.; et al. Identification and clinical impact of potentially actionable somatic oncogenic mutations in solid tumor samples. J. Transl. Med. 2020, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, K. Quantifying the Contributions of Environmental Factors to Prostate Cancer and Detecting Risk-Related Diet Metrics and Racial Disparities. Cancer Inform. 2023, 22, 11769351231168006. [Google Scholar] [CrossRef]
- Morton, L.M.; Dores, G.M.; Schonfeld, S.J.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Curtis, R.E. Association of Chemotherapy for Solid Tumors with Development of Therapy-Related Myelodysplastic Syndrome or Acute Myeloid Leukemia in the Modern Era. JAMA Oncol. 2019, 5, 318–325. [Google Scholar] [CrossRef]
- Kahi, C.J.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Robertson, D.J.; Rex, D.K. Colonoscopy Surveillance After Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2016, 150, 758–768.e711. [Google Scholar] [CrossRef]
- Halamkova, J.; Kazda, T.; Pehalova, L.; Gonec, R.; Kozakova, S.; Bohovicova, L.; Krakorova, D.A.; Slaby, O.; Demlova, R.; Svoboda, M.; et al. Second primary malignancies in colorectal cancer patients. Sci. Rep. 2021, 11, 2759. [Google Scholar] [CrossRef]
- Costa, A.R.; Lança de Oliveira, M.; Cruz, I.; Gonçalves, I.; Cascalheira, J.F.; Santos, C.R.A. The Sex Bias of Cancer. Trends Endocrinol. Metab. 2020, 31, 785–799. [Google Scholar] [CrossRef]
- Hyde, Z.; Flicker, L.; McCaul, K.A.; Almeida, O.P.; Hankey, G.J.; Chubb, S.A.; Yeap, B.B. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1319–1329. [Google Scholar] [CrossRef]
- Czaderny, K. Gender gap in cancer prevention and mortality. A multidimensional analysis. Aging Male 2020, 23, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, L.; Chen, H.; Wang, Y.; Xu, Y.; Mao, H.; Li, J.; Mills, G.B.; Shu, Y.; Li, L.; et al. Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell 2016, 29, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Caramia, F.; Herschtal, A.; Soussi, T.; Lozano, G.; Chen, H.; Liang, H.; Speed, T.P.; Haupt, Y. Identification of cancer sex-disparity in the functional integrity of p53 and its X chromosome network. Nat. Commun. 2019, 10, 5385. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Haider, S.; Shiah, Y.J.; Thai, K.; Boutros, P.C. Sex Differences in Cancer Driver Genes and Biomarkers. Cancer Res. 2018, 78, 5527–5537. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Park, M.D.; Le Berichel, J.; Hamon, P.; Wilk, C.M.; Belabed, M.; Yatim, N.; Saffon, A.; Boumelha, J.; Falcomatà, C.; Tepper, A.; et al. Hematopoietic aging promotes cancer by fueling IL-1⍺-driven emergency myelopoiesis. Science 2024, 386, eadn0327. [Google Scholar] [CrossRef]
- Shi, M.; Luo, C.; Oduyale, O.K.; Zong, X.; LoConte, N.K.; Cao, Y. Alcohol Consumption Among Adults with a Cancer Diagnosis in the All of Us Research Program. JAMA Netw. Open 2023, 6, e2328328. [Google Scholar] [CrossRef]
- Paul, C.L.; Tzelepis, F.; Boyes, A.W.; D’Este, C.; Sherwood, E.; Girgis, A. Continued smoking after a cancer diagnosis: A longitudinal study of intentions and attempts to quit. J. Cancer Surviv. 2019, 13, 687–694. [Google Scholar] [CrossRef]
- Heymer, E.J.; Jóźwiak, K.; Kremer, L.C.; Winter, D.L.; de Vathaire, F.; Sunguc, C.; Sugden, E.; Kok, J.L.; van der Pal, H.J.H.; Hjorth, L.; et al. Cumulative Absolute Risk of Subsequent Colorectal Cancer After Abdominopelvic Radiotherapy Among Childhood Cancer Survivors: A PanCareSurFup Study. J. Clin. Oncol. 2024, 42, 336–347. [Google Scholar] [CrossRef]
- Yang, J.; Du, X.L.; Li, S.; Wu, Y.; Lv, M.; Dong, D.; Zhang, L.; Chen, Z.; Wang, B.; Wang, F.; et al. The risk and survival outcome of subsequent primary colorectal cancer after the first primary colorectal cancer: Cases from 1973 to 2012. BMC Cancer 2017, 17, 783. [Google Scholar] [CrossRef]
- Nors, J.; Iversen, L.H.; Erichsen, R.; Gotschalck, K.A.; Andersen, C.L. Incidence of Recurrence and Time to Recurrence in Stage I to III Colorectal Cancer: A Nationwide Danish Cohort Study. JAMA Oncol. 2024, 10, 54–62. [Google Scholar] [CrossRef]
- Jia, H.; Li, Q.; Yuan, J.; Sun, X.; Wu, Z. Second Primary Malignancies in Patients with Colorectal Cancer: A Population-Based Analysis. Oncologist 2020, 25, e644–e650. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M.; Jung, B.H. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology 2015, 149, 1177–1190.e1173. [Google Scholar] [CrossRef]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Whiting, J.; Xie, H.; Imanirad, I.; Carballido, E.; Felder, S.; Frakes, J.; Mo, Q.; Walko, C.; Permuth, J.B.; et al. BRAF Mutations Are Associated with Poor Survival Outcomes in Advanced-stage Mismatch Repair-deficient/Microsatellite High Colorectal Cancer. Oncologist 2022, 27, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.C. DNA mismatch repair proteins: Scientific update and practical guide. J. Clin. Pathol. 2021, 74, 264–268. [Google Scholar] [CrossRef]
- Martin, S.; Katainen, R.; Taira, A.; Välimäki, N.; Ristimäki, A.; Seppälä, T.; Renkonen-Sinisalo, L.; Lepistö, A.; Tahkola, K.; Mattila, A.; et al. Lynch syndrome-associated and sporadic microsatellite unstable colorectal cancers: Different patterns of clonal evolution yield highly similar tumours. Hum. Mol. Genet. 2024, 33, 1858–1872. [Google Scholar] [CrossRef]
- Poulogiannis, G.; Frayling, I.M.; Arends, M.J. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology 2010, 56, 167–179. [Google Scholar] [CrossRef]
- Willis, J.A.; Reyes-Uribe, L.; Chang, K.; Lipkin, S.M.; Vilar, E. Immune Activation in Mismatch Repair-Deficient Carcinogenesis: More Than Just Mutational Rate. Clin. Cancer Res. 2020, 26, 11–17. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 554) | Index CRC Only (n = 490) | MPC (n = 64) | p-Value | |
|---|---|---|---|---|---|
| Age at diagnosis of the index CRC (years) | Median (IQR) | 65.4 (55.3–73.4) | 64.5 (53.6–72.8) | 71.4 (66.1–77.5) | <0.001 * |
| Sex | Male | 332 (59.9%) | 287 (58.5%) | 45 (70.3%) | 0.070 |
| Female | 222 (40.1%) | 203 (41.4%) | 19 (29.7%) | ||
| Socioeconomic status (quintile) | Lowest to low | 286 (51.6%) | 149 (50.8%) | 37 (57.8%) | 0.292 |
| Middle to highest | 268(48.4%) | 241(49.2%) | 27 (42.2%) | ||
| Smoking habit | Current/previous smoker | 299(54.0%) | 264 (53.9%) | 35 (54.7%) | 0.903 |
| No smoking | 255 (46.0%) | 226 (46.1%) | 29 (45.3%) | ||
| Alcohol consumption | Risky alcohol consumption | 93 (16.8%) | 78 (15.9%) | 15 (23.4%) | 0.130 |
| Less risky or no alcohol consumption | 461 (83.2%) | 412 (84.1%) | 49 (76.6%) | ||
| Site of index CRC | Colon | 360 (65.0%) | 310 (63.3%) | 50 (78.1%) | 0.019 |
| Rectum | 194 (35.0%) | 180 (36.7%) | 14 (21.9% | ||
| Tumour differentiation | Moderately to well differentiated | 459 (82.9%) | 407 (83.1%) | 52 (81.2%) | 0.718 |
| Poorly differentiated | 95 (17.1%) | 83 (16.9%) | 12 (18.8%) | ||
| Stage of CRC at diagnosis | Stage I and II | 264 (47.7%) | 219 (44.7%) | 45 (70.3%) | <0.001 |
| Stage III and IV | 290 (52.3%) | 271 (55.3%) | 19 (29.7%) | ||
| MMR protein expression by IHC | Loss | 49 (8.8%) | 40 (8.2%) | 9 (14.1%) | 0.118 |
| Normal | 505 (91.2%) | 450 (91.8%) | 55 (85.9%) | ||
| Surgery for index CRC | Yes | 381 (68.6%) | 327 (66.7%) | 54 (84.5%) | 0.004 |
| No | 173 (31.4%) | 163 (33.3%) | 10 (15.6%) | ||
| Chemotherapy for index CRC | Yes | 324 (58.5%) | 301(61.4%) | 23 (35.9%) | <0.001 |
| No | 230 (41.5%) | 189 (38.6%) | 41 (64.1%) | ||
| Radiotherapy for index CRC | Yes | 143 (25.8%) | 135 (27.6%) | 8 (12.5%) | 0.01 |
| No | 411 (74.2%) | 355 (72.4%) | 56 (87.5%) | ||
| BMI | <25 kg/m2 | 152 (27.4%) | 135 (27.6%) | 17 (26.6%) | 0.868 |
| ≥25 kg/m2 | 402 (72.6%) | 355 (72.4%) | 47 (73.4%) | ||
| Hypertension | Yes | 259 (46.8%) | 220 (44.9%) | 39 (60.4%) | 0.016 |
| No | 295 (53.2%) | 270 (55.1%) | 25 (39.1%) | ||
| Cardiac diseases | Yes | 136 (24.5%) | 113 (23.0%) | 23 (35.9%) | 0.024 |
| No | 418 (75.5%) | 377 (76.9%) | 41 (64.1%) | ||
| Chronic renal disease | Yes | 29 (5.2%) | 27 (5.5%) | 2 (3.1%) | 0.561 # |
| No | 525 (94.8%) | 463 (94.5%) | 62 (96.9%) | ||
| Diabetes mellitus | Yes | 102 (18.4%) | 87 (17.8%) | 15 (23.4%) | 0.270 |
| No | 452 (81.6%) | 403 (82.2%) | 49 (76.6%) | ||
| Chronic respiratory disease | Yes | 127 (22.9%) | 109 (22.2%) | 18 (28.1%) | 0.293 |
| No | 427 (77.1%) | 381 (77.8%) | 46 (71.9%) |
| Variables | Categories | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| SHR (95% CI) | p-Value | SHR (95% CI) | p-Value | ||
| Age at index CRC diagnosis, years | <65 | 1.0 | 1.0 | ||
| ≥65 | 3.61 (2.00, 6.49) | <0.001 | 3.04 (1.56, 5.95) | 0.001 | |
| Sex | Female | 1.0 | 1.0 | ||
| Male | 1.44 (0.85, 2.45) | 0.181 | 1.75 (1.01, 3.01) | 0.045 | |
| Site of index CRC | Rectum | 1.0 | 1.0 | ||
| Colon | 1.82 (1.01, 3.) | 0.047 | 0.90 (0.35, 2.37) | 0.837 | |
| Stage of index CRC at diagnosis | Stage III and IV | 1.0 | 1.0 | ||
| Stage I and II | 2.34 (1.37, 3.99) | 0.002 | 2.20 (1.20, 4.03) | 0.011 | |
| Expression of MMR protein(s) | Normal | 1.0 | 1.0 | ||
| Loss | 2.47 (1.23, 4.96) | 0.011 | 2.08 (1.04, 4.13) | 0.037 | |
| Surgery for index CRC | No | 1.0 | 1.0 | ||
| Yes | 2.03 (1.05, 3.93) | 0.036 | 1.01 (0.33, 3.10) | 0.989 | |
| Chemotherapy for index CRC | Yes | 1.0 | 1.0 | ||
| No | 2.07 (1.24, 3.45) | 0.005 | 0.83 (0.44, 1.56) | 0.559 | |
| Radiotherapy for index CRC | Yes | 1.0 | 1.0 | ||
| No | 2.52 (1.20, 5.30) | 0.014 | 1.98 (0.49, 8.06) | 0.340 | |
| Hypertension | Yes | 1.0 | 1.0 | ||
| No | 1.75 (1.06, 2.89) | 0.028 | 1.08 (0.61, 1.91) | 0.797 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melku, M.; Best, O.G.; Winter, J.M.; Thurgood, L.A.; Ahmed, M.; Kichenadasse, G.; Wassie, M.M.; Symonds, E.L. Risk Factors of Multiple Primary Cancers Among Colorectal Cancer Survivors. Cancers 2025, 17, 2145. https://doi.org/10.3390/cancers17132145
Melku M, Best OG, Winter JM, Thurgood LA, Ahmed M, Kichenadasse G, Wassie MM, Symonds EL. Risk Factors of Multiple Primary Cancers Among Colorectal Cancer Survivors. Cancers. 2025; 17(13):2145. https://doi.org/10.3390/cancers17132145
Chicago/Turabian StyleMelku, Mulugeta, Oliver G. Best, Jean M. Winter, Lauren A. Thurgood, Muktar Ahmed, Ganessan Kichenadasse, Molla M. Wassie, and Erin L. Symonds. 2025. "Risk Factors of Multiple Primary Cancers Among Colorectal Cancer Survivors" Cancers 17, no. 13: 2145. https://doi.org/10.3390/cancers17132145
APA StyleMelku, M., Best, O. G., Winter, J. M., Thurgood, L. A., Ahmed, M., Kichenadasse, G., Wassie, M. M., & Symonds, E. L. (2025). Risk Factors of Multiple Primary Cancers Among Colorectal Cancer Survivors. Cancers, 17(13), 2145. https://doi.org/10.3390/cancers17132145






