A Multicenter Machine Learning-Based Predictive Model of Acute Toxicity in Prostate Cancer Patients Undergoing Salvage Radiotherapy (ICAROS Study)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Endpoints
2.2. Inclusion and Exclusion Criteria
2.3. Evaluated Parameters
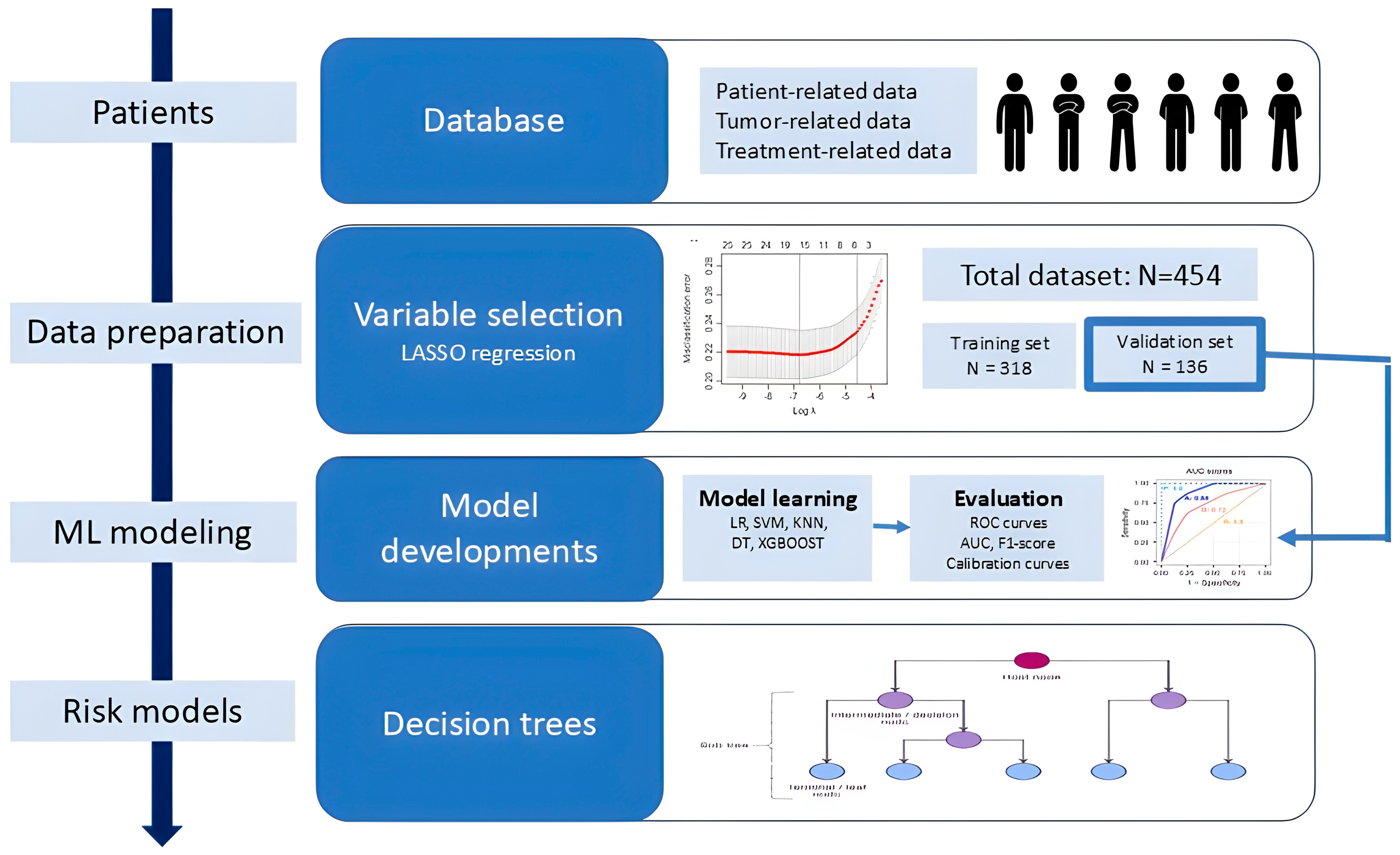
2.4. Flowchart and Data Preparation
2.5. Machine Learning Modeling and Statistical Analysis
2.6. Ethical Issues
3. Results
3.1. Patients’ Characteristics
3.2. Acute Toxicity
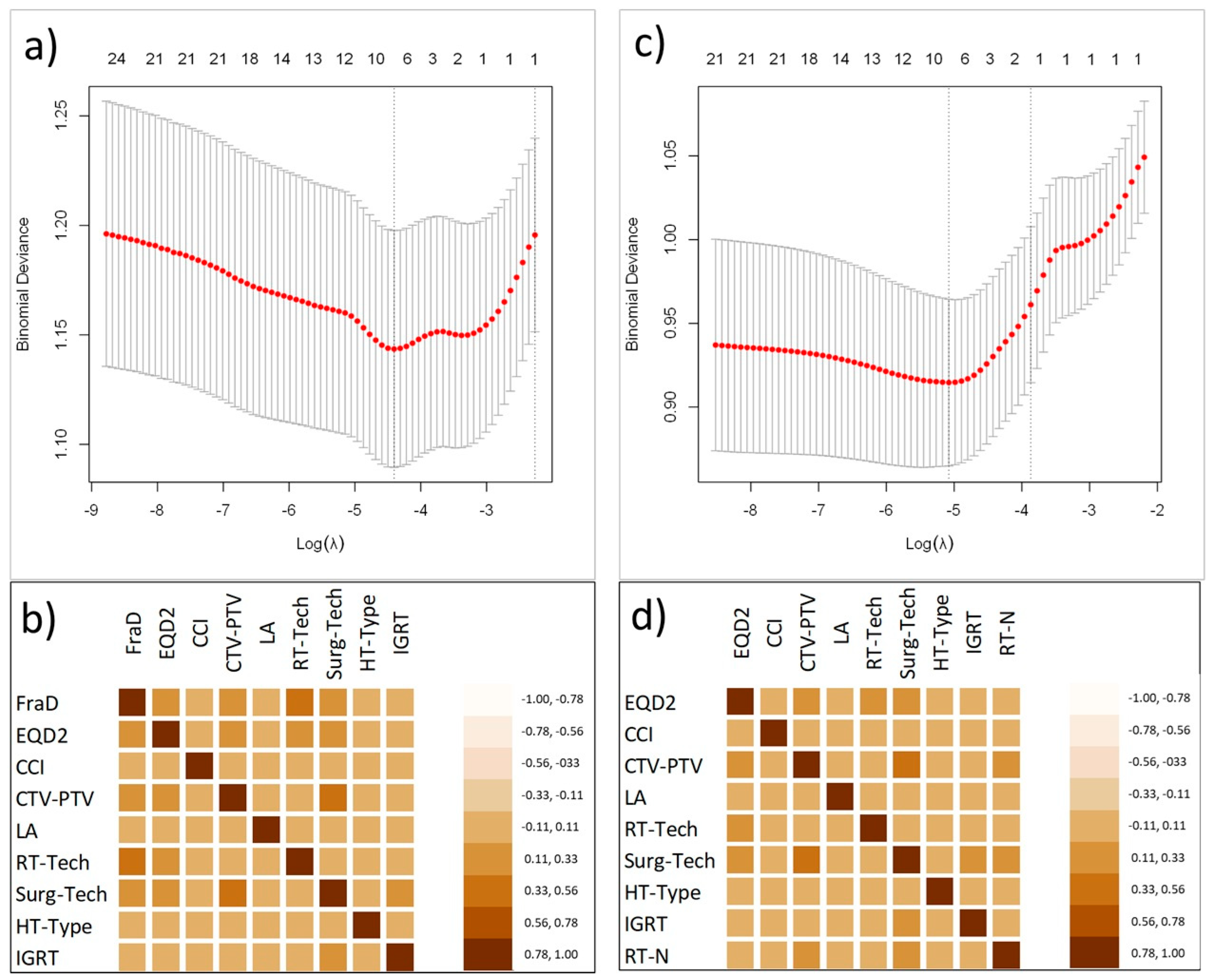
3.3. Variables Selection
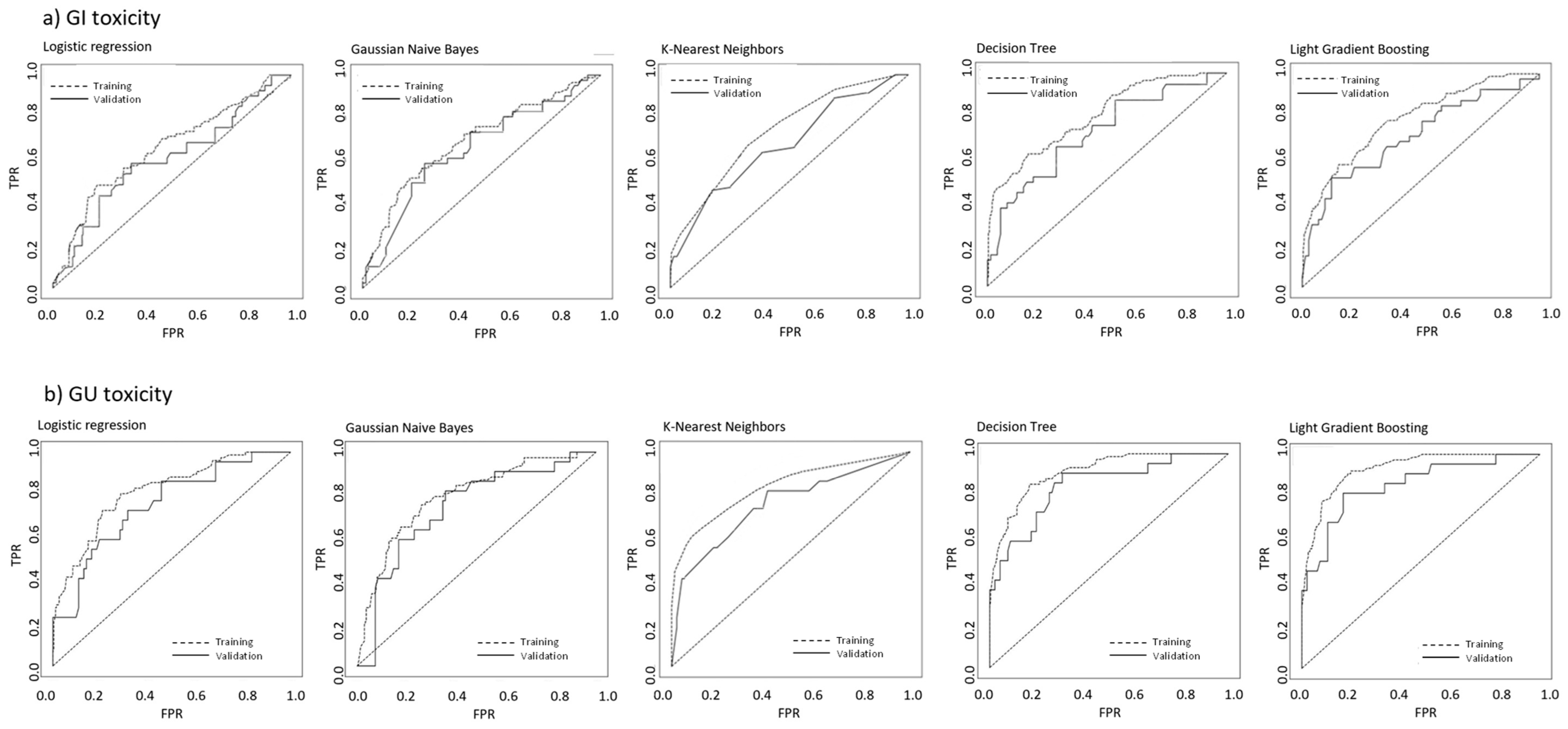
3.4. Machine Learning Models
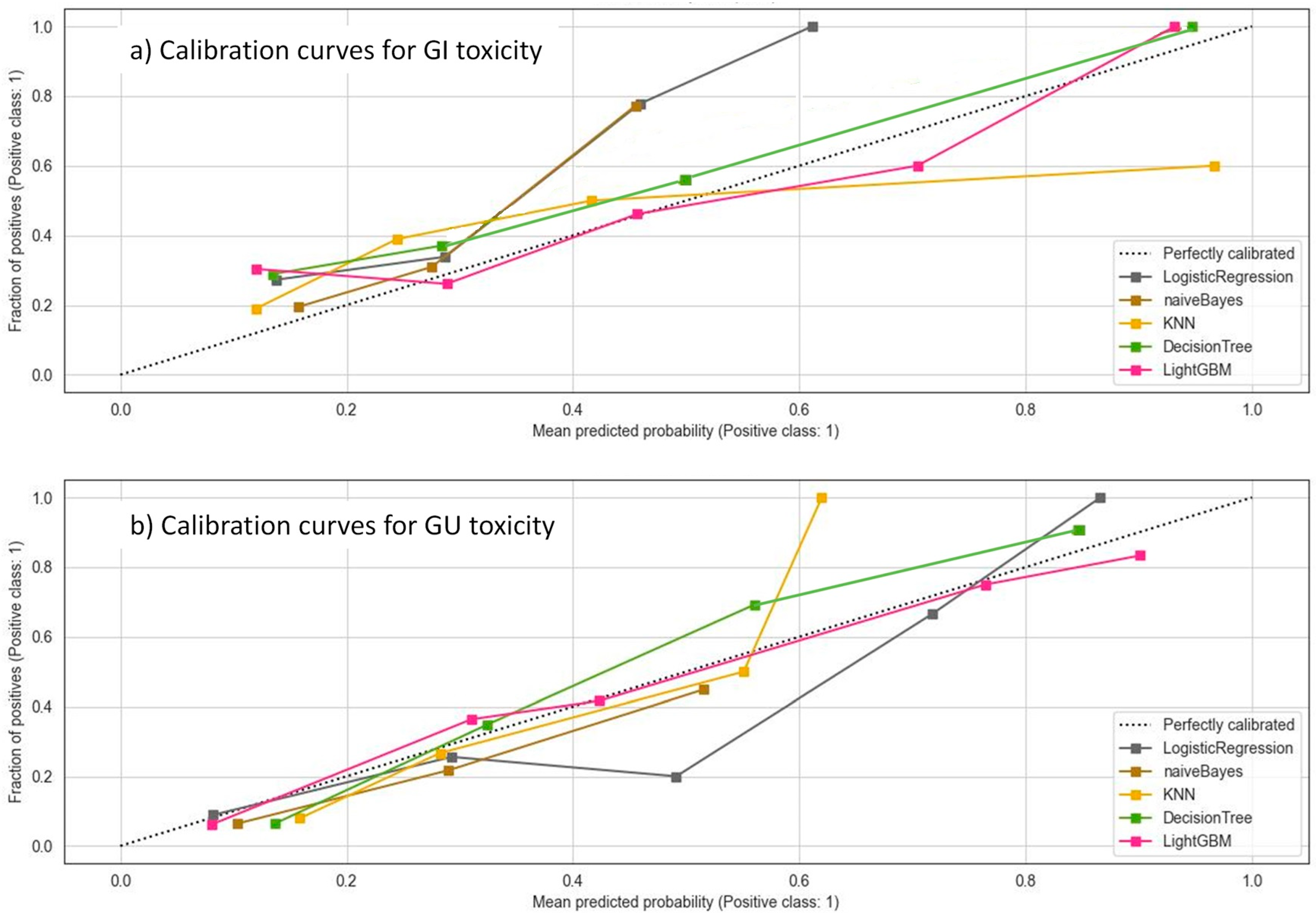
3.5. Predictive Model of Gastrointestinal Toxicity
3.6. Predictive Model of Genitourinary Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Kotb, A.F.; Elabbady, A.A. Prognostic factors for the development of biochemical recurrence after radical prostatectomy. Prostate Cancer 2011, 2011, 485189. [Google Scholar] [CrossRef] [PubMed]
- González-San Segundo, C.; Gómez-Iturriaga, A.; Couñago, F. Are all prostate cancer patients “fit” for salvage radiotherapy? World J. Clin. Oncol. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heemsbergen, W.D.; Peeters, S.T.; Koper, P.C.; Hoogeman, M.S.; Lebesque, J.V. Acute and late gastrointestinal toxicity after radiotherapy in prostate cancer patients: Consequential late damage. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 3–10. [Google Scholar] [CrossRef]
- Ost, P.; De Gersem, W.; De Potter, B.; Fonteyne, V.; De Neve, W.; De Meerleer, G. A comparison of the acute toxicity profile between two-dimensional and three-dimensional image-guided radiotherapy for postoperative prostate cancer. Clin. Oncol. (R Coll. Radiol.) 2011, 23, 344–349. [Google Scholar] [CrossRef]
- Ghadjar, P.; Hayoz, S.; Bernhard, J.; Zwahlen, D.R.; Hölscher, T.; Gut, P.; Guckenberger, M.; Hildebrandt, G.; Müller, A.C.; Plasswilm, L.; et al. Acute Toxicity and Quality of Life After Dose-Intensified Salvage Radiation Therapy for Biochemically Recurrent Prostate Cancer After Prostatectomy: First Results of the Randomized Trial SAKK 09/10. J. Clin. Oncol. 2015, 33, 4158–4166. [Google Scholar] [CrossRef]
- Goenka, A.; Magsanoc, J.M.; Pei, X.; Schechter, M.; Kollmeier, M.; Cox, B.; Scardino, P.T.; Eastham, J.A.; Zelefsky, M.J. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur. Urol. 2011, 60, 1142–1148. [Google Scholar] [CrossRef]
- Waldstein, C.; Dörr, W.; Pötter, R.; Widder, J.; Goldner, G. Postoperative radiotherapy for prostate cancer: Morbidity of local-only or local-plus-pelvic radiotherapy. Strahlenther. Onkol. 2018, 194, 23–30. [Google Scholar] [CrossRef]
- Flores-Balcázar, C.H.; Urías-Arce, D.M.; Bourlon, M.T.; Gabilondo-Navarro, F.; Pérez-Álvarez, S.I.; Ramos-Prudencio, R.; Rodríguez-Covarrubias, F. Transitioning from conformal radiotherapy to intensity-modulated radiotherapy after radical prostatectomy: Clinical benefit, oncologic outcomes and incidence of gastrointestinal and urinary toxicities. Rep. Pract. Oncol. Radiother. 2020, 25, 568–573. [Google Scholar] [CrossRef]
- Buwenge, M.; Macchia, G.; Cavallini, L.; Cortesi, A.; Malizia, C.; Bianchi, L.; Ntreta, M.; Arcelli, A.; Capocaccia, I.; Natoli, E. Unraveling the safety of adjuvant radiotherapy in prostate cancer: Impact of older age and hypofractionated regimens on acute and late toxicity—A multicenter comprehensive analysis. Front. Oncol. 2023, 13, 1281432. [Google Scholar] [CrossRef] [PubMed]
- Hasterok, M.; Szołtysik, M.; Nowicka, Z.; Goc, B.; Gräupner, D.; Majewski, W.; Rasławski, K.; Rajwa, P.; Jabłońska, I.; Magrowski, Ł.; et al. Rectum and Bladder Toxicity in Postoperative Prostate Bed Irradiation: Dose-Volume Parameters Analysis. Cancers 2023, 15, 5334. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Fiorino, C.; Cozzarini, C.; Broggi, S.; Perna, L.; Cattaneo, G.M.; Calandrino, R.; Di Muzio, N. IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with post-operative adjuvant or salvage radiotherapy after radical prostatectomy. Radiother. Oncol. 2009, 93, 207–212. [Google Scholar] [CrossRef]
- Deville, C.; Vapiwala, N.; Hwang, W.T.; Broggi, S.; Perna, L.; Cattaneo, G.M.; Calandrino, R.; Di Muzio, N. Comparative toxicity and dosimetric profile of whole-pelvis versus prostate bed-only intensity-modulated radiation therapy after prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arribas, C.M.; González-San Segundo, C.; Cuesta-Álvaro, P.; Calvo-Manuel, F.A. Predictors of urinary and rectal toxicity after external conformed radiation therapy in prostate cancer: Correlation between clinical, tumour and dosimetric parameters and radical and postoperative radiation therapy. Actas Urológicas Españolas (Engl. Ed.) 2017, 41, 615–623. (In Spanish) [Google Scholar] [CrossRef]
- Fiorino, C.; Alongi, F.; Perna, L.; Broggi, S.; Cattaneo, G.M.; Cozzarini, C.; Di Muzio, N.; Fazio, F.; Calandrino, R. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 29–35. [Google Scholar] [CrossRef]
- Deodato, F.; Cilla, S.; Massaccesi, M.; Macchia, G.; Ippolito, E.; Caravatta, L.; Picardi, V.; Romanella, M.; Di Falco, C.; Bartollino, A.; et al. Daily on-line set-up correction in 3D-conformal radiotherapy: Is it feasible? Tumori J. 2012, 98, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Seikkula, H.; Janssen, P.; Tutolo, M.; Tosco, L.; Battaglia, A.; Moris, L.; Van den Broeck, T.; Albersen, M.; De Meerleer, G.; Van Poppel, H.; et al. Comparison of Functional Outcome after Extended versus Super-Extended Pelvic Lymph Node Dissection during Radical Prostatectomy in High-Risk Localized Prostate Cancer. Front. Oncol. 2017, 7, 280. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidance on Ethics and Governance of Artificial Intelligence for Health; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240029200 (accessed on 24 February 2025).
- Collins, G.S.; Moons, K.G.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; Van Smeden, M.; et al. TRIPOD+AI statement: Updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Borghesi, M.; Casablanca, C.; Chessa, F.; Mineo Bianchi, F.; Pultrone, C.; Vagnoni, V.; Ercolino, A.; Dababneh, H.; et al. Patterns of positive surgical margins after open radical prostatectomy and their association with clinical recurrence. Minerva Urol. Nefrol. 2020, 72, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Gandaglia, G.; Fossati, N.; Larcher, A.; Pultrone, C.; Turri, F.; Selli, C.; de Groote, R.; de Naeyer, G.; Borghesi, M.; et al. Oncologic outcomes in prostate cancer patients treated with robot-assisted radical prostatectomy: Results from a single institution series with more than 10 years follow up. Minerva Urol. Nefrol. 2019, 71, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Maas, M.; Nassiri, N.; Ortega, D.; Gill, K.; Dell’Oglio, P.; Thalmann, G.N.; Heidenreich, A.; Eastham, J.A.; Evans, C.P.; et al. Impact of Pelvic Lymph Node Dissection and Its Extent on Perioperative Morbidity in Patients Undergoing Radical Prostatectomy for Prostate Cancer: A Comprehensive Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2021, 4, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, K.; Wang, Y.; Wu, Y.; Bao, E.; Wang, J.; Tan, C.; Tang, T. Robot-assisted versus open radical prostatectomy: A systematic review and meta-analysis of prospective studies. J. Robot. Surg. 2023, 17, 2617–2631. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Olszewski, D.; He, C.; Pintea, G.; Lian, J.; Chou, T.; Chen, R.C.; Shtylla, B. Machine learning and statistical prediction of patient quality-of-life after prostate radiation therapy. Comput. Biol. Med. 2021, 129, 104127. [Google Scholar] [CrossRef]
- Pella, A.; Cambria, R.; Riboldi, M.; Jereczek-Fossa, B.A.; Fodor, C.; Zerini, D.; Torshabi, A.E.; Cattani, F.; Garibaldi, C.; Pedroli, G.; et al. Use of machine learning methods for prediction of acute toxicity in organs at risk following prostate radiotherapy. Med. Phys. 2011, 38, 2859–2867. [Google Scholar] [CrossRef]
- Ospina, J.D.; Zhu, J.; Chira, C.; Bossi, A.; Delobel, J.B.; Beckendorf, V.; Dubray, B.; Lagrange, J.L.; Correa, J.C.; Simon, A.; et al. Random forests to predict rectal toxicity following prostate cancer radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1024–1031. [Google Scholar] [CrossRef]
- Carrara, M.; Massari, E.; Cicchetti, A.; Giandini, T.; Avuzzi, B.; Palorini, F.; Stucchi, C.; Fellin, G.; Gabriele, P.; Vavassori, V.; et al. Development of a ready-to-use graphical tool based on artificial neural network classification: Application for the prediction of late fecal incontinence after prostate cancer radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1533–1542. [Google Scholar] [CrossRef]
- Ozkan, E.E.; Serel, T.A.; Soyupek, A.S.; Kaymak, Z.A. Utilization of machine learning methods for prediction of acute and late rectal toxicity due to curative prostate radiotherapy. Radiat. Prot. Dosim. 2024, 200, 1244–1250. [Google Scholar] [CrossRef]
- Jones, S.; Hargrave, C.; Deegan, T.; Holt, T.; Mengersen, K. Comparison of statistical machine learning models for rectal protocol compliance in prostate external beam radiation therapy. Med. Phys. 2020, 47, 1452–1459. [Google Scholar] [CrossRef]
- Lee, S.; Kerns, S.; Ostrer, H.; Rosenstein, B.; Deasy, J.O.; Oh, J.H. Machine learning on a genome-wide association study to predict late genitourinary toxicity after prostate radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Noble, D.J.; Shelley, L.; Berger, T.; Jena, R.; McLaren, D.B.; Burnet, N.G.; Nailon, W.H. Machine-learning with region-level radiomic and dosimetric features for predicting radiotherapy-induced rectal toxicities in prostate cancer patients. Radiother. Oncol. 2023, 183, 109593. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, M.A.; Kneebone, A.B.; Lehman, M.; Wiltshire, K.L.; Millar, J.L.; Mukherjee, R.K.; Shakespeare, T.P.; Tai, K.H. Post-prostatectomy radiation therapy: Consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother. Oncol. 2008, 88, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, B.S.; Yu, N.Y.; Lo, S.; Duan, J.; Welchel, Z.; Tinnon, K.; Beckett, M.; Schild, S.E.; Wong, W.W.; Keole, S.R.; et al. Clinical Practice Evolvement for Post-Operative Prostate Cancer Radiotherapy-Part 2: Feasibility of Margin Reduction for Fractionated Radiation Treatment with Advanced Image Guidance. Cancers 2022, 15, 40. [Google Scholar] [CrossRef]
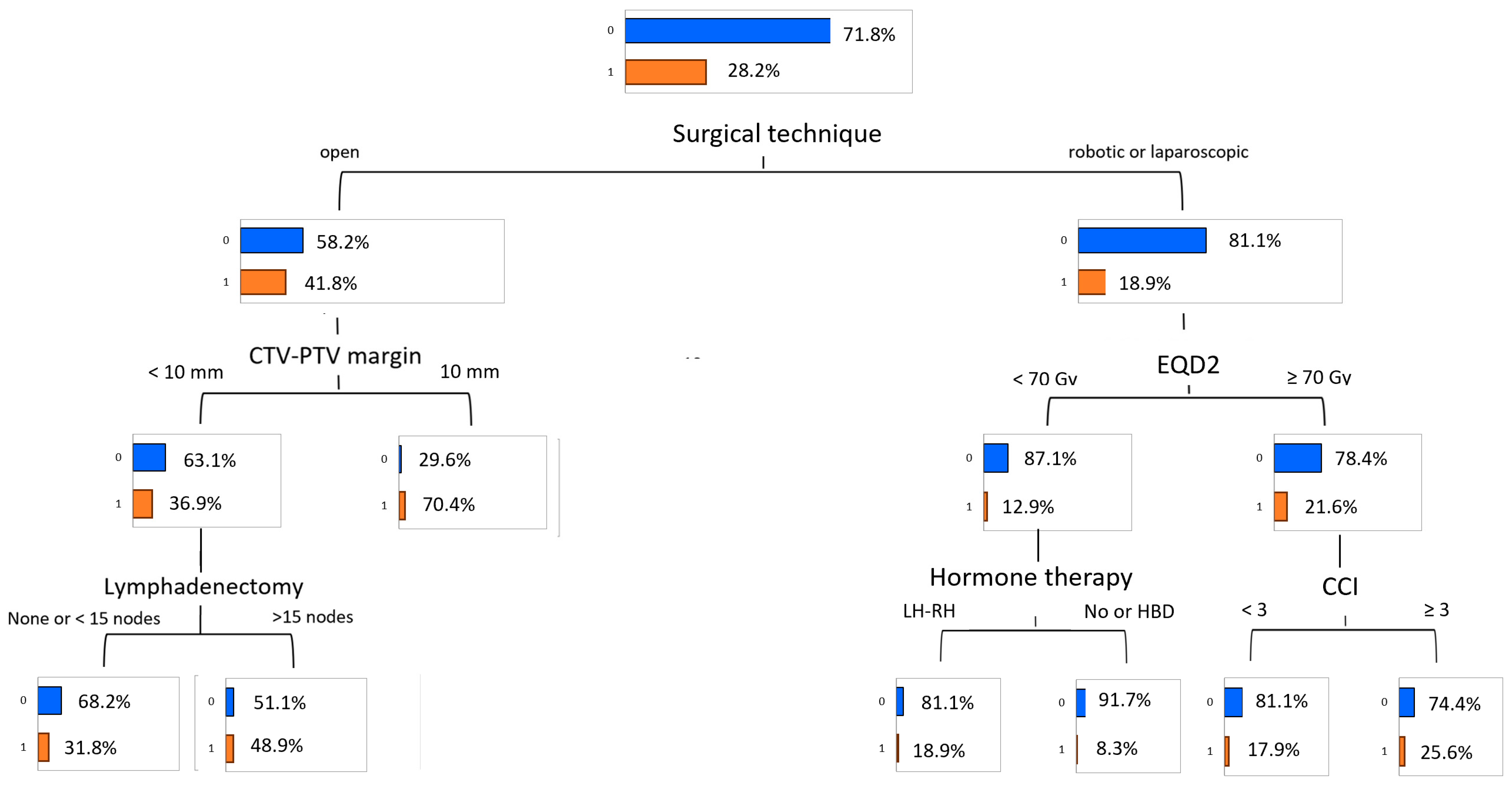
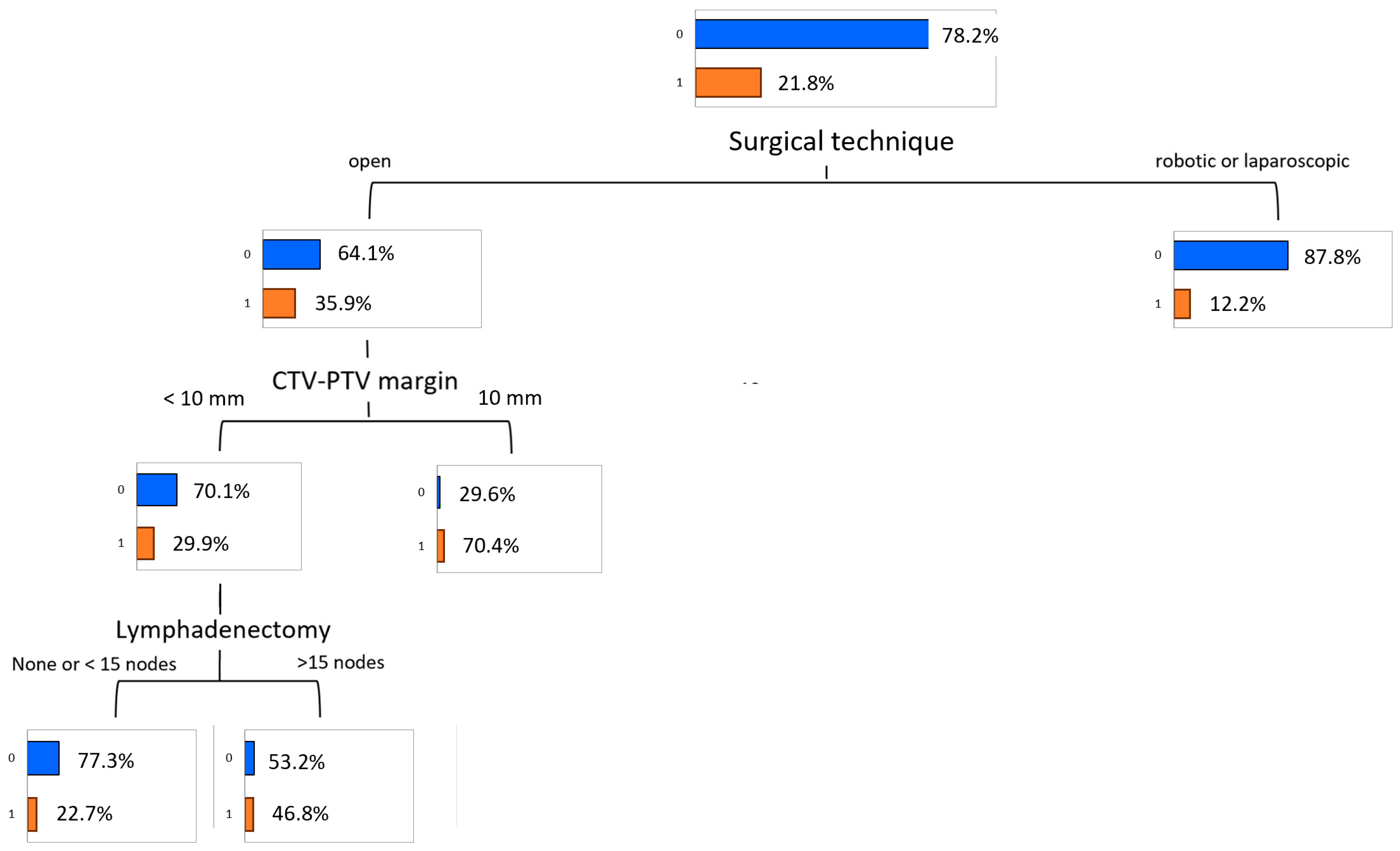
| Variable | Value | Total (N, %) | Training (N, %) | Validation (N, %) | p-Value |
|---|---|---|---|---|---|
| GI toxicity | <2 | 326 (71.8) | 228 (71.7) | 98 (72.1) | 0.812 |
| ≥2 | 128 (28.2) | 90 (28.3) | 38 (27.9) | ||
| GU toxicity | <2 | 355 (78.2) | 250 (78.6) | 105 (77.2) | 0.752 |
| ≥2 | 99 (21.8) | 68 (21.4) | 31 (22.8) | ||
| Age-adjusted Charlson Comorbidity Index | <3 | 198 (43.6) | 138 (43.4) | 60 (44.1) | 0.802 |
| ≥3 | 256 (56.4) | 180 (56.6) | 76 (55.9) | ||
| ISUP Grade | 1 | 77 (17.0) | 57 (17.9) | 20 (14.7) | 0.114 |
| 2 | 83 (18.3) | 55 (17.3) | 28 (20.6) | ||
| 3 | 117 (25.8) | 75 (23.6) | 42 (30.9) | ||
| 4 | 86 (18.9) | 68 (21.4) | 18 (13.2) | ||
| 5 | 91 (20.0) | 61 (19.2) | 30 (22.1) | ||
| pT stage | 1 | 4 (0.9) | 3 (0.9) | 1 (0.7) | 0.414 |
| 2 | 183 (40.3) | 124 (39.0) | 59 (43.4) | ||
| 3 | 261 (57.5) | 187 (58.8) | 74 (54.4) | ||
| 4 | 6 (1.3) | 4 (1.3) | 2 (1.5) | ||
| pN stage | 0 | 392 (86.3) | 272 (85.6) | 120 (88.2) | 0.888 |
| 1 | 62 (13.7) | 46 (14.4) | 16 (11.8) | ||
| EAU risk category | Very low/low | 11 (2.4) | 9 (2.8) | 2 (1.5) | 0.228 |
| Intermediate | 38 (8.4) | 24 (7.5) | 14 (10.3) | ||
| High | 263 (57.9) | 195 (61.4) | 68 (50.0) | ||
| Locally advanced | 142 (31.3) | 90 (28.3) | 52 (38.2) | ||
| Surgical technique | Open | 184 (40.5) | 135 (42.5) | 49 (36.0) | 0.175 |
| Laparoscopic | 216 (47.6) | 155 (48.7) | 61 (44.9) | ||
| Robotic | 54 (11.9) | 28 (8.8) | 26 (19.1) | ||
| Nodal irradiation | No | 184 (40.5) | 138 (43.4) | 46 (33.8) | 0.151 |
| Yes | 270 (59.5) | 180 (56.6) | 90 (66.2) | ||
| Lymphadenectomy | No | 178 (39.2) | 112 (35.2) | 66 (48.5) | 0.369 |
| <15 nodes | 137 (30.2) | 104 (32.7) | 33 (24.3) | ||
| ≥15 nodes | 139 (30.6) | 102 (32.1) | 37 (27.2) | ||
| Macroscopic recurrence in the prostate bed | No | 371 (81.7) | 268 (84.3) | 103 (75.7) | 0.378 |
| Yes | 83 (18.3) | 50 (15.7) | 33 (24.3) | ||
| Previous abdominal–pelvic surgery | No | 417 (91.9) | 299 (94.0) | 118 (86.8) | 0.275 |
| Yes | 37 (8.1) | 19 (6.0) | 18 (13.2) | ||
| Androgen-deprivation therapy | No | 163 (35.9) | 117 (36.8) | 46 (33.8) | 0.775 |
| Yes | 291 (64.1) | 201 (63.2) | 90 (66.2) | ||
| Type of androgen-deprivation therapy | Not prescribed | 163 (35.9) | 125 (39.3) | 38 (27.9) | 0.188 |
| LH-RH analog | 215 (47.4) | 150 (47.2) | 65 (47.8) | ||
| High-dose Bicalutamide | 76 (16.7) | 43 (13.5) | 33 (24.3) | ||
| Radiotherapy technique | 3D-CRT | 119 (26.2) | 74 (23.3) | 45 (33.1) | 0.942 |
| IMRT/VMAT | 335 (73.8) | 244 (76.7) | 91 (66.9) | ||
| Image guidance | EPID | 367 (80.8) | 251 (78.9) | 116 (85.3) | 0.782 |
| CBCT | 87 (19.2) | 67 (21.1) | 20 (14.7) | ||
| CTV-to-PTV margin (mm) | Median (range) | 10 (6–10) | 10 (6–10) | 10 (6–10) | 0.512 |
| EQD2 to the prostatic fossa α/β10 (Gy) | Median (range) | 69.0 (60.0–80.0) | 69.0 (60.0–80.0) | 68.3 (65.1–80.0) | 0.612 |
| EQD2 to the lymph nodes α/β10 (Gy) | Median (range) | 44.3 (44.3–53.1) | 44.3 (44.3–53.1) | 44.3 (44.3–53.1) | 0.950 |
| Total dose to the prostatic fossa (Gy) | <70 Gy | 264 (58.1) | 190 (59.7) | 74 (54.4) | 0.973 |
| ≥70 Gy | 190 (41.9) | 128 (40.3) | 62 (45.6) | ||
| Dose per fraction to the prostatic fossa (Gy) | ≤2.0 Gy | 168 (37.0) | 113 (35.5) | 55 (40.4) | 0.368 |
| 2.1–2.4 Gy | 122 (26.9) | 90 (28.3) | 32 (23.5) | ||
| >2.4 Gy | 164 (36.1) | 115 (36.2) | 49 (36.0) |
| Assessment Metrics (95% CI) | ||||
|---|---|---|---|---|
| ML Model | ROC AUC | Accuracy | F1-Score | Brier Score |
| GI toxicity | ||||
| Logistic regression | 0.632 (0.578–0.686) | 0.638 (0.597–0.679) | 0.712 (0.666–0.758) | 0.194 (0.180–0.208) |
| Naive Bayes | 0.624 (0.572–0.676) | 0.599 (0.563–0.635) | 0.646 (0.607–0.685) | 0.201 (0.186–0.214) |
| K-nearest neighbors | 0.649 (0.597–0.701) | 0.633 (0.601–0.665) | 0.721 (0.685–0.757) | 0.215 (0.201–0.229) |
| Decision tree | 0.690 (0.641–0.739) | 0.668 (0.638–0.698) | 0.755 (0.721–0.789) | 0.123 (0.116–0.131) |
| LightGBM | 0.706 (0.657–0.755) | 0.703 (0.675–0.731) | 0.766 (0.735–0.797) | 0.121 (0.114–0.128) |
| GU toxicity | ||||
| Logistic regression | 0.751 (0.699–0.803) | 0.722 (0.675–0.769) | 0.788 (0.737–0.839) | 0.181 (0.169–0.193) |
| Naive Bayes | 0.738 (0.688–0.788) | 0.685 (0.644–0.726) | 0.752 (0.707–0.797) | 0.192 (0.179–0.205) |
| K-nearest neighbors | 0.769 (0.719–0.819) | 0.751 (0.713–0.789) | 0.816 (0.775–0.857) | 0.188 (0.176–0.200) |
| Decision tree | 0.848 (0.805–0.891) | 0.784 (0.749–0.819) | 0.854 (0.816–0.892) | 0.092 (0.087–0.098) |
| LightGBM | 0.855 (0.814–0.896) | 0.821 (0.780–0.862) | 0.878 (0.843–0.913) | 0.088 (0.083–0.093) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deodato, F.; Macchia, G.; Duhanxhiu, P.; Mammini, F.; Cavallini, L.; Ntreta, M.; Zamfir, A.A.; Buwenge, M.; Cellini, F.; Ciabatti, S.; et al. A Multicenter Machine Learning-Based Predictive Model of Acute Toxicity in Prostate Cancer Patients Undergoing Salvage Radiotherapy (ICAROS Study). Cancers 2025, 17, 2142. https://doi.org/10.3390/cancers17132142
Deodato F, Macchia G, Duhanxhiu P, Mammini F, Cavallini L, Ntreta M, Zamfir AA, Buwenge M, Cellini F, Ciabatti S, et al. A Multicenter Machine Learning-Based Predictive Model of Acute Toxicity in Prostate Cancer Patients Undergoing Salvage Radiotherapy (ICAROS Study). Cancers. 2025; 17(13):2142. https://doi.org/10.3390/cancers17132142
Chicago/Turabian StyleDeodato, Francesco, Gabriella Macchia, Patrick Duhanxhiu, Filippo Mammini, Letizia Cavallini, Maria Ntreta, Arina Alexandra Zamfir, Milly Buwenge, Francesco Cellini, Selena Ciabatti, and et al. 2025. "A Multicenter Machine Learning-Based Predictive Model of Acute Toxicity in Prostate Cancer Patients Undergoing Salvage Radiotherapy (ICAROS Study)" Cancers 17, no. 13: 2142. https://doi.org/10.3390/cancers17132142
APA StyleDeodato, F., Macchia, G., Duhanxhiu, P., Mammini, F., Cavallini, L., Ntreta, M., Zamfir, A. A., Buwenge, M., Cellini, F., Ciabatti, S., Bianchi, L., Schiavina, R., Brunocilla, E., D’Angelo, E., Morganti, A. G., & Cilla, S. (2025). A Multicenter Machine Learning-Based Predictive Model of Acute Toxicity in Prostate Cancer Patients Undergoing Salvage Radiotherapy (ICAROS Study). Cancers, 17(13), 2142. https://doi.org/10.3390/cancers17132142









