Impact of Tumor Location on Survival Outcomes in Pancreatic Head Versus Body/Tail Cancer: Institutional Experience
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Population
2.2. Treatment Protocol and Assessment
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
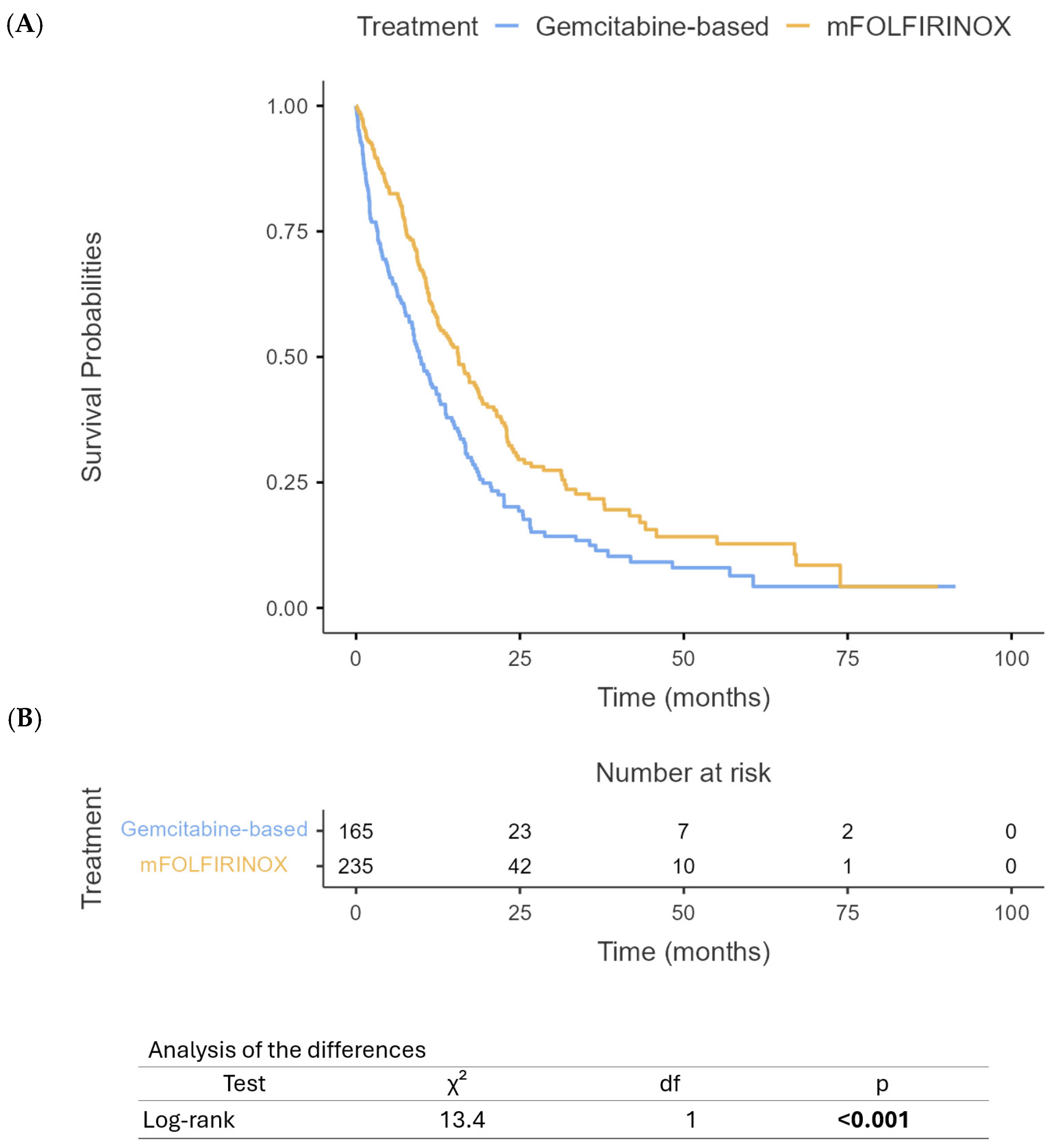
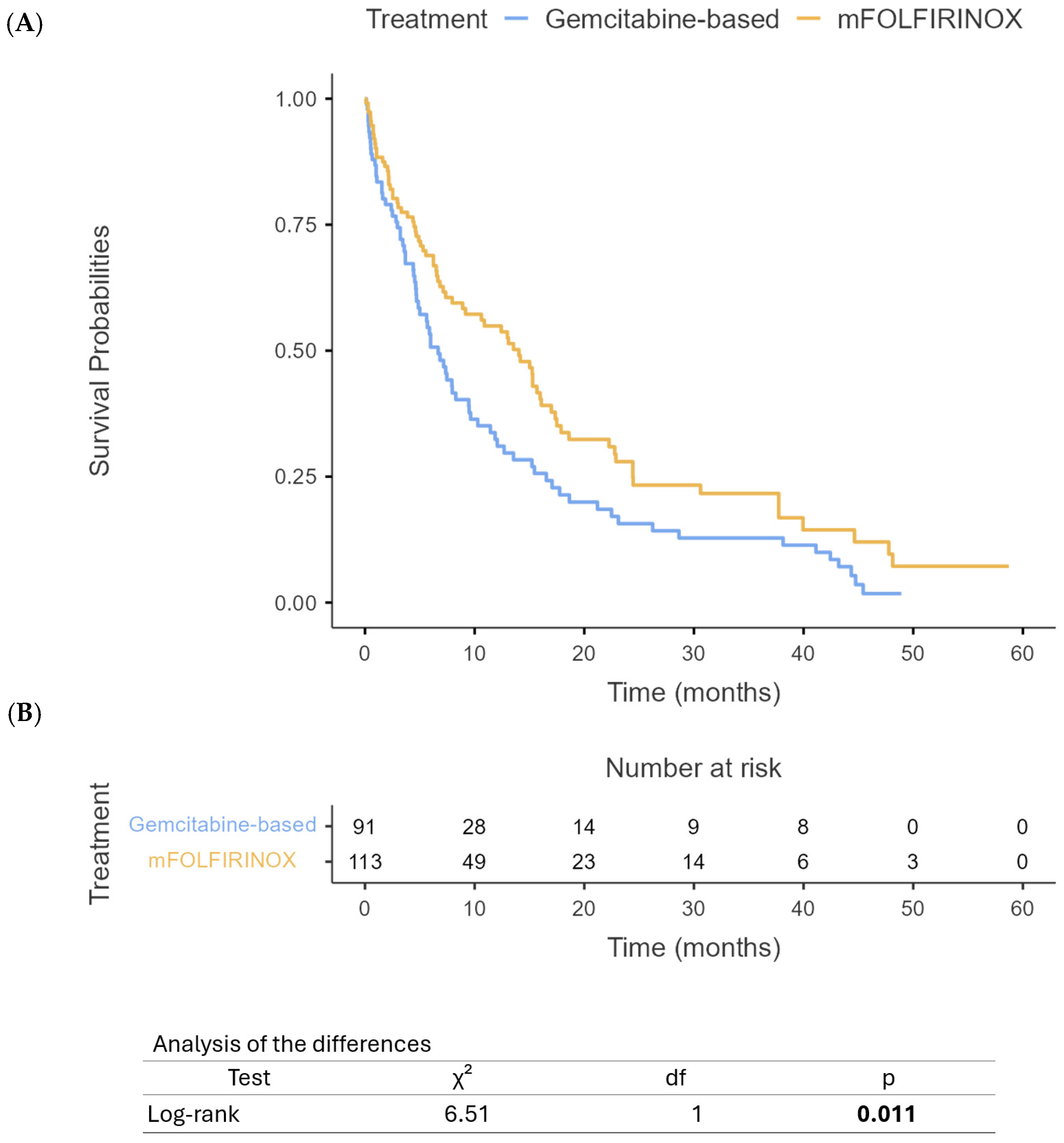
3.2. Treatment Characteristics
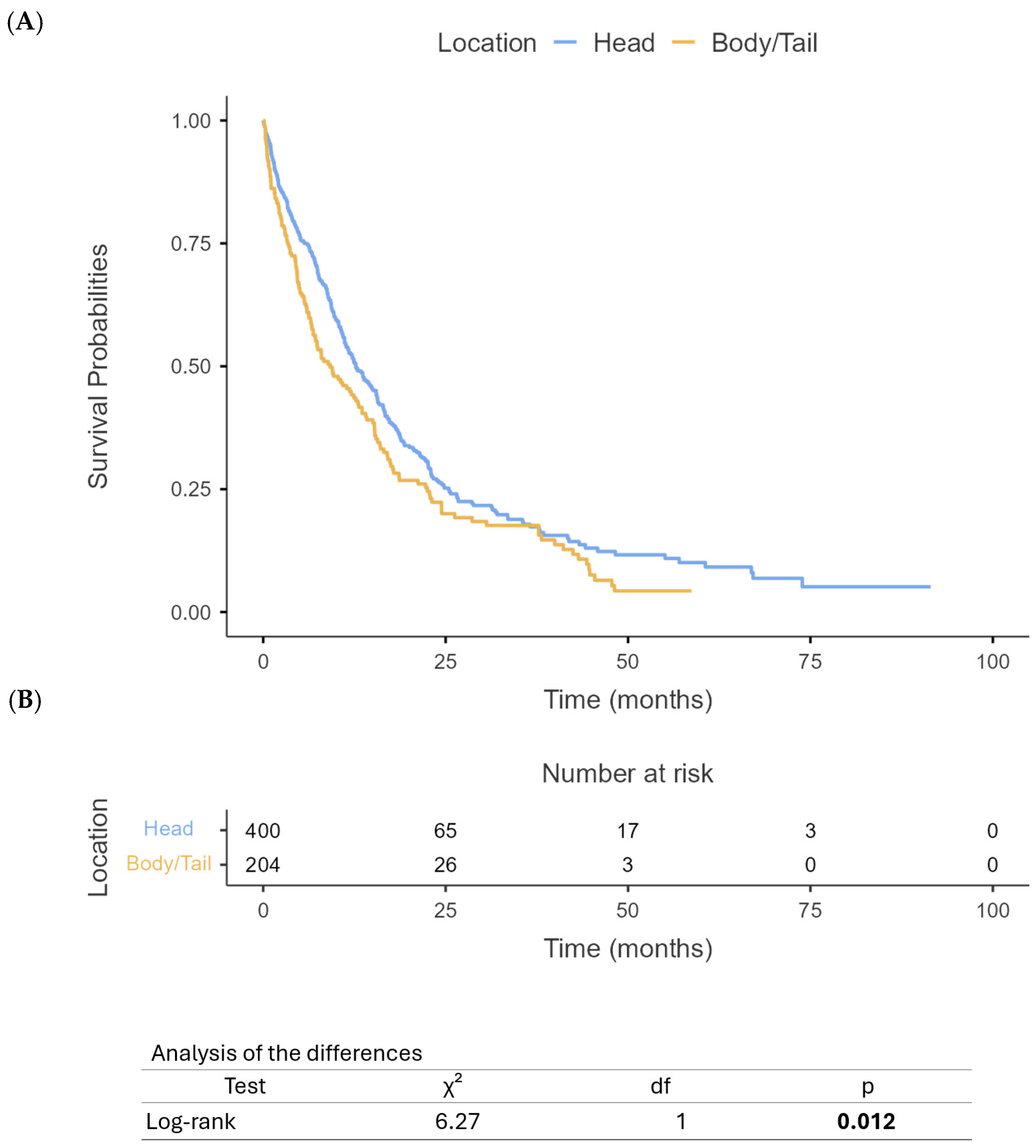
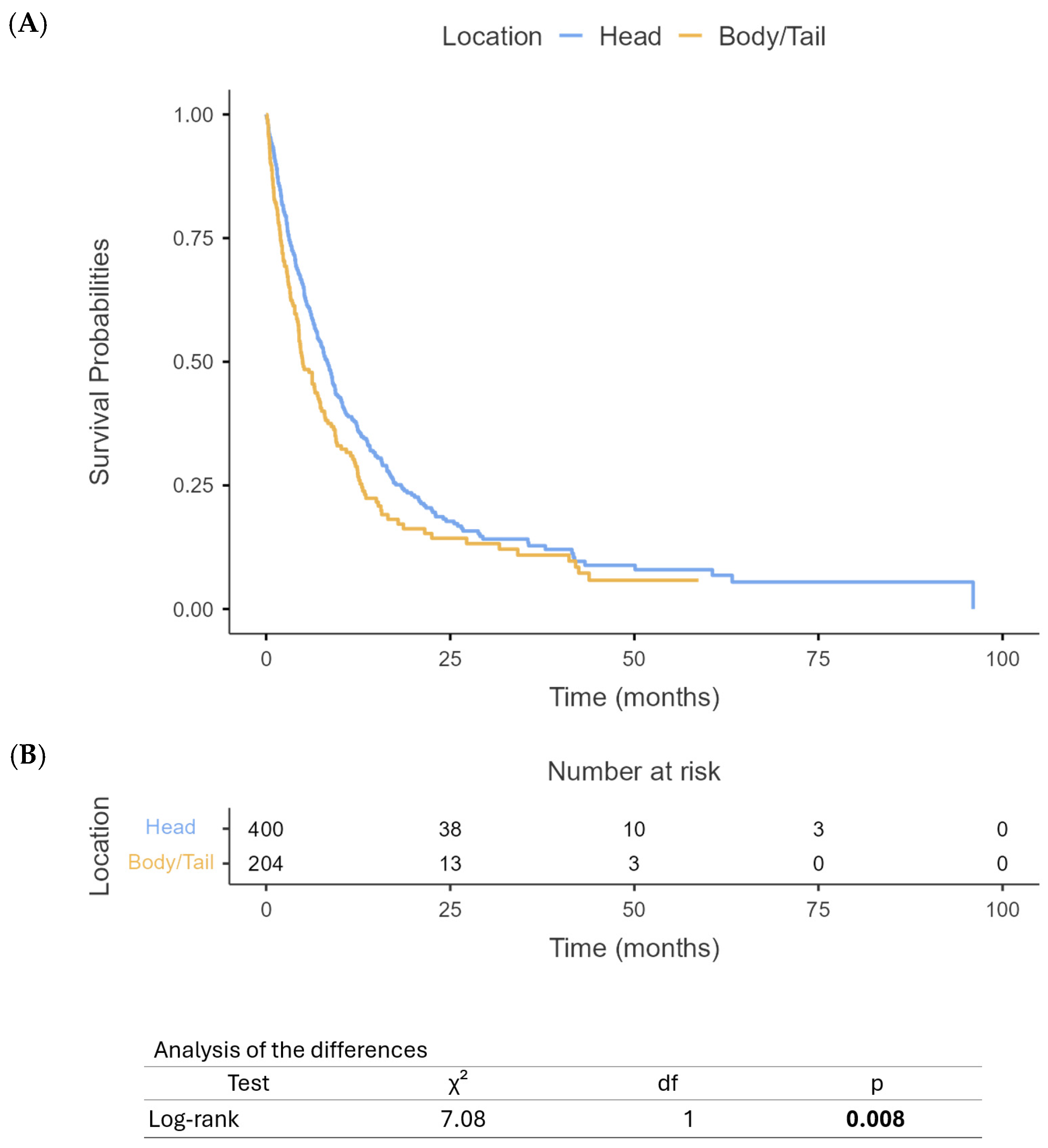
3.3. Survival Outcomes
3.4. Latent Class Analysis
3.4.1. Pancreatic Head Cancer Classes
3.4.2. Pancreatic Body/Tail Cancer Classes
3.4.3. Clinical Implications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farshadi, E.A.; Wang, W.; Mohammad, F.; van der Oost, E.; Doukas, M.; van Eijck, C.H.; van de Werken, H.J.; Katsikis, P.D. Tumor organoids improve mutation detection of pancreatic ductal adenocarcinoma. Sci. Rep. 2024, 14, 25468. [Google Scholar] [CrossRef] [PubMed]
- Mosalem, O.M.; Abdelhakeem, A.; Abdel-Razeq, N.H.; Babiker, H. Pancreatic ductal adenocarcinoma (PDAC): Clinical progress in the last five years. Expert. Opin. Investig. Drugs 2025, 34, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Mukund, A.; Afridi, M.A.; Karolak, A.; Park, M.A.; Permuth, J.B.; Rasool, G. Pancreatic Ductal Adenocarcinoma (PDAC): A review of recent advancements enabled by artificial intelligence. Cancers 2024, 16, 2240. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Javed, A.A.; Mahmud, O.; Fatimi, A.S.; Habib, A.; Grewal, M.; He, J.; Wolfgang, C.L.; Besselink, M.G.; PANC-PALS Consortium. Predictors for Long-Term Survival After Resection of Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2024, 31, 4673–4687. [Google Scholar] [CrossRef]
- van Erning, F.N.; Mackay, T.M.; van der Geest, L.G.M.; Groot Koerkamp, B.; van Laarhoven, H.W.M.; Bonsing, B.A.; Wilmink, J.W.; van Santvoort, H.C.; de Vos-Geelen, J.; van Eijck, C.H.J.; et al. Association of the location of pancreatic ductal adenocarcinoma (head, body, tail) with tumor stage, treatment, and survival: A population-based analysis. Acta Oncol. 2018, 57, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Bugazia, D.; Al-Najjar, E.; Esmail, A.; Abdelrahim, S.; Abboud, K.; Abdelrahim, A.; Umoru, G.; Rayyan, H.A.; Abudayyeh, A.; Al Moustafa, A.E.; et al. Pancreatic ductal adenocarcinoma: The latest on diagnosis, molecular profiling, and systemic treatments. Front. Oncol. 2024, 14, 1386699. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Qiao, G.; Ni, J.; Chen, T.; Wang, Y.; Wu, C.; Zhang, Q.; Ma, T.; Gao, S. 5-Year survival rate over 20% in pancreatic ductal adenocarcinoma: A retrospective study from a Chinese high-volume center. Cancer Lett. 2025, 619, 217658. [Google Scholar] [CrossRef]
- Yasinzai, A.Q.K.; Tareen, B.; Tracy, K.; Jamil, N.; Khan, M.; Ullah, H.; Raza, M.; Khan, A.U.; Arif, D.; Waheed, A. Pancreatic ductal adenocarcinoma: Exploring clinicopathological trends and racial disparities in a comprehensive US population-based study. Clin. Transl. Oncol. 2024, 26, 2618–2628. [Google Scholar] [CrossRef]
- Mas, L.; Castelli, C.; Coffy, A.; Tretarre, B.; Piquemal, D.; Bachet, J.-B. Nationwide trends over 10 years in epidemiology and management of pancreatic ductal adenocarcinoma: A real-world study from the French administrative database. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102426. [Google Scholar] [CrossRef]
- Shin, K.I.; Yoon, M.S.; Kim, J.H.; Jang, W.J.; Leem, G.; Jo, J.H.; Chung, M.J.; Park, J.Y.; Park, S.W.; Hwang, H.K. Long-Term Outcomes of Neoadjuvant Therapy Versus Upfront Surgery for Resectable Pancreatic Ductal Adenocarcinoma. Cancer Med. 2024, 13, e70363. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, B.; Esmail, A.; Al-Najjar, E.; El Beheary, S.; Abdelrahim, M. An 81-Year-Old Geriatric Patient with Metastatic Pancreatic Cancer Demonstrating Excellent Response and Well Tolerance to NALIRIFOX: A Case Report and Literature Review. Reports 2025, 8, 69. [Google Scholar] [CrossRef]
- Ushio, J.; Kanno, A.; Ikeda, E.; Ando, K.; Nagai, H.; Miwata, T.; Kawasaki, Y.; Tada, Y.; Yokoyama, K.; Numao, N.; et al. Pancreatic Ductal Adenocarcinoma: Epidemiology and Risk Factors. Diagnostics 2021, 11, 562. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Kasi, A.; Esnaola, N.F.; Xiu, J.; Baca, Y.; Weinberg, B.A. Comparative molecular profiling of pancreatic ductal adenocarcinoma of the head versus body and tail. NPJ Precis. Oncol. 2024, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Katz, T.A.; Sharma, S.; Kalashnikova, E.; Malhotra, M.; Olshan, P.; Billings, P.R.; Aleshin, A. Early relapse detection and monitoring disease status in patients with early-stage pancreatic adenocarcinoma using circulating tumor DNA. J. Surg. Res. 2021, 4, 602–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Esmail, A.; Hassanain, H.; Dhillon, V.; Abdelrahim, W.; Al-Najjar, E.; Khasawneh, B.; Abdelrahim, M. Correlative Analysis of Tumor-Informed Circulating Tumor DNA (ctDNA) and the Survival Outcomes of Patients with Pancreatic Adenocarcinoma. Biomedicines 2025, 13, 1124. [Google Scholar] [CrossRef]
- Dreyer, S.B.; Upstill-Goddard, R.; Legrini, A.; Biankin, A.V.; Jamieson, N.B.; Chang, D.K.; Jamieson, N.B.; Chang, D.K. Genomic and Molecular Analyses Identify Molecular Subtypes of Pancreatic Cancer Recurrence. Gastroenterology 2022, 162, 320–324.e324. [Google Scholar] [CrossRef]
- Botta, G.P.; Abdelrahim, M.; Aushev, V.N.; Esmail, A.; Drummond, B.; Sharma, S.; Kalashnikova, E.; Hook, N.; Chandana, S.R.; Tejani, M.A.; et al. Association of personalized and tumor-informed ctDNA with patient survival outcomes in pancreatic adenocarcinoma. J. Clin. Oncol. 2022, 40, 517. [Google Scholar] [CrossRef]
- Botta, G.P.; Abdelrahim, M.; Drengler, R.L.; Aushev, V.N.; Esmail, A.; Laliotis, G.; Brewer, C.M.; George, G.V.; Abbate, S.M.; Chandana, S.R.; et al. Association of personalized and tumor-informed ctDNA with patient survival outcomes in pancreatic adenocarcinoma. Oncologist 2024, 29, 859–869. [Google Scholar] [CrossRef]
- Esmail, A.; Guan, J.; Xu, J.; Al-Rawi, H.; Parks, B.; Al Saadi, N.; Khan, T.; Madoux, L.; Taha, M.; Abdelrahim, M. Prognostic value of molecular response via ctDNA measurement in predicating response of systemic therapy in patients with advanced solid cancer. J. Clin. Oncol. 2022, 40, e13001. [Google Scholar] [CrossRef]
- Micaily, I.; Blais, E.M.; Carhart, R.; Lam, S.; Cohen, S.J.; Cannaday, S.J.; Halverson, D.; Matrisian, L.M.; DeArbeloa, P.; Thach, D.; et al. Association of Pancreatic Adenocarcinoma Location With DNA Damage Response Status and Response to Platinum-Based Therapy. JCO Precis. Oncol. 2023, 7, e2200648. [Google Scholar] [CrossRef] [PubMed]
- Malleo, G.; Maggino, L.; Ferrone, C.R.; Marchegiani, G.; Luchini, C.; Mino-Kenudson, M.; Paiella, S.; Qadan, M.; Scarpa, A.; Lillemoe, K.D. Does site matter? Impact of tumor location on pathologic characteristics, recurrence, and survival of resected pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 3898–3912. [Google Scholar] [CrossRef]
- Targarona, E.M.; Balague, C.; Pernas, J.C.; Martinez, C.; Berindoague, R.; Gich, I.; Trias, M. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann. Surg. 2008, 247, 642–649. [Google Scholar] [CrossRef]
- Sung, M.K.; Park, Y.; Kwak, B.J.; Jun, E.; Lee, W.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Kim, S.C. Comparison of Characteristics and Survival Rates of Resectable Pancreatic Ductal Adenocarcinoma according to Tumor Location. Biomedicines 2021, 9, 1706. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Lambert, A.; Tarabay, A.; Hollebecque, A.; Smolenschi, C.; Valery, M.; Pudlarz, T.; Fuerea, A.; Camilleri, G.M.; Delaye, M. Heads or Tails? In-Depth Analysis of Pancreatic Cancer Outcome According to Tumor Location. Ann. Oncol. 2024, 35, S142. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Q.; Xiang, C.; Liu, X.; Zheng, Z. Increased risk of multiple metastases and worse overall survival of metastatic pancreatic body and tail cancer: A retrospective cohort study. Gland. Surg. 2024, 13, 480. [Google Scholar] [CrossRef]
- Murakawa, Y.; Ootsuka, K.; Abue, M. The Appropriate First-Line Chemotherapy Regimen for Incurable Pancreatic Cancer in Clinical Practice: A Consideration of Patients’ Overall Survival and Quality of Life. J. Pancreat. Cancer 2021, 7, 48–56. [Google Scholar] [CrossRef]
- Badheeb, M.; Abdelrahim, A.; Esmail, A.; Umoru, G.; Abboud, K.; Al-Najjar, E.; Rasheed, G.; Alkhulaifawi, M.; Abudayyeh, A.; Abdelrahim, M. Pancreatic Tumorigenesis: Precursors, Genetic Risk Factors and Screening. Curr. Oncol. 2022, 29, 8693–8719. [Google Scholar] [CrossRef]
- Esmail, A.; Badheeb, M.; Abdelrahim, M. Pancreatic Tumorigenesis: Precursors, Genetic Risk Factors and Screening. In Pancreatic Cancer-Updates in Pathogenesis, Diagnosis and Therapies; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; Fouchardière, C.d.l.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ramanathan, R.K.; Borad, M.J.; Laheru, D.A.; Smith, L.S.; Wood, T.E.; Korn, R.L.; Desai, N.; Trieu, V.; Iglesias, J.L.; et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J. Clin. Oncol. 2011, 29, 4548–4554. [Google Scholar] [CrossRef]
- Ko, A.H. Updates in Clinical Management of Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2024, 22, e245019. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Melisi, D.; Macarulla, T.; Pazo Cid, R.; Chandana, S.R.; De La Fouchardière, C.; Dean, A.; Kiss, I.; Lee, W.J.; Goetze, T.O.; et al. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): A randomised, open-label, phase 3 trial. Lancet 2023, 402, 1272–1281. [Google Scholar] [CrossRef]
- Bengtsson, A.; Andersson, R.; Ansari, D. Histological variants of pancreatic ductal adenocarcinoma: A survival analysis. Langenbeck’s Arch. Surg. 2024, 409, 312. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Séne, M.; Taylor, J.M.; Jacqmin-Gadda, H. Joint latent class models for longitudinal and time-to-event data: A review. Stat. Methods Med. Res. 2014, 23, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Chu, L.C.; Deig, C.R.; Fishman, E.K.; Hwang, W.L.; Maitra, A.; Marks, D.L.; Mehta, A.; Nabavizadeh, N.; Simeone, D.M.; et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J. Clin. 2020, 70, 375–403. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Cao, M.; Zhang, Y.; Liu, Z.; Wu, S.; Wu, H. Tumor location as an indicator of survival in T1 resectable pancreatic ductal adenocarcinoma: A propensity score-matched analysis. BMC Gastroenterol. 2019, 19, 59. [Google Scholar] [CrossRef]
- Tomasello, G.; Ghidini, M.; Costanzo, A.; Ghidini, A.; Russo, A.; Barni, S.; Passalacqua, R.; Petrelli, F. Outcome of head compared to body and tail pancreatic cancer: A systematic review and meta-analysis of 93 studies. J. Gastrointest. Oncol. 2019, 10, 259–269. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, S.; Wang, Q.; Huang, H.; Chen, R.; Xie, Q.; Zhang, W.; Wang, A.; Zhang, S.; Wang, L.; et al. Comparative genomic analysis of head and body/tail of pancreatic ductal adenocarcinoma at early and late stages. J. Cell. Mol. Med. 2021, 25, 1750–1758. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
| Category | Subcategory | C1 | C2 | C3 |
|---|---|---|---|---|
| Gender Breakdown | Female | 160 | 26 | 30 |
| Male | 144 | 22 | 19 | |
| Ethnicity Breakdown | Caucasian | 236 | 37 | 42 |
| Black | 47 | 10 | 3 | |
| Asian | 14 | 0 | 3 | |
| Hispanic | 6 | 1 | 0 | |
| Other | 0 | 0 | 1 | |
| Treatment Breakdown | mFOLFIRINOX | 173 | 27 | 37 |
| Gemcitabine based | 131 | 21 | 12 | |
| Setting Breakdown | Palliative | 169 | 18 | 8 |
| Adjuvant | 99 | 3 | 1 | |
| Curative | 35 | 26 | 6 | |
| Neoadjuvant | 1 | 1 | 34 | |
| Number of Cycles | Mean | 5.7 | 8.5 | 5.5 |
| Max | 28 | 43 | 12 | |
| Pathologic Stage Breakdown | IV | 139 | 3 | 1 |
| III | 54 | 8 | 7 | |
| IIB | 53 | 10 | 11 | |
| IB | 18 | 10 | 14 | |
| IIA | 11 | 10 | 4 | |
| IA | 5 | 0 | 6 | |
| II | 3 | 4 | 2 | |
| IIIA | 1 | 0 | 0 | |
| IIIB | 2 | 0 | 0 | |
| Radiation | Yes | 0 | 46 | 16 |
| No | 304 | 2 | 33 | |
| Median OS | Median | 9 | 13.5 | 18 |
| Category | Subcategory | C1 | C2 | C3 |
|---|---|---|---|---|
| Gender Breakdown | Female | 28 | 47 | 7 |
| Male | 47 | 60 | 13 | |
| Ethnicity Breakdown | Caucasian | 63 | 89 | 14 |
| Black | 10 | 14 | 4 | |
| Asian | 2 | 2 | 2 | |
| Hispanic | 0 | 0 | 0 | |
| Other | 0 | 0 | 0 | |
| Treatment Breakdown | mFOLFIRINOX | 51 | 46 | 14 |
| Gemcitabine based | 24 | 61 | 6 | |
| Setting Breakdown | Palliative | 48 | 90 | 9 |
| Adjuvant | 17 | 7 | 0 | |
| Curative | 6 | 3 | 11 | |
| Neoadjuvant | 4 | 7 | 0 | |
| Number of Cycles | Mean | 10.39 | 2.70 | 7.4 |
| Max | 52 | 9 | 12 | |
| Pathologic Stage Breakdown | IV | 46 | 78 | 3 |
| III | 7 | 9 | 7 | |
| IIB | 7 | 7 | 1 | |
| IB | 7 | 2 | 6 | |
| IIA | 3 | 2 | 0 | |
| IA | 2 | 4 | 1 | |
| II | 0 | 0 | 0 | |
| IIIA | 0 | 0 | 0 | |
| IIIB | 0 | 0 | 0 | |
| Radiation | Yes | 0 | 0 | 20 |
| No | 75 | 107 | 0 | |
| Median OS | Median (months) | 16 | 3 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmail, A.; Dhillon, V.; Al-Najjar, E.; Khasawneh, B.; Alghamdi, M.; Ibnshamsah, F.; Abdelrahim, M. Impact of Tumor Location on Survival Outcomes in Pancreatic Head Versus Body/Tail Cancer: Institutional Experience. Cancers 2025, 17, 1777. https://doi.org/10.3390/cancers17111777
Esmail A, Dhillon V, Al-Najjar E, Khasawneh B, Alghamdi M, Ibnshamsah F, Abdelrahim M. Impact of Tumor Location on Survival Outcomes in Pancreatic Head Versus Body/Tail Cancer: Institutional Experience. Cancers. 2025; 17(11):1777. https://doi.org/10.3390/cancers17111777
Chicago/Turabian StyleEsmail, Abdullah, Vikram Dhillon, Ebtesam Al-Najjar, Bayan Khasawneh, Mohammed Alghamdi, Fahad Ibnshamsah, and Maen Abdelrahim. 2025. "Impact of Tumor Location on Survival Outcomes in Pancreatic Head Versus Body/Tail Cancer: Institutional Experience" Cancers 17, no. 11: 1777. https://doi.org/10.3390/cancers17111777
APA StyleEsmail, A., Dhillon, V., Al-Najjar, E., Khasawneh, B., Alghamdi, M., Ibnshamsah, F., & Abdelrahim, M. (2025). Impact of Tumor Location on Survival Outcomes in Pancreatic Head Versus Body/Tail Cancer: Institutional Experience. Cancers, 17(11), 1777. https://doi.org/10.3390/cancers17111777








