Association of Percutaneous Endoscopic Gastrostomy (PEG) Tube Placement with Unplanned Hospitalization for Head and Neck Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
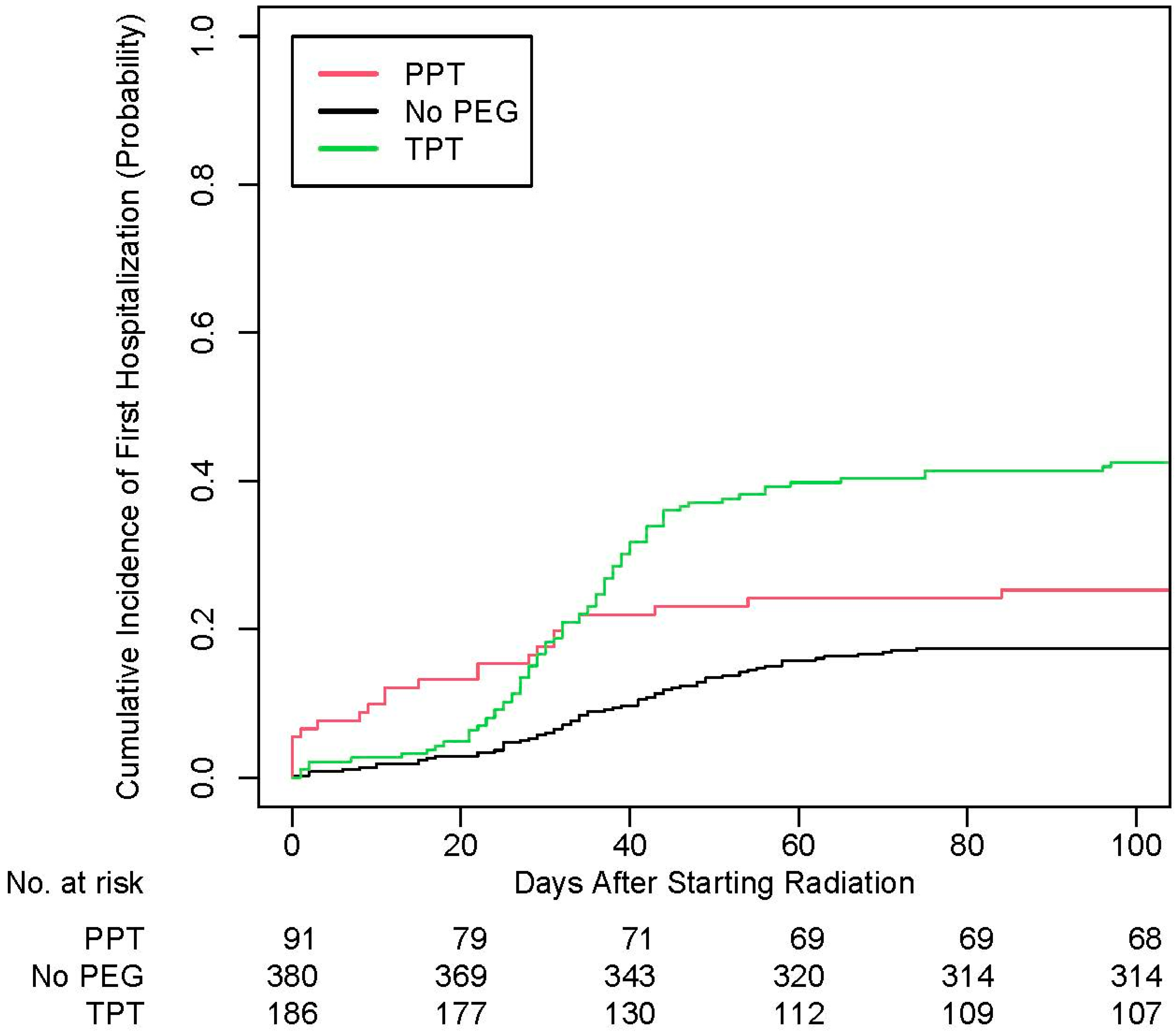
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iovoli, A.J.; Turecki, L.; Qiu, M.L.; Khan, M.; Smith, K.; Yu, H.; Singh, A.K. Severe Oral Mucositis After Intensity-Modulated Radiation Therapy for Head and Neck Cancer. JAMA Netw. Open 2023, 6, e2337265. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.D.; Yu, H.; Iovoli, A.J.; Fang, M.; Schrand, T.V.; Pepin, A.; Singh, A.K. Prophylactic gastrostomy tube during chemoradiation for head and neck cancer decreases weight loss but increases rate of tube use beyond six months. Oral Oncol. 2025, 160, 107136. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Machtay, M.; Unger, L.D.; Weinstein, G.S.; Weber, R.S.; Chalian, A.A.; Rosenthal, D.I. Prophylactic gastrostomy tubes in patients undergoing intensive irradiation for cancer of the head and neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; North, D.; Smith, H.J.; Dutta, S.; Alfieri, A.; Karlsson, U.; Sallah, S. Safety and effectiveness of prophylactic gastrostomy tubes for head and neck cancer patients undergoing chemoradiation. Surg. Oncol. 2006, 15, 199–203. [Google Scholar] [CrossRef]
- Piquet, M.A.; Ozsahin, M.; Larpin, I.; Zouhair, A.; Coti, P.; Monney, M.; Roulet, M. Early nutritional intervention in oropharyngeal cancer patients undergoing radiotherapy. Support. Care Cancer 2002, 10, 502–504. [Google Scholar] [CrossRef]
- Wiggenraad, R.G.J.; Flierman, L.; Goossens, A.; Brand, R.; Verschuur, H.P.; Croll, G.A.; Vriesendorp, R. Prophylactic gastrostomy placement and early tube feeding may limit loss of weight during chemoradiotherapy for advanced head and neck cancer, a preliminary study. Clin. Otolaryngol. 2007, 32, 384–390. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Le, Q.T. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Alderman, B.; Allan, L.; Amano, K.; Bouleuc, C.; Davis, M.; Lister-Flynn, S.; Mukhopadhyay, S.; Davies, A. Multinational Association of Supportive Care in Cancer (MASCC) expert opinion/guidance on the use of clinically assisted nutrition in patients with advanced cancer. Support. Care Cancer 2022, 30, 2983–2992. [Google Scholar] [CrossRef]
- Network NCC. Head and Neck Cancers (Version 2.2025). 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 18 June 2025).
- Fung-Kee-Fung, S.D.; Hackett, R.; Hales, L.; Warren, G.; Singh, A.K. A prospective trial of volumetric intensity-modulated arc therapy vs. conventional intensity modulated radiation therapy in advanced head and neck cancer. World J. Clin. Oncol. 2012, 3, 57–62. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Han, H.R.; Hermann, G.M.; Ma, S.J.; Iovoli, A.J.; Wooten, K.E.; Arshad, H.; Gupta, V.; McSpadden, R.P.; Kuriakose, M.A.; Markiewicz, M.R.; et al. Matched pair analysis to evaluate the impact of hospitalization during radiation therapy as an early marker of survival in head and neck cancer patients. Oral Oncol. 2020, 109, 104854. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Khan, M.; Chatterjee, U.; Santhosh, S.; Hashmi, M.; Gill, J.; Yu, B.; Iovoli, A.; Farrugia, M.; Wooten, K.; et al. Association of Body Mass Index With Outcomes Among Patients With Head and Neck Cancer Treated With Chemoradiotherapy. JAMA Netw. Open 2023, 6, e2320513. [Google Scholar] [CrossRef] [PubMed]
- Baine, M.J.; Dorius, T.; Bennion, N.; Smith, L.; Zhen, W.; Ganti, A.K. Weight Loss and Percutaneous Endoscopic Gastrostomy Tube Placement during Chemoradiotherapy for Locally Advanced Cancer of the Oropharynx Do Not Negatively Impact Outcomes. Front. Oncol. 2017, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Loimu, V.; Collan, J.; Vaalavirta, L.; Bäck, L.; Kapanen, M.; Mäkitie, A.; Tenhunen, M.; Saarilahti, K. Patterns of relapse following definitive treatment of head and neck squamous cell cancer by intensity modulated radiotherapy and weekly cisplatin. Radiother. Oncol. 2011, 98, 34–37. [Google Scholar] [CrossRef]
- Vangelov, B.; Kotevski, D.P.; Williams, J.R.; Smee, R.I. The impact of HPV status on weight loss and feeding tube use in oropharyngeal carcinoma. Oral Oncol. 2018, 79, 33–39. [Google Scholar] [CrossRef]
- Hanna, E.; Alexiou, M.; Morgan, J.; Badley, J.; Maddox, A.M.; Penagaricano, J.; Fan, C.-Y.; Breau, R.; Suen, J. Intensive chemoradiotherapy as a primary treatment for organ preservation in patients with advanced cancer of the head and neck: Efficacy, toxic effects, and limitations. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 861–867. [Google Scholar] [CrossRef]
- Lee, H.; Havrila, C.; Bravo, V.; Shantz, K.; Diaz, K.; Larner, J.; Read, P. Effect of oral nutritional supplementation on weight loss and percutaneous endoscopic gastrostomy tube rates in patients treated with radiotherapy for oropharyngeal carcinoma. Support. Care Cancer 2008, 16, 285–289. [Google Scholar] [CrossRef]
- Rivers, C.I.; Iovoli, A.J.; Chatterjee, U.; Hermann, G.M.; Singh, A.K. Intravenous fluids for pain management in head and neck cancer patients undergoing chemoradiation. Ann. Transl. Med. 2021, 9, 912. [Google Scholar] [CrossRef]
- Madhoun, M.F.; Blankenship, M.M.; Blankenship, D.M.; Krempl, G.A.; Tierney, W.M. Prophylactic PEG placement in head and neck cancer: How many feeding tubes are unused (and unnecessary)? World J. Gastroenterol. 2011, 17, 1004–1008. [Google Scholar] [CrossRef]
- Pih, G.Y.; Na, H.K.; Ahn, J.Y.; Jung, K.W.; Kim, D.H.; Lee, J.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; Jung, H.-Y. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy insertion. BMC Gastroenterol. 2018, 18, 101. [Google Scholar] [CrossRef]
- Sealock, R.J.; Munot, K. Common Gastrostomy Feeding Tube Complications and Troubleshooting. Clin. Gastroenterol. Hepatol. 2018, 16, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Eclov, N.C.W.; Dalal, N.H.; Thomas, S.M.; Stephens, S.J.; Malicki, M.; Shields, S.; Cobb, A.; Mowery, Y.M.; Niedzwiecki, D.; et al. System for High-Intensity Evaluation During Radiation Therapy (SHIELD-RT): A Prospective Randomized Study of Machine Learning-Directed Clinical Evaluations During Radiation and Chemoradiation. J. Clin. Oncol. 2020, 38, 3652–3661. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.; Finneran, L.; Roe, J.; Sydenham, M.A.; Beasley, M.; Bhide, S.; Boon, C.; Cook, A.; De Winton, E.; Emson, M.; et al. Dysphagia-optimised intensity-modulated radiotherapy versus standard intensity-modulated radiotherapy in patients with head and neck cancer (DARS): A phase 3, multicentre, randomised, controlled trial. Lancet Oncol. 2023, 24, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Friesner, I.D.; Feng, J.; Kalnicki, S.; Garg, M.; Ohri, N.; Hong, J.C. Machine Learning-Based Prediction of Hospitalization During Chemoradiotherapy With Daily Step Counts. JAMA Oncol. 2024, 10, 642–647. [Google Scholar] [CrossRef]
- Sher, D.J.; Radpour, S.; Shah, J.L.; Pham, N.-L.; Jiang, S.; Vo, D.; Sumer, B.D.; Day, A.T. Pilot Study of a Wearable Activity Monitor During Head and Neck Radiotherapy to Predict Clinical Outcomes. JCO Clin. Cancer Inform. 2022, 6, e2100179. [Google Scholar] [CrossRef]
- Anderson, B.J.; Moreno, A.C.; Qing, Y.; Lee, J.J.; Johnson, F.M.; Lango, M.N.; Barbon, C.E.A.; Tripuraneni, L.; Sahli, A.; Piper, V.; et al. Revisiting Feeding Tube Utilization in Oropharynx Cancer: 6-Year Prospective Registry Analysis. Otolaryngol. Head Neck Surg. 2024, 170, 1319–1330. [Google Scholar] [CrossRef]
- Mays, A.C.; Worley, M.; Ackall, F.; D’Agostino, R., Jr.; Waltonen, J.D. The association between gastrostomy tube placement, poor post-operative outcomes, and hospital re-admissions in head and neck cancer patients. Surg. Oncol. 2015, 24, 248–257. [Google Scholar] [CrossRef]
- Ma, S.J.; Zhu, S.; Virk, J.; Koempel, A.; Bhateja, P.; Gogineni, E.; Baliga, S.; Konieczkowski, D.; Mitchell, D.; Jhawar, S.; et al. Weekly Cisplatin Cycles and Outcomes for Chemoradiation in Head and Neck Cancer. JAMA Netw. Open 2024, 7, e2450272. [Google Scholar] [CrossRef]
- Virk, J.; Gill, J.; Fekrmandi, F.; Iovoli, A.; Farrugia, M.; Al-Afif, A.; Wooten, K.; Gupta, V.; McSpadden, R.; Kuriakose, M.A.; et al. Association of low adherence to weekly cisplatin with outcomes in patients with head and neck squamous cell carcinoma: A retrospective cohort study. BMC Cancer 2024, 24, 838. [Google Scholar] [CrossRef]
- Yang, Z.; Mansour, J.; Sun, P.; Wei, P.; Dahlstrom, K.R.; Zafereo, M.; Li, G.; Gross, N.D. Impact of pretreatment body mass index on the survival of head and neck cancer patients. Head Neck 2024, 46, 1881–1892. [Google Scholar] [CrossRef]

| Before Matching | After Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Hospitalization | Hospitalization | No Hospitalization | Hospitalization | |||||||
| n | % | n | % | p | n | % | n | % | p | |
| Age | 0.03 | 0.66 | ||||||||
| <65 years | 332 | 70.8 | 116 | 61.7 | 110 | 61.1 | 115 | 63.9 | ||
| 65 years or older | 137 | 29.2 | 72 | 38.3 | 70 | 38.9 | 65 | 36.1 | ||
| Gender | <0.001 | 0.71 | ||||||||
| Female | 71 | 15.1 | 51 | 27.1 | 41 | 22.8 | 45 | 25.0 | ||
| Male | 398 | 84.9 | 137 | 72.9 | 139 | 77.2 | 135 | 75.0 | ||
| Race | 0.38 | 0.55 | ||||||||
| White | 409 | 87.2 | 159 | 84.6 | 157 | 87.2 | 152 | 84.4 | ||
| Other | 60 | 12.8 | 29 | 15.4 | 23 | 12.8 | 28 | 15.6 | ||
| Smoking | 0.99 | 0.98 | ||||||||
| Never | 121 | 25.8 | 48 | 25.5 | 47 | 26.1 | 48 | 26.7 | ||
| Former | 266 | 56.7 | 108 | 57.4 | 100 | 55.6 | 101 | 56.1 | ||
| Current | 82 | 17.5 | 32 | 17.0 | 33 | 18.3 | 31 | 17.2 | ||
| ECOG PS | 0.85 | 0.39 | ||||||||
| 0 | 125 | 26.7 | 52 | 27.7 | 41 | 22.8 | 49 | 27.2 | ||
| >0 | 344 | 73.3 | 136 | 72.3 | 139 | 77.2 | 131 | 72.8 | ||
| Primary site | 0.65 | 0.92 | ||||||||
| Oropharynx | 273 | 58.2 | 102 | 54.3 | 99 | 55.0 | 98 | 54.4 | ||
| Larynx | 100 | 21.3 | 44 | 23.4 | 45 | 25.0 | 43 | 23.9 | ||
| Other | 96 | 20.5 | 42 | 22.3 | 36 | 20.0 | 39 | 21.7 | ||
| BMI | 0.25 | 1 | ||||||||
| Normal | 114 | 24.3 | 41 | 21.8 | 41 | 22.8 | 41 | 22.8 | ||
| Underweight | 13 | 2.8 | 8 | 4.3 | 8 | 4.4 | 8 | 4.4 | ||
| Overweight | 181 | 38.6 | 62 | 33.0 | 62 | 34.4 | 61 | 33.9 | ||
| Obese | 161 | 34.3 | 77 | 41.0 | 69 | 38.3 | 70 | 38.9 | ||
| T staging | 0.49 | 0.4 | ||||||||
| 1–2 | 247 | 52.7 | 93 | 49.5 | 97 | 53.9 | 88 | 48.9 | ||
| 3–4 | 222 | 47.3 | 95 | 50.5 | 83 | 46.1 | 92 | 51.1 | ||
| N staging | 0.63 | 0.91 | ||||||||
| 0–1 | 126 | 26.9 | 54 | 28.7 | 51 | 28.3 | 53 | 29.4 | ||
| 2–3 | 343 | 73.1 | 134 | 71.3 | 129 | 71.7 | 127 | 70.6 | ||
| HPV | 0.6 | 0.72 | ||||||||
| Negative | 182 | 38.8 | 81 | 43.1 | 79 | 43.9 | 77 | 42.8 | ||
| Positive | 247 | 52.7 | 93 | 49.5 | 83 | 46.1 | 89 | 49.4 | ||
| Not available | 40 | 8.5 | 14 | 7.4 | 18 | 10.0 | 14 | 7.8 | ||
| Cisplatin | 0.91 | 0.67 | ||||||||
| No | 76 | 16.2 | 31 | 16.5 | 27 | 15.0 | 31 | 17.2 | ||
| Yes | 393 | 83.8 | 157 | 83.5 | 153 | 85.0 | 149 | 82.8 | ||
| PEG tube placement | <0.001 | 0.91 | ||||||||
| Prophylactic | 63 | 13.4 | 28 | 14.9 | 30 | 16.7 | 28 | 15.6 | ||
| No | 307 | 65.5 | 73 | 38.8 | 69 | 38.3 | 73 | 40.6 | ||
| Therapeutic | 99 | 21.1 | 87 | 46.3 | 81 | 45.0 | 79 | 43.9 | ||
| Reasons for Hospitalization | ||
|---|---|---|
| n | % | |
| Dehydration | 58 | 19.9 |
| Fever | 41 | 14.1 |
| Dysphagia or odynophagia | 27 | 9.3 |
| Pneumonia | 24 | 8.2 |
| Altered mental status | 10 | 3.4 |
| PE or DVT | 7 | 2.4 |
| Other | 124 | 42.6 |
| Total | 291 | 100.0 |
| p16-Negative Tumors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall survival | Progression-free survival | Locoregional failure | Distant failure | |||||||||
| aHR | 95% CI | p | aHR | 95% CI | p | aHR | 95% CI | p | aHR | 95% CI | p | |
| Hospitalization | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.92 | 1.24–2.97 | 0.003 | 1.84 | 1.24–2.74 | 0.002 | 0.83 | 0.40–1.73 | 0.62 | 1.05 | 0.46–2.39 | 0.91 |
| p16-positive tumors | ||||||||||||
| Overall survival | Progression-free survival | Locoregional failure | Distant failure | |||||||||
| aHR | 95% CI | p | aHR | 95% CI | p | aHR | 95% CI | p | aHR | 95% CI | p | |
| Hospitalization | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 2.47 | 1.38–4.40 | 0.002 | 1.8 | 1.11–2.92 | 0.02 | 0.25 | 0.02–2.77 | 0.26 | 1.44 | 0.65–3.16 | 0.37 |
| First Hospitalization | Multiple Hospitalization | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p | aOR | 95% CI | p | |
| PEG tube placement | ||||||
| Prophylactic | Reference | Reference | ||||
| No | 0.48 | 0.27–0.86 | 0.01 | 0.86 | 0.35–2.31 | 0.76 |
| Therapeutic | 1.96 | 1.10–3.54 | 0.02 | 1.81 | 0.74–4.81 | 0.21 |
| Age | ||||||
| For every increase by 1 | 1.01 | 0.99–1.03 | 0.2 | 1.01 | 0.98–1.05 | 0.39 |
| Gender | ||||||
| Female | Reference | Reference | ||||
| Male | 0.46 | 0.29–0.72 | <0.001 | 0.6 | 0.31–1.23 | 0.15 |
| Race | ||||||
| White | Reference | Reference | ||||
| Other | 1.32 | 0.77–2.21 | 0.3 | 0.53 | 0.17–1.30 | 0.2 |
| Smoking | ||||||
| Never | Reference | Reference | ||||
| Former | 1.03 | 0.66–1.62 | 0.88 | 0.87 | 0.42–1.85 | 0.7 |
| Current | 0.91 | 0.49–1.65 | 0.75 | 0.71 | 0.26–1.88 | 0.49 |
| ECOG PS | ||||||
| 0 | Reference | Reference | ||||
| >0 | 0.8 | 0.53–1.21 | 0.29 | 2.24 | 1.02–5.62 | 0.06 |
| Primary site | ||||||
| Oropharynx | Reference | Reference | ||||
| Larynx | 1.23 | 0.64–2.38 | 0.53 | 1.34 | 0.47–3.79 | 0.58 |
| Other | 1.24 | 0.70–2.18 | 0.45 | 1.37 | 0.54–3.31 | 0.49 |
| BMI | ||||||
| Normal | Reference | Reference | ||||
| Underweight | 1.31 | 0.45–3.67 | 0.6 | 1.19 | 0.24–4.52 | 0.81 |
| Overweight | 0.98 | 0.59–1.65 | 0.95 | 0.36 | 0.15–0.82 | 0.02 |
| Obese | 1.54 | 0.92–2.60 | 0.1 | 0.71 | 0.33–1.54 | 0.39 |
| T staging | ||||||
| 1–2 | Reference | Reference | ||||
| 3–4 | 1.01 | 0.67–1.50 | 0.97 | 1.13 | 0.59–2.17 | 0.72 |
| N staging | ||||||
| 0–1 | Reference | Reference | ||||
| 2–3 | 1.02 | 0.63–1.65 | 0.94 | 0.77 | 0.37–1.63 | 0.49 |
| HPV | ||||||
| Negative | Reference | Reference | ||||
| Positive | 1.24 | 0.70–2.20 | 0.45 | 1.47 | 0.59–3.68 | 0.41 |
| Not available | 0.89 | 0.38–2.04 | 0.79 | 1.52 | 0.39–5.37 | 0.53 |
| Cisplatin | ||||||
| No | Reference | Reference | ||||
| Yes | 1.09 | 0.65–1.84 | 0.75 | 1.34 | 0.59–3.41 | 0.51 |
| Time to First Hospitalization | |||
|---|---|---|---|
| aHR | 95% CI | p | |
| PEG tube placement | |||
| Prophylactic | Reference | ||
| No | 0.52 | 0.32–0.85 | 0.008 |
| Therapeutic | 1.59 | 1.00–2.54 | 0.05 |
| Age | |||
| For every increase by 1 | 1.01 | 0.99–1.03 | 0.18 |
| Gender | |||
| Female | Reference | ||
| Male | 0.57 | 0.41–0.80 | 0.001 |
| Race | |||
| White | Reference | ||
| Other | 1.17 | 0.78–1.76 | 0.45 |
| Smoking | |||
| Never | Reference | ||
| Former | 1.01 | 0.70–1.45 | 0.95 |
| Current | 0.91 | 0.55–1.52 | 0.73 |
| ECOG PS | |||
| 0 | Reference | ||
| >0 | 0.84 | 0.61–1.16 | 0.29 |
| Primary site | |||
| Oropharynx | Reference | ||
| Larynx | 1.18 | 0.71–1.97 | 0.52 |
| Other | 1.14 | 0.73–1.77 | 0.58 |
| BMI | |||
| Normal | Reference | ||
| Underweight | 1.21 | 0.54–2.70 | 0.64 |
| Overweight | 0.94 | 0.62–1.41 | 0.75 |
| Obese | 1.27 | 0.84–1.92 | 0.26 |
| T staging | |||
| 1–2 | Reference | ||
| 3–4 | 1.01 | 0.73–1.38 | 0.98 |
| N staging | |||
| 0–1 | Reference | ||
| 2–3 | 1.01 | 0.70–1.47 | 0.95 |
| HPV | |||
| Negative | Reference | ||
| Positive | 1.22 | 0.78–1.91 | 0.38 |
| Not available | 0.97 | 0.47–2.00 | 0.93 |
| Cisplatin | |||
| No | Reference | ||
| Yes | 1.05 | 0.67–1.63 | 0.84 |
| aOR | 95% CI | p | |
|---|---|---|---|
| Age | |||
| For every increase by 1 | 1.01 | 0.99–1.03 | 0.25 |
| Gender | |||
| Female | Reference | ||
| Male | 0.93 | 0.58–1.54 | 0.79 |
| Race | |||
| White | Reference | ||
| Other | 0.69 | 0.37–1.25 | 0.24 |
| Smoking | |||
| Never | Reference | ||
| Former | 0.84 | 0.54–1.32 | 0.45 |
| Current | 1.22 | 0.66–2.23 | 0.52 |
| ECOG PS | |||
| 0 | Reference | ||
| >0 | 1.35 | 0.90–2.06 | 0.15 |
| Primary site | |||
| Oropharynx | Reference | ||
| Larynx | 0.68 | 0.34–1.34 | 0.26 |
| Other | 0.71 | 0.39–1.26 | 0.25 |
| BMI | |||
| Normal | Reference | ||
| Underweight | 2.26 | 0.60–8.78 | 0.22 |
| Overweight | 1.47 | 0.88–2.49 | 0.15 |
| Obese | 1.25 | 0.74–2.15 | 0.41 |
| T staging | |||
| 1–2 | Reference | ||
| 3–4 | 1.36 | 0.91–2.02 | 0.13 |
| N staging | |||
| 0–1 | Reference | ||
| 2–3 | 1.15 | 0.70–1.90 | 0.58 |
| HPV | |||
| Negative | Reference | ||
| Positive | 0.51 | 0.28–0.92 | 0.03 |
| Not available | 1.6 | 0.67–3.88 | 0.29 |
| Cisplatin | |||
| No | Reference | ||
| Yes | 1.19 | 0.71–2.03 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.J.; Virk, J.; Schrand, T.V.; Gill, J.; Almeida, N.; Cheruvu, H.K.; Gupta, V.; Wooten, K.E.; Kuriakose, M.A.; Markiewicz, M.R.; et al. Association of Percutaneous Endoscopic Gastrostomy (PEG) Tube Placement with Unplanned Hospitalization for Head and Neck Cancer. Cancers 2025, 17, 2066. https://doi.org/10.3390/cancers17132066
Ma SJ, Virk J, Schrand TV, Gill J, Almeida N, Cheruvu HK, Gupta V, Wooten KE, Kuriakose MA, Markiewicz MR, et al. Association of Percutaneous Endoscopic Gastrostomy (PEG) Tube Placement with Unplanned Hospitalization for Head and Neck Cancer. Cancers. 2025; 17(13):2066. https://doi.org/10.3390/cancers17132066
Chicago/Turabian StyleMa, Sung Jun, Jas Virk, Tyler V. Schrand, Jasmin Gill, Neil Almeida, Harshini K. Cheruvu, Vishal Gupta, Kimberly E. Wooten, Moni A. Kuriakose, Michael R. Markiewicz, and et al. 2025. "Association of Percutaneous Endoscopic Gastrostomy (PEG) Tube Placement with Unplanned Hospitalization for Head and Neck Cancer" Cancers 17, no. 13: 2066. https://doi.org/10.3390/cancers17132066
APA StyleMa, S. J., Virk, J., Schrand, T. V., Gill, J., Almeida, N., Cheruvu, H. K., Gupta, V., Wooten, K. E., Kuriakose, M. A., Markiewicz, M. R., McSpadden, R. P., Hicks, W. L., Farrugia, M. K., & Singh, A. K. (2025). Association of Percutaneous Endoscopic Gastrostomy (PEG) Tube Placement with Unplanned Hospitalization for Head and Neck Cancer. Cancers, 17(13), 2066. https://doi.org/10.3390/cancers17132066






