High Tumoral CD24 Expression and Low CD3+ Tumor-Infiltrating Lymphocytes as a Biomarker for High-Risk Locally Advanced Nasopharyngeal Carcinoma
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Immunohistochemistry
2.3. Verification of Antibodies Used in Manual Immunohistochemistry
2.4. Pathologic Scoring
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. CD24 Expression Is Significantly Associated with Survival
3.3. Expression of Vimentin Is Associated with Shorter DFS
3.4. Low CD3+ TIL Density Is Associated with Shorter Survival
3.5. CD24 Expression Is an Independent Prognostic Marker in LA-NPC
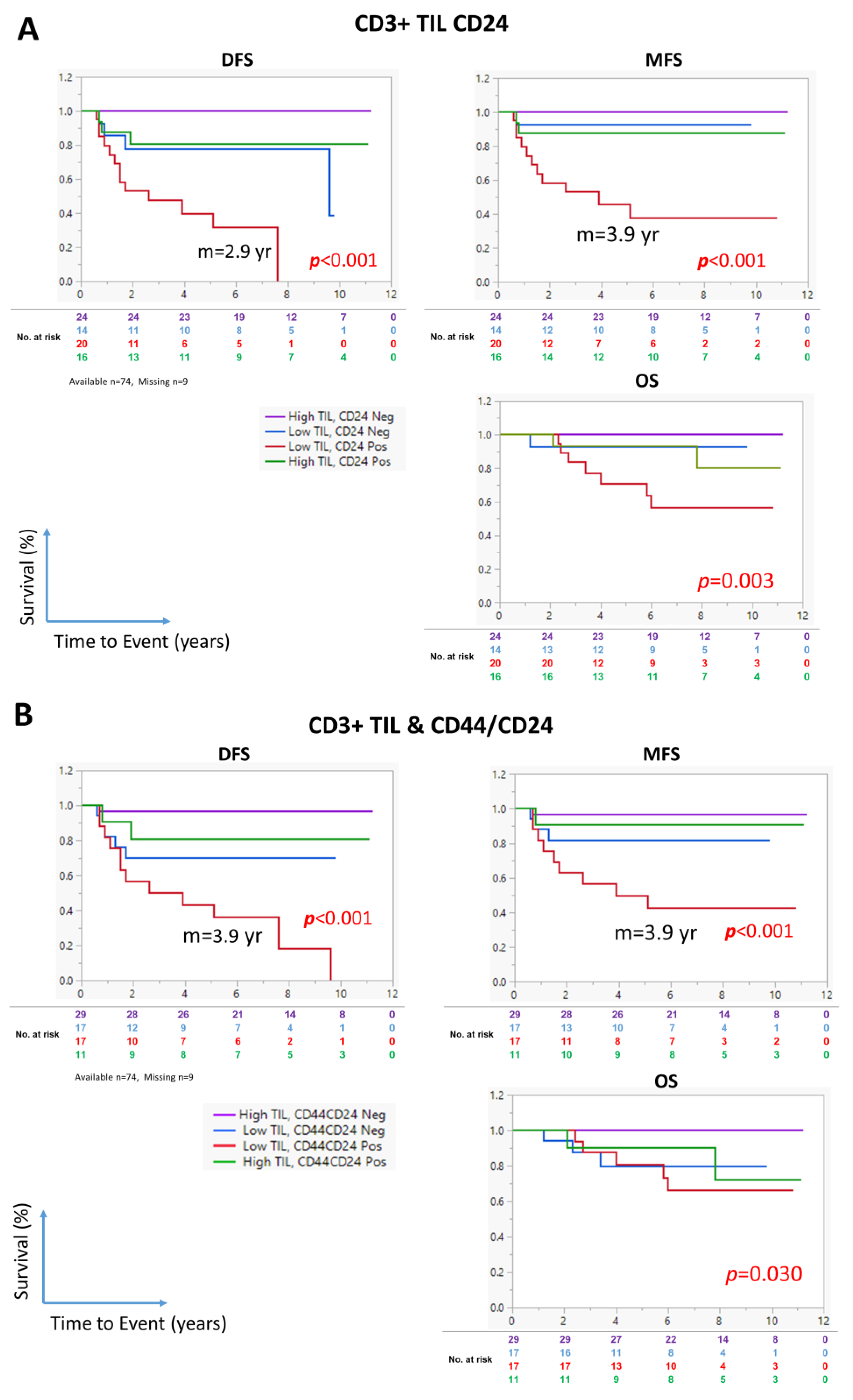
3.6. The Combination of CD24 Expression and CD3+ TIL Density Identifies High-Risk NPC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCRT | concurrent chemoradiotherapy |
| DFS | disease-free survival |
| LA | locally advanced |
| MFS | metastasis-free survival |
| NK | natural killer |
| NPC | nasopharyngeal carcinoma |
| OS | overall survival |
| TIL | tumor-infiltrating lymphocytes |
References
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, N.; Soudy, H.; Ahmed, S.A.; Elhassan, T.; Mohammed, S.F.; Khoja, H.A.; Ghebeh, H. CD3+ T-lymphocyte infiltration is an independent prognostic factor for advanced nasopharyngeal carcinoma. BMC Cancer 2020, 20, 240. [Google Scholar] [CrossRef]
- Liu, S.; Wicha, M.S. Targeting breast cancer stem cells. J. Clin. Oncol. 2010, 28, 4006–4012. [Google Scholar] [CrossRef] [PubMed]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Lei, Y.; Shen, H.F.; Li, Q.W.; Yang, S.; Xie, H.T.; Li, X.F.; Chen, M.L.; Xia, J.W.; Wang, S.C.; Dai, G.Q.; et al. Hairy gene homolog increases nasopharyngeal carcinoma cell stemness by upregulating Bmi-1. Aging 2023, 15, 4391–4410. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.R.; Yao, K.T. Cancer stem cell characteristics, ALDH1 expression in the invasive front of nasopharyngeal carcinoma. Virchows Arch. 2014, 464, 35–43. [Google Scholar] [CrossRef]
- Lun, S.W.; Cheung, S.T.; Cheung, P.F.; To, K.F.; Woo, J.K.; Choy, K.W.; Chow, C.; Cheung, C.C.; Chung, G.T.; Cheng, A.S.; et al. CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma. PLoS ONE 2012, 7, e52426. [Google Scholar] [CrossRef]
- Shen, Y.A.; Wang, C.Y.; Chuang, H.Y.; Hwang, J.J.; Chi, W.H.; Shu, C.H.; Ho, C.Y.; Li, W.Y.; Chen, Y.J. CD44 and CD24 coordinate the reprogramming of nasopharyngeal carcinoma cells towards a cancer stem cell phenotype through STAT3 activation. Oncotarget 2016, 7, 58351–58366. [Google Scholar] [CrossRef]
- Shen, Y.-A.; Wei, Y.-H.; Chen, Y.-J. Abstract 482: High CD44/CD24 expressive cells presented cancer stem cell characteristics and undergo mitochondrial resetting and metabolic shift in nasopharyngeal carcinoma. Cancer Res. 2011, 71, 482. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Dong, Z.; Vodopyanov, D.; Imai, A.; Helman, J.I.; Prince, M.E.; Wicha, M.S.; Nor, J.E. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010, 70, 9969–9978. [Google Scholar] [CrossRef]
- Al-Rajhi, N.M.; Khalil, E.M.; Ahmad, S.; Soudy, H.; AlGhazi, M.; Fatani, D.M.; Memon, M.; Abouzied, M.; Khafaga, Y.M. Low-dose fractionated radiation with induction docetaxel and cisplatin followed by concurrent cisplatin and radiation therapy in locally advanced nasopharyngeal cancer: A randomized phase II-III trial. Hematol. Oncol. Stem Cell Ther. 2020, 14, 199–205. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Xu, X.; Qian, H.; Xu, W. Anti-proliferation effect of BMI-1 in U937 cells with siRNA. Int. J. Mol. Med. 2010, 25, 889–895. [Google Scholar] [PubMed]
- Yuan, W.; Yuan, Y.; Zhang, T.; Wu, S. Role of Bmi-1 in regulation of ionizing irradiation-induced epithelial-mesenchymal transition and migration of breast cancer cells. PLoS ONE 2015, 10, e0118799. [Google Scholar] [CrossRef]
- Park, J.W.; Jung, K.H.; Lee, J.H.; Moon, S.H.; Cho, Y.S.; Lee, K.H. Inhibition of aldehyde dehydrogenase 1 enhances the cytotoxic effect of retinaldehyde on A549 cancer cells. Oncotarget 2017, 8, 99382–99393. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Huang, S.; An, G.; Wang, G.; Gu, S.; Zhao, X. Identification of new cancer stem cell markers and signaling pathways in HER-2-positive breast cancer by transcriptome sequencing. Int. J. Oncol. 2019, 55, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Ma, H.L.; Zhang, J.; Zhu, L.; Wang, C.; Yang, Y.L. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 2017, 7, 13856. [Google Scholar] [CrossRef]
- Al-Rajhi, N.; Mohammed, S.F.; Khoja, H.A.; Al-Dehaim, M.; Ghebeh, H. Prognostic markers compared to CD3+ TIL in locally advanced nasopharyngeal carcinoma. Medicine 2021, 100, e27956. [Google Scholar] [CrossRef]
- Wu, A.; Luo, W.; Zhang, Q.; Yang, Z.; Zhang, G.; Li, S.; Yao, K. Aldehyde dehydrogenase 1, a functional marker for identifying cancer stem cells in human nasopharyngeal carcinoma. Cancer Lett. 2013, 330, 181–189. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Su, J.; Xu, X.H.; Huang, Q.A.; Lu, M.Q.; Li, D.J.; Xue, F.; Yi, F.; Ren, J.H.; Wu, Y.P. Identification of Cancer Stem-like CD44 Cells in Human Nasopharyngeal Carcinoma Cell Line. Arch. Med. Res. 2011, 42, 15–21. [Google Scholar] [CrossRef]
- Hoe, S.L.L.; Tan, L.P.; Abdul Aziz, N.; Liew, K.; Teow, S.Y.; Abdul Razak, F.R.; Chin, Y.M.; Mohamed Shahrehan, N.A.; Chu, T.L.; Mohd Kornain, N.K.; et al. CD24, CD44 and EpCAM enrich for tumour-initiating cells in a newly established patient-derived xenograft of nasopharyngeal carcinoma. Sci. Rep. 2017, 7, 12372. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.R.; Gao, F.; Li, S.Y.; Yao, K.T. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology 2012, 61, 1072–1081. [Google Scholar] [CrossRef]
- Janisiewicz, A.M.; Shin, J.H.; Murillo-Sauca, O.; Kwok, S.; Le, Q.T.; Kong, C.; Kaplan, M.J.; Sunwoo, J.B. CD44+ cells have cancer stem cell-like properties in nasopharyngeal carcinoma. Int. Forum Allergy Rhinol. 2012, 2, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Karran, L.; Jones, M.; Morley, G.; van Noorden, S.; Smith, P.; Lampert, I.; Griffin, B.E. Expression of a B-cell marker, CD24, on nasopharyngeal carcinoma cells. Int. J. Cancer 1995, 60, 562–566. [Google Scholar] [CrossRef]
- Pandey, V.; Jung, Y.; Kang, J.; Steiner, M.; Qian, P.X.; Banerjee, A.; Mitchell, M.D.; Wu, Z.S.; Zhu, T.; Liu, D.X.; et al. Artemin Reduces Sensitivity to Doxorubicin and Paclitaxel in Endometrial Carcinoma Cells through Specific Regulation of CD24. Transl. Oncol. 2010, 3, 218–229. [Google Scholar] [CrossRef]
- Koh, J.; Lee, S.B.; Park, H.; Lee, H.J.; Cho, N.H.; Kim, J. Susceptibility of CD24 ovarian cancer cells to anti-cancer drugs and natural killer cells. Biochem. Biophys. Res. Commun. 2012, 427, 373–378. [Google Scholar] [CrossRef]
- Jia, Y.F.; Gu, D.S.; Wan, J.; Yu, B.Q.; Zhang, X.L.; Chiorean, E.G.; Wang, Y.S.; Xie, J.W. The role of GLI-SOX2 signaling axis for gemcitabine resistance in pancreatic cancer. Oncogene 2019, 38, 1764–1777. [Google Scholar] [CrossRef]
- Modur, V.; Joshi, P.; Nie, D.T.; Robbins, K.T.; Khan, A.U.; Rao, K. CD24 Expression May Play a Role as a Predictive Indicator and a Modulator of Cisplatin Treatment Response in Head and Neck Squamous Cellular Carcinoma. PLoS ONE 2016, 11, e0156651. [Google Scholar] [CrossRef] [PubMed]
- Sinnung, S.; Janvilisri, T.; Kiatwuthinon, P. Reversal of cisplatin sensitization and abrogation of cisplatin-enriched cancer stem cells in 5-8F nasopharyngeal carcinoma cell line through a suppression of Wnt/β-catenin-signaling pathway. Mol. Cell. Biochem. 2021, 476, 1663–1672. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Wang, Z.; Jiang, J.; Yu, S.C.; Ping, Y.F.; Yang, J.; Xu, S.L.; Ye, X.Z.; Xu, C.; et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014, 74, 5746–5757. [Google Scholar] [CrossRef]
- Hsu, J.M.; Xia, W.Y.; Hsu, Y.H.; Chan, L.C.; Yu, W.H.; Cha, J.H.; Chen, C.T.; Liao, H.W.; Kuo, C.W.; Khoo, K.H.; et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018, 9, 1908. [Google Scholar] [CrossRef] [PubMed]
- Mansour, F.A.; Al-Mazrou, A.; Al-Mohanna, F.; Al-Alwan, M.; Ghebeh, H. PD-L1 is overexpressed on breast cancer stem cells through notch3/mTOR axis. Oncoimmunology 2020, 9, 1729299. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Hubbe, M. Heat-Stable Antigen/Cd24 on Mouse T-Lymphocytes—Evidence for a Costimulatory Function. Eur. J. Immunol. 1994, 24, 731–737. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, X.M.; Tao, K.X.; Shi, L.; Li, W.; Wang, G.B.; Wu, K. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. J. Surg. Res. 2015, 194, 107–113. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]




| Relapse | DFS | Death | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | HR | 95% CI | * p | − | + | HR | 95% CI | * p | |
| BMI1 | ||||||||||
| Neg (score 1 or 2) | 22 (76) | 7 (24) | 1 | 26 (90) | 3 (10) | 1 | ||||
| Pos (score 3 or 4) | 37 (70) | 16 (30) | 1.2 | 0.5–2.9 | 0.700 | 44 (83) | 9 (17) | 1.4 | 0.4–5.3 | 0.596 |
| ◊ CD44 | ||||||||||
| Neg (<70%) | 41 (84) | 8 (16) | 1 | 44 (90) | 5 (10) | 1 | ||||
| Pos (≥70%) | 13 (52) | 12 (48) | 3.2 | 1.3–7.9 | 0.011 | 20 (80) | 5 (20) | 1.9 | 0.6–6.7 | 0.298 |
| ◊ ALDH1 | ||||||||||
| Neg (<10%) | 15 (83) | 3 (17) | 1 | 33 (87) | 5 (13) | 1 | ||||
| Pos (≥10%) | 39 (70) | 17 (30) | 2.0 | 0.6–7.0 | 0.256 | 31 (86) | 5 (14) | 1.4 | 0.3–6.7 | 0.659 |
| ◊ ALDH1/CD44 | ||||||||||
| Neg (<10%) | 30 (81) | 7 (19) | 1 | 37 (86) | 6 (14) | 1 | ||||
| Pos (≥10%) | 24 (65) | 13 (35) | 1.8 | 0.7–4.5 | 0.211 | 27 (87) | 4 (13) | 0.9 | 0.3–3.2 | 0.932 |
| ◊ CD24 | ||||||||||
| Neg (<30%) | 33 (89) | 4 (11) | 1 | 43 (93) | 3 (7) | 1 | ||||
| Pos (≥30%) | 21 (57) | 16 (43) | 5.3 | 1.8–16.0 | 0.003 | 21 (75) | 7 (25) | 10.8 | 1.4–85.5 | 0.024 |
| ◊ CD24/CD44 | ||||||||||
| Neg (<10%) | 40 (87) | 6 (13) | 1 | 43 (93) | 3 (7) | 1 | ||||
| Pos (≥10%) | 14 (50) | 14 (50) | 4.6 | 1.7–11.9 | 0.002 | 21 (75) | 7 (25) | 4.0 | 1.0–15.6 | 0.044 |
| Vimentin | ||||||||||
| Neg (score 1 or 2) | 46 (81) ♣ | 11 (19) | 1 | 51 (89) | 6 (11) | 1 | ||||
| Pos (score 3 or 4) | 14 (54) | 12 (46) | 2.6 | 1.2–6.0 | 0.021 | 20 (77) | 6 (23) | 2.4 | 0.8–7.4 | 0.134 |
| Relapse | DFS | Death | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | HR | 95% CI | * p | − | + | HR | 95% CI | * p | |
| WHO Type | ||||||||||
| III | 59 (76) | 19 (24) | 1 | 67 (86) | 11 (14) | 1 | ||||
| I and II | 1 (20) ♣ | 4 (80) | 3.2 | 0.9–11.7 | 0.080 | 4 (80) | 1 (20) | 2.1 | 0.2–21.8 | 0.541 |
| Vimentin | ||||||||||
| Negative | 46 (81) | 11 (19) | 1 | 51 (89) | 6 (11) | 1 | ||||
| Positive | 14 (54) | 12 (46) | 1.1 | 0.4–3.2 | 0.819 | 20 (77) | 6 (23) | 1.3 | 0.3–5.3 | 0.714 |
| ◊ CD44 | ||||||||||
| <70% | 41 (84) | 8 (16) | 1 | 44 (90) | 5 (10) | 1 | ||||
| ≥70% | 13 (52) | 12 (48) | 2.0 | 0.7–5.1 | 0.178 | 20 (80) | 5 (20) | 1.0 | 0.2–3.7 | 0.952 |
| ◊ CD24 | ||||||||||
| <30% | 33 (89) | 4 (11) | 1 | 36 (97) | 1 (3) | 1 | ||||
| ≥30% | 21 (57) | 16 (43) | 4.3 | 1.3–13.9 | 0.015 | 28 (76) | 9 (24) | 8.9 | 1.1–73.0 | 0.041 |
| CD3+ TIL | ||||||||||
| High | 42 (89) | 5 (11) | 1 | 44 (94) | 3 (6) | 1 | ||||
| Low | 18 (50) | 18 (50) | 7.4 | 1.9–28.6 | 0.004 | 27 (75) | 9 (25) | 4.9 | 0.9–26.9 | 0.068 |
| Relapse | DFS | Death | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | HR | 95% CI | * p | − | + | HR | 95% CI | * p | |
| ◊ CD24 | ||||||||||
| Negative | 33 (89) | 4 (11) | 1 | 36 (97) | 1 (3) | 1 | ||||
| Positive | 21 (57) | 16 (43) | 4.8 | 1.6–15.0 | 0.007 | 28 (76) | 9 (24) | 8.6 | 1.1–69.0 | 0.042 |
| CD3+ TIL | ||||||||||
| High | 42 (89) | 5 (11) | 1 | 44 (94) | 3 (6) | 1 | ||||
| Low | 18 (50) | 18 (50) | 11.1 | 2.7–35.0 | <0.001 | 27 (75) | 9 (25) | 4.6 | 1.0–22.0 | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghebeh, H.; Mirza, J.Y.; Mohammed, S.F.; Khoja, H.A.; Dababo, M.A.; Tukruni, M.E.; Anwar, M.S.; Aldehaim, M.; Al-Rajhi, N. High Tumoral CD24 Expression and Low CD3+ Tumor-Infiltrating Lymphocytes as a Biomarker for High-Risk Locally Advanced Nasopharyngeal Carcinoma. Cancers 2025, 17, 2094. https://doi.org/10.3390/cancers17132094
Ghebeh H, Mirza JY, Mohammed SF, Khoja HA, Dababo MA, Tukruni ME, Anwar MS, Aldehaim M, Al-Rajhi N. High Tumoral CD24 Expression and Low CD3+ Tumor-Infiltrating Lymphocytes as a Biomarker for High-Risk Locally Advanced Nasopharyngeal Carcinoma. Cancers. 2025; 17(13):2094. https://doi.org/10.3390/cancers17132094
Chicago/Turabian StyleGhebeh, Hazem, Jumanah Y. Mirza, Shamayel F. Mohammed, Hatim A. Khoja, Mohammad Anas Dababo, Muruj E. Tukruni, Muhammad S. Anwar, Mohammed Aldehaim, and Nasser Al-Rajhi. 2025. "High Tumoral CD24 Expression and Low CD3+ Tumor-Infiltrating Lymphocytes as a Biomarker for High-Risk Locally Advanced Nasopharyngeal Carcinoma" Cancers 17, no. 13: 2094. https://doi.org/10.3390/cancers17132094
APA StyleGhebeh, H., Mirza, J. Y., Mohammed, S. F., Khoja, H. A., Dababo, M. A., Tukruni, M. E., Anwar, M. S., Aldehaim, M., & Al-Rajhi, N. (2025). High Tumoral CD24 Expression and Low CD3+ Tumor-Infiltrating Lymphocytes as a Biomarker for High-Risk Locally Advanced Nasopharyngeal Carcinoma. Cancers, 17(13), 2094. https://doi.org/10.3390/cancers17132094





