Synthesis and Preliminary Cytotoxicity Evaluation of 3-Lup-20(29)-Ene-3β,28-Diol Glycoconjugates Containing a Succinic Linker and a 1,2,3-Triazole Ring
Simple Summary
Abstract
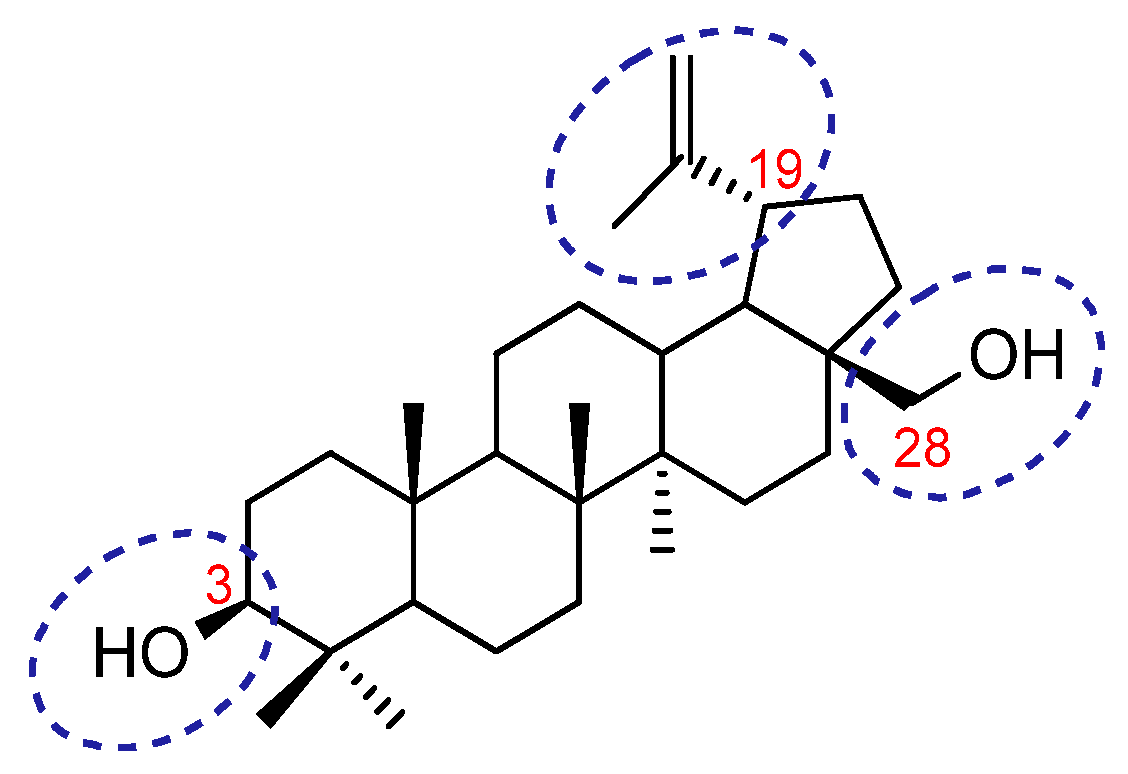
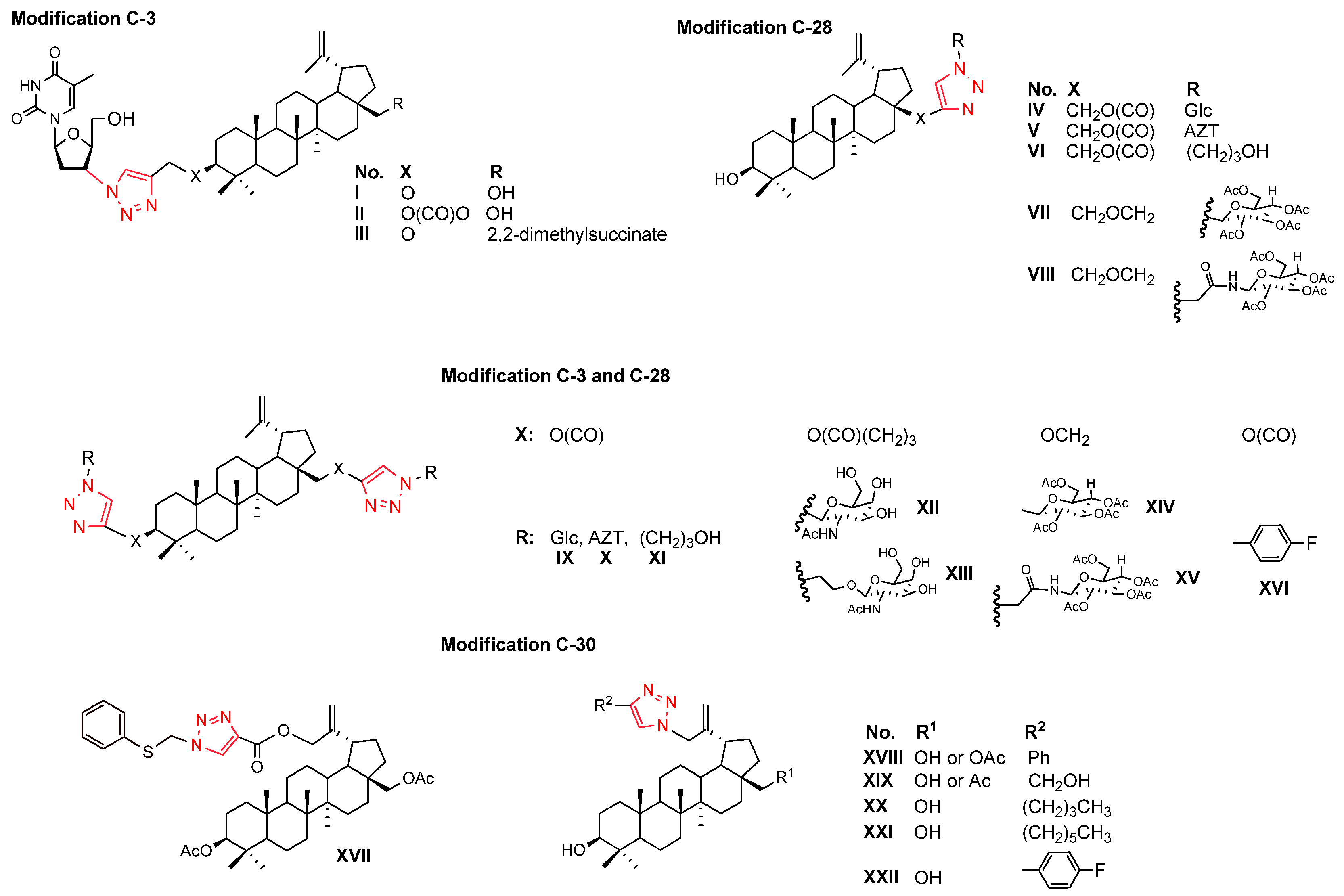
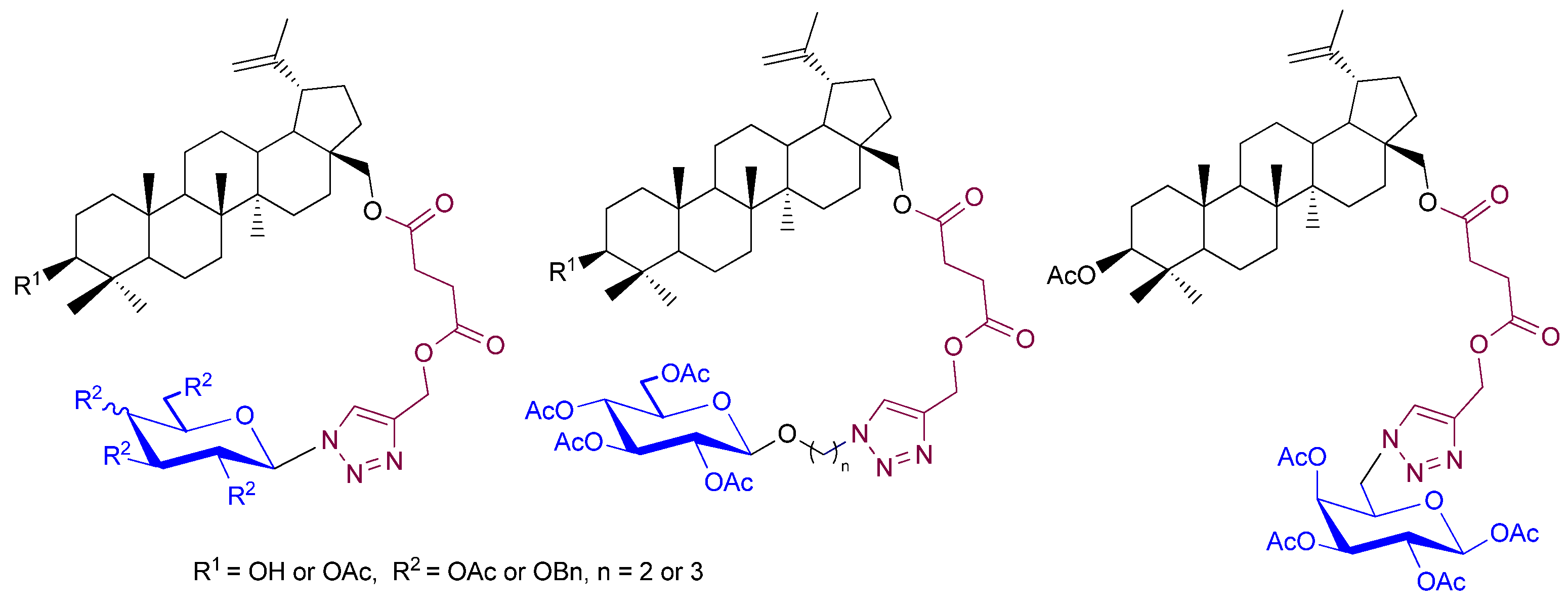
1. Introduction
2. Materials and Methods
2.1. General Experimental Informations
2.2. Biological Studies
2.3. In Silico Analysis
3. Results and Discussion
3.1. Betulin Analogues with Alkyne Moiety (3)
3.2. Sugar Derivatives (4)
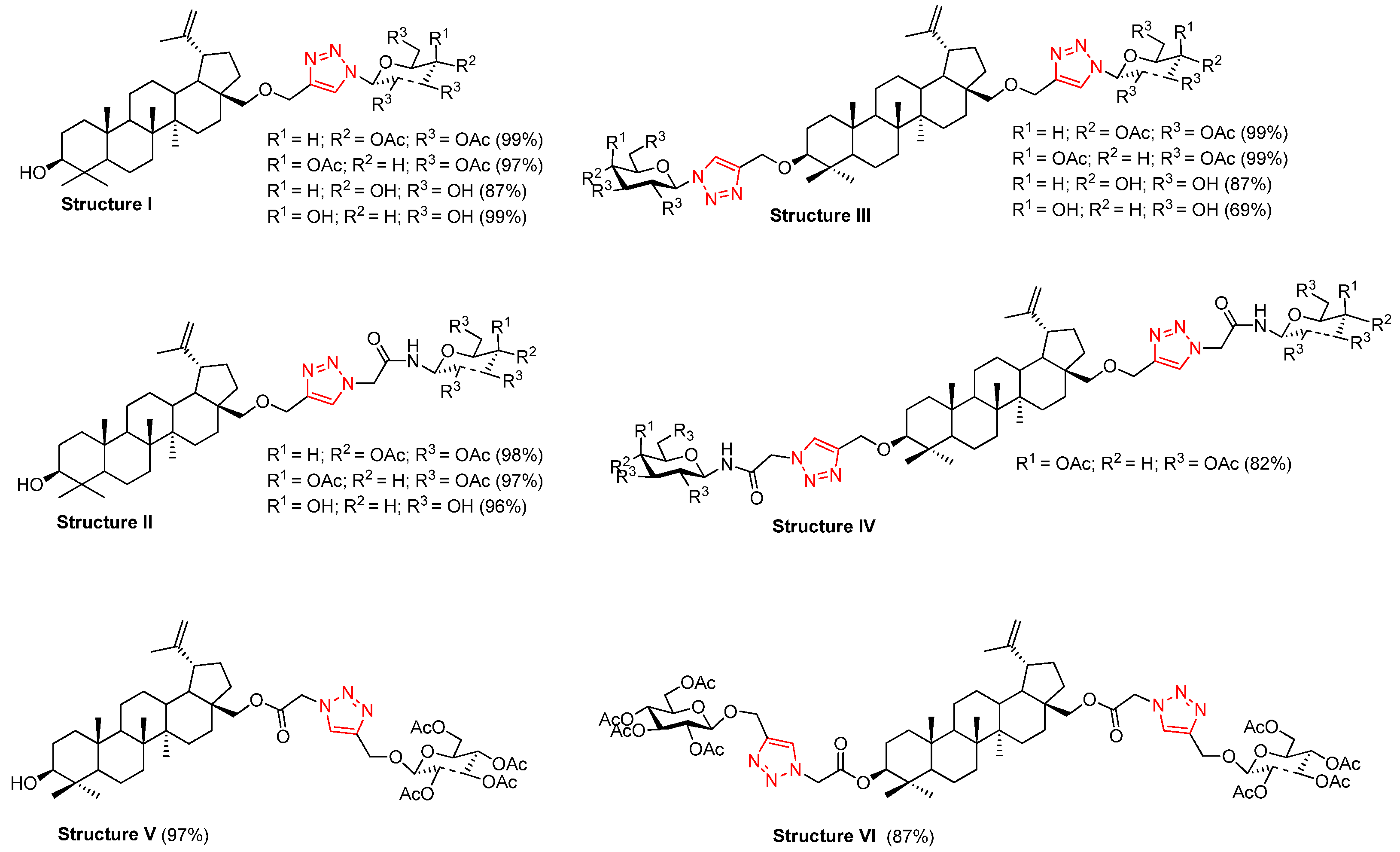
3.3. Synthesis of Betulin Glycoconjugates via Click Chemistry (5–8)
3.4. Synthesis of Metabolite (9a)
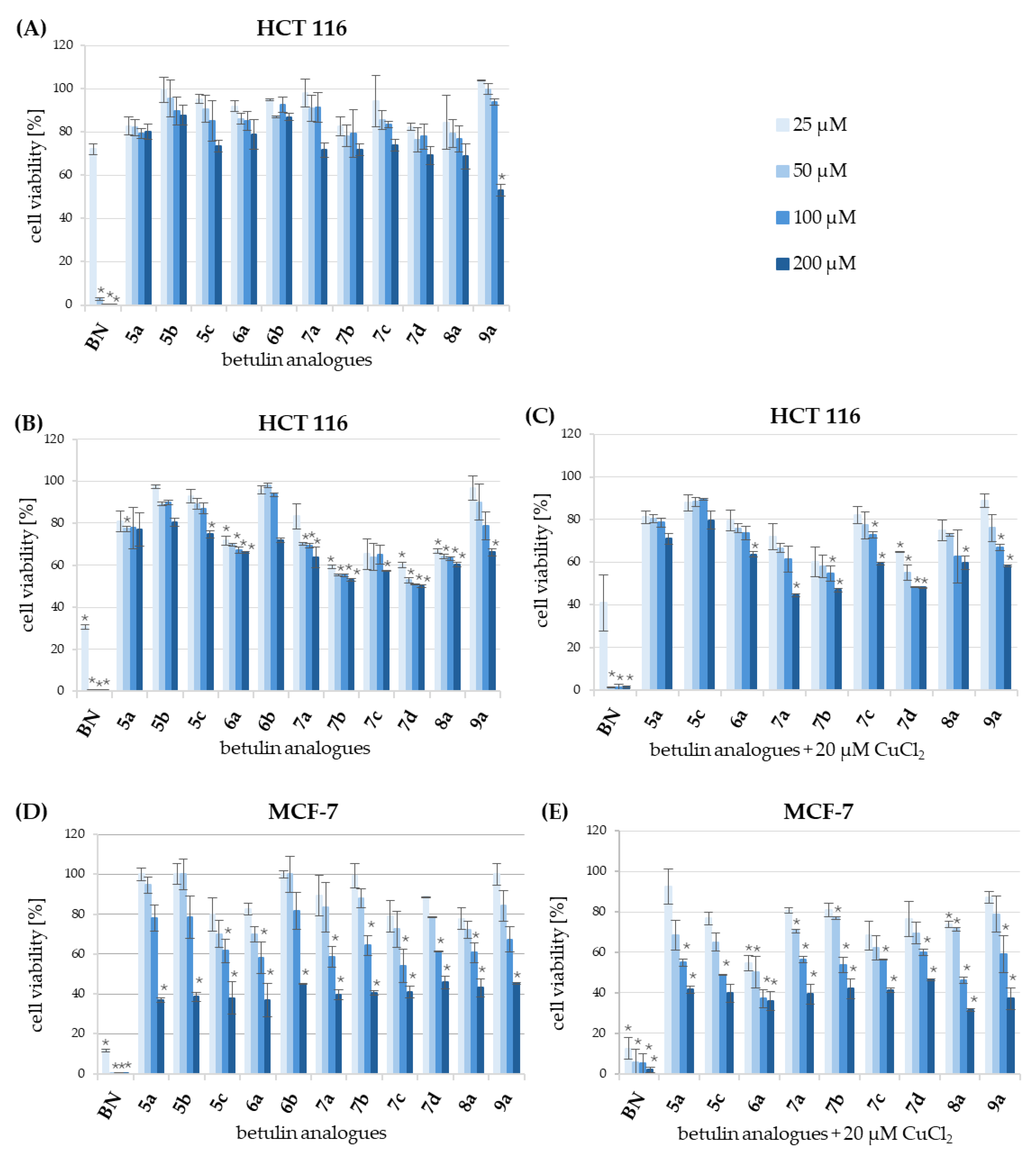
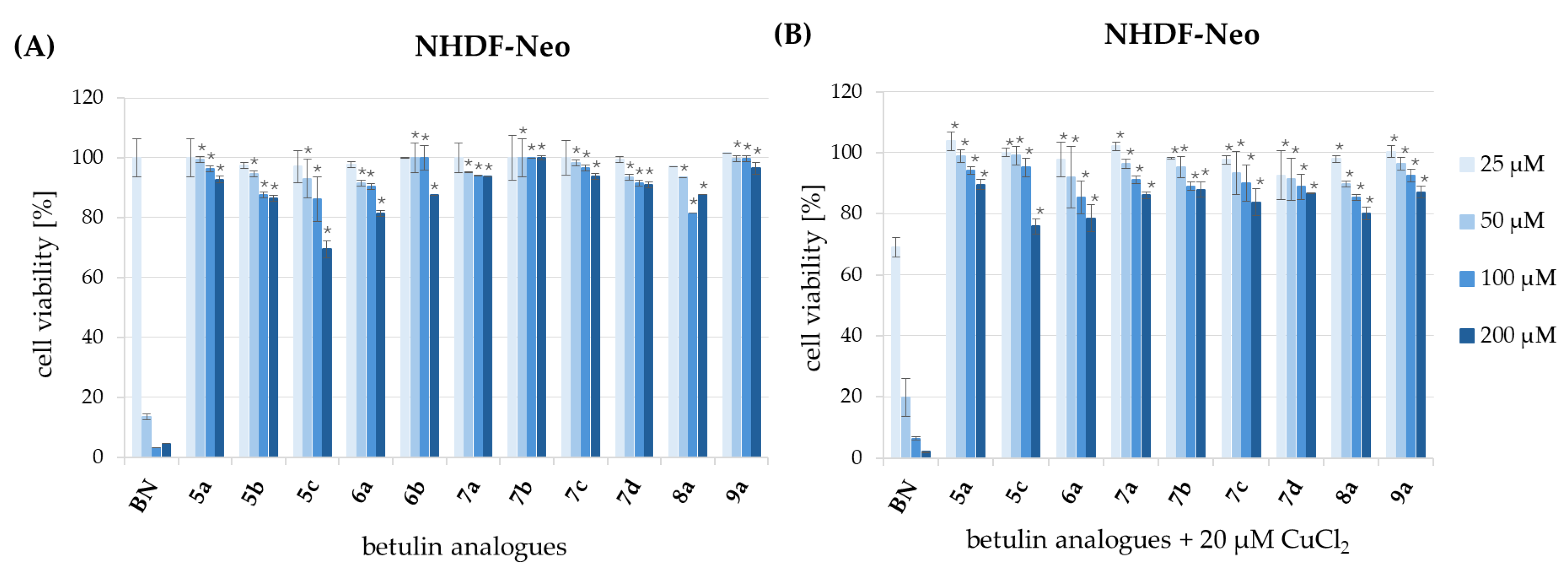
3.5. Evaluation of Cytotoxicity
3.6. Molecular Descriptors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adepoju, F.O.; Duru, K.C.; Li, E.; Kovaleva, E.G.; Tsurkan, M.V. Pharmacological Potential of Betulin as a Multitarget Compound. Biomolecules 2023, 13, 1105. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and Its Derivatives as Novel Compounds with Different Pharmacological Effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef] [PubMed]
- Jonnalagadda, S.C.; Suman, P.; Morgan, D.C.; Seay, J.N. Recent Developments on the Synthesis and Applications of Betulin and Betulinic Acid Derivatives as Therapeutic Agents. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 53, pp. 45–84. ISBN 978-0-444-63930-1. [Google Scholar]
- Martin, H.; Lázaro, L.R.; Gunnlaugsson, T.; Scanlan, E.M. Glycosidase Activated Prodrugs for Targeted Cancer Therapy. Chem. Soc. Rev. 2022, 51, 9694–9716. [Google Scholar] [CrossRef] [PubMed]
- Pastuch-Gawołek, G.; Szreder, J.; Domińska, M.; Pielok, M.; Cichy, P.; Grymel, M. A Small Sugar Molecule with Huge Potential in Targeted Cancer Therapy. Pharmaceutics 2023, 15, 913. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, W.; Garcia-Prieto, C.; Huang, P. The Warburg Effect and Its Cancer Therapeutic Implications. J. Bioenerg. Biomembr. 2007, 39, 267–274. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Pliszka, M.; Szablewski, L. Glucose Transporters as a Target for Anticancer Therapy. Cancers 2021, 13, 4184. [Google Scholar] [CrossRef]
- Ancey, P.; Contat, C.; Meylan, E. Glucose Transporters in Cancer—From Tumor Cells to the Tumor Microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.B.; Figueroa, A.; Pulido, E.G.; Campelo, R.G.; Aparicio, L.A. Potential Role of Sugar Transporters in Cancer and Their Relationship with Anticancer Therapy. Int. J. Endocrinol. 2010, 2010, 205357. [Google Scholar] [CrossRef]
- Sakashita, M.; Aoyama, N.; Minami, R.; Maekawa, S.; Kuroda, K.; Shirasaka, D.; Ichihara, T.; Kuroda, Y.; Maeda, S.; Kasuga, M. Glut1 Expression in T1 and T2 Stage Colorectal Carcinomas. Eur. J. Cancer 2001, 37, 204–209. [Google Scholar] [CrossRef]
- Grover-McKay, M.; Walsh, S.A.; Seftor, E.A.; Thomas, P.A.; Hendrix, M.J. Role for Glucose Transporter 1 Protein in Human Breast Cancer. Pathol. Oncol. Res. 1998, 4, 115–120. [Google Scholar] [CrossRef]
- Wu, M.; Neilson, A.; Swift, A.L.; Moran, R.; Tamagnine, J.; Parslow, D.; Armistead, S.; Lemire, K.; Orrell, J.; Teich, J.; et al. Multiparameter Metabolic Analysis Reveals a Close Link between Attenuated Mitochondrial Bioenergetic Function and Enhanced Glycolysis Dependency in Human Tumor Cells. Am. J. Physiol. Cell Physiol. 2007, 292, C125–C136. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-H.; Jan, H.-J.; Liu, L.-W.; Lee, C.-C.; Wang, S.-G.; Hueng, D.-Y.; Cheng, Y.-Y.; Lee, H.-M.; Ma, H.-I. Nodal Regulates Energy Metabolism in Glioma Cells by Inducing Expression of Hypoxia-Inducible Factor 1. Neuro-Oncol. 2013, 15, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, D. Glufosfamide: Can We Improve the Process of Anticancer Agent Development? Expert Opin. Investig. Drugs 2012, 21, 749–754. [Google Scholar] [CrossRef]
- Porter, J.; Noble, A.R.; Signoret, N.; Fascione, M.A.; Miller, G.J. Exploring a Gemcitabine-Glucose Hybrid as a Glycoconjugate Prodrug. ACS Omega 2024, 9, 31703–31713. [Google Scholar] [CrossRef]
- Vaidya, S.P.; Patra, M. Platinum Glycoconjugates: “Sweet Bullets” for Targeted Cancer Therapy? Curr. Opin. Chem. Biol. 2023, 72, 102236. [Google Scholar] [CrossRef]
- Iorio, A.L.; Lenci, E.; Marzano, C.; Bucaletti, E.; Tirinnanzi, B.; Casati, G.; Giunti, L.; Dallari, C.; Credi, C.; Sardi, I.; et al. Oxime Linked Doxorubicin Glycoconjugates Improve the Specific Targeting of Glioblastoma in High-Grade Glioma Therapy. ACS Med. Chem. Lett. 2024, 15, 1953–1960. [Google Scholar] [CrossRef]
- Meng, X.; Lian, X.; Li, X.; Ya, Q.; Li, T.; Zhang, Y.; Yang, Y.; Zhang, Y. Synthesis of 2′-Paclitaxel 2-Deoxy-2-Fluoro-Glucopyranosyl Carbonate for Specific Targeted Delivery to Cancer Cells. Carbohydr. Res. 2020, 493, 108034. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals: Miniperspective. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar Jain, S. A Comprehensive Review of N-Heterocycles as Cytotoxic Agents. Curr. Med. Chem. 2016, 23, 4338–4394. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef] [PubMed]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal Attributes of 1,2,3-Triazoles: Current Developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Da S. M. Forezi, L.; Lima, C.G.S.; Amaral, A.A.P.; Ferreira, P.G.; De Souza, M.C.B.V.; Cunha, A.C.; De C. Da Silva, F.; Ferreira, V.F. Bioactive 1,2,3-Triazoles: An Account on Their Synthesis, Structural Diversity and Biological Applications. Chem. Rec. 2021, 21, 2782–2807. [Google Scholar] [CrossRef]
- Markham, A. Savolitinib: First Approval. Drugs 2021, 81, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Yamansarov, E.Y.; Lopatukhina, E.V.; Evteev, S.A.; Skvortsov, D.A.; Lopukhov, A.V.; Kovalev, S.V.; Vaneev, A.N.; Shkil’, D.O.; Akasov, R.A.; Lobov, A.N.; et al. Discovery of Bivalent GalNAc-Conjugated Betulin as a Potent ASGPR-Directed Agent against Hepatocellular Carcinoma. Bioconjugate Chem. 2021, 32, 763–781. [Google Scholar] [CrossRef]
- Bori, I.D.; Hung, H.-Y.; Qian, K.; Chen, C.-H.; Morris-Natschke, S.L.; Lee, K.-H. Anti-AIDS Agents 88. Anti-HIV Conjugates of Betulin and Betulinic Acid with AZT Prepared via Click Chemistry. Tetrahedron Lett. 2012, 53, 1987–1989. [Google Scholar] [CrossRef]
- Grymel, M.; Pastuch-Gawołek, G.; Lalik, A.; Zawojak, M.; Boczek, S.; Krawczyk, M.; Erfurt, K. Glycoconjugation of Betulin Derivatives Using Copper-Catalyzed 1,3-Dipolar Azido-Alkyne Cycloaddition Reaction and a Preliminary Assay of Cytotoxicity of the Obtained Compounds. Molecules 2020, 25, 6019. [Google Scholar] [CrossRef]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef]
- Shi, W.; Tang, N.; Yan, W.-D. Synthesis and Cytotoxicity of Triterpenoids Derived from Betulin and Betulinic Acid via Click Chemistry. J. Asian Nat. Prod. Res. 2015, 17, 159–169. [Google Scholar] [CrossRef]
- Antimonova, A.N.; Petrenko, N.I.; Shakirov, M.M.; Rybalova, T.V.; Frolova, T.S.; Shul’ts, E.E.; Kukina, T.P.; Sinitsyna, O.I.; Tolstikov, G.A. Synthesis and Study of Mutagenic Properties of Lupane Triterpenoids Containing 1,2,3-Triazole Fragments in the C-30 Position. Chem. Nat. Compd. 2013, 49, 657–664. [Google Scholar] [CrossRef]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Jastrzębska, M.; Książek, M. Synthesis and Structural Characterization of a New 1,2,3-Triazole Derivative of Pentacyclic Triterpene. Crystals 2022, 12, 422. [Google Scholar] [CrossRef]
- Khalil, M.M.; Radalla, A.M.; Abd Elnaby, N.M. Solution Equilibria and Thermodynamic Studies of Complexation of Divalent Transition Metal Ions with Some Triazoles and Biologically Important Aliphatic Dicarboxylic Acids in Aqueous Media. J. Solut. Chem. 2013, 42, 1123–1145. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatent. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting Copper in Cancer Therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Dufour, P.; Pichette, A. Synthesis and Structure–Activity Relationship Study of Cytotoxic Germanicane- and Lupane-Type 3β-O-Monodesmosidic Saponins Starting from Betulin. Bioorg. Med. Chem. 2007, 15, 6144–6157. [Google Scholar] [CrossRef]
- Grymel, M.; Lalik, A.; Kazek-Kęsik, A.; Szewczyk, M.; Grabiec, P.; Erfurt, K. Design, Synthesis and Preliminary Evaluation of the Cytotoxicity and Antibacterial Activity of Novel Triphenylphosphonium Derivatives of Betulin. Molecules 2022, 27, 5156. [Google Scholar] [CrossRef]
- Domińska, M.; Pastuch-Gawołek, G.; Skonieczna, M.; Szeja, W.; Domiński, A.; Kurcok, P. Glycoconjugation of Quinoline Derivatives Using the C-6 Position in Sugars as a Strategy for Improving the Selectivity and Cytotoxicity of Functionalized Compounds. Molecules 2022, 27, 6918. [Google Scholar] [CrossRef]
- Le Roux, A.; Meunier, S.; Le Gall, T.; Denis, J.; Bischoff, P.; Wagner, A. Synthesis and Radioprotective Properties of Pulvinic Acid Derivatives. ChemMedChem 2011, 6, 561–569. [Google Scholar] [CrossRef]
- Ibatullin, F.M.; Shabalin, K.A. A Simple and Convenient Synthesis of Glycosyl Azides. Synth. Commun. 2000, 30, 2819–2823. [Google Scholar] [CrossRef]
- Brzuska, G.; Pastuch-Gawolek, G.; Krawczyk, M.; Szewczyk, B.; Krol, E. Anti-Tick-Borne Encephalitis Virus Activity of Novel Uridine Glycoconjugates Containing Amide or/and 1,2,3-Triazole Moiety in the Linker Structure. Pharmaceuticals 2020, 13, 460. [Google Scholar] [CrossRef]
- Pastuch-Gawołek, G.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Musioł, M.; Serda, M.; Czaplinska, B.; Musiol, R. Small Molecule Glycoconjugates with Anticancer Activity. Eur. J. Med. Chem. 2016, 112, 130–144. [Google Scholar] [CrossRef]
- Joosten, J.A.F.; Loimaranta, V.; Appeldoorn, C.C.M.; Haataja, S.; El Maate, F.A.; Liskamp, R.M.J.; Finne, J.; Pieters, R.J. Inhibition of Streptococcus s Uis Adhesion by Dendritic Galabiose Compounds at Low Nanomolar Concentration. J. Med. Chem. 2004, 47, 6499–6508. [Google Scholar] [CrossRef]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose Conjugation for the Specific Targeting and Treatment of Cancer. Chem. Sci. 2013, 4, 2319. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, J.; Seeberger, P.H.; Yin, J. Glycoconjugates for Glucose Transporter-Mediated Cancer-Specific Targeting and Treatment. Carbohydr. Res. 2020, 498, 108195. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Andreana, P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef]
- Barnett, J.E.G.; Holman, G.D.; Munday, K.A. Structural Requirements for Binding to the Sugar-Transport System of the Human Erythrocyte. Biochem. J. 1973, 131, 211–221. [Google Scholar] [CrossRef]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A Potent Glucose–Platinum Conjugate Exploits Glucose Transporters and Preferentially Accumulates in Cancer Cells. Angew. Chem. Int. Ed. 2016, 55, 2550–2554. [Google Scholar] [CrossRef]
- Tanasova, M.; Fedie, J.R. Molecular Tools for Facilitative Carbohydrate Transporters (Gluts). ChemBioChem 2017, 18, 1774–1788. [Google Scholar] [CrossRef]
- Deng, D.; Xu, C.; Sun, P.; Wu, J.; Yan, C.; Hu, M.; Yan, N. Crystal Structure of the Human Glucose Transporter GLUT1. Nature 2014, 510, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wen, J.; Tian, T.; Lu, Z.; Wang, Y.; Wang, Z.; Wang, X.; Yang, Y. GLUT-1 Overexpression as an Unfavorable Prognostic Biomarker in Patients with Colorectal Cancer. Oncotarget 2017, 8, 11788–11796. [Google Scholar] [CrossRef]
- Shin, E.; Koo, J.S. Glucose Metabolism and Glucose Transporters in Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 728759. [Google Scholar] [CrossRef] [PubMed]
- Laudański, P.; Swiatecka, J.; Kovalchuk, O.; Wołczyński, S. Expression of GLUT1 Gene in Breast Cancer Cell Lines MCF-7 and MDA-MB-231. Ginekol. Pol. 2003, 74, 782–785. [Google Scholar]
- O’Connor, P.M.; Jackman, J.; Bae, I.; Myers, T.G.; Fan, S.; Mutoh, M.; Scudiero, D.A.; Monks, A.; Sausville, E.A.; Weinstein, J.N.; et al. Characterization of the P53 Tumor Suppressor Pathway in Cell Lines of the National Cancer Institute Anticancer Drug Screen and Correlations with the Growth-Inhibitory Potency of 123 Anticancer Agents. Cancer Res. 1997, 57, 4285–4300. [Google Scholar]
- Koygun, G.; Arslan, E.; Zengin, G.; Orlando, G.; Ferrante, C. Comparison of Anticancer Activity of Dorycnium Pentaphyllum Extract on MCF-7 and MCF-12A Cell Line: Correlation with Invasion and Adhesion. Biomolecules 2021, 11, 671. [Google Scholar] [CrossRef]
- Larsson, S.; Rydén, T.; Holst, U.; Oredsson, S.; Johansson, M. Estimating the Total Rate of DNA Replication Using Branching Processes. Bull. Math. Biol. 2008, 70, 2177–2194. [Google Scholar] [CrossRef]
- Feng, L.; Dong, Z.; Tao, D.; Zhang, Y.; Liu, Z. The Acidic Tumor Microenvironment: A Target for Smart Cancer Nano-Theranostics. Natl. Sci. Rev. 2018, 5, 269–286. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic Extracellular Microenvironment and Cancer. Cancer Cell. Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Hu, K.; Guo, J.; Zeng, J.; Shao, Y.; Wu, B.; Mo, J.; Mo, G. Current State of Research on Copper Complexes in the Treatment of Breast Cancer. Open Life Sci. 2024, 19, 20220840. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Zhao, L.; Shi, X.; Ma, X.; Chen, Z. Copper in Cancer: From Pathogenesis to Therapy. Biomed. Pharmacother. 2023, 163, 114791. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Martel, F. Targeting Glucose Transporters for Breast Cancer Therapy: The Effect of Natural and Synthetic Compounds. Cancers 2020, 12, 154. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef]
- Price, E.; Weinheimer, M.; Rivkin, A.; Jenkins, G.; Nijsen, M.; Cox, P.B.; DeGoey, D. Beyond Rule of Five and PROTACs in Modern Drug Discovery: Polarity Reducers, Chameleonicity, and the Evolving Physicochemical Landscape. J. Med. Chem. 2024, 67, 5683–5698. [Google Scholar] [CrossRef]



| BN Analogue | Sugar | Product | HRMS ([M+Na]+) | |||||
|---|---|---|---|---|---|---|---|---|
| No. | R1 | R2 | Yield (%) | Summary Formula | Calc. | Found. | ||
| 3a | 4a | 5a | OAc | OAc | 72 | C53H75N3O15 | 1018.5252 | 1018.5353 |
| 3a | 4b | 5b | OAc | OBn | 60 | C73H93N3O11 | 1210.6708 | 1210.7081 |
| 3b | 4a | 5c | OH | OAc | 64 | C51H75N3O14 | 976.5147 | 976.5180 |
| 3a | 4c | 6a | OAc | OAc | 71 | C53H75N3O15 | 1018.5252 | 1018.5360 |
| 3a | 4d | 6b | OAc | OBn | 53 | C73H93N3O11 | 1210.6708 | 1210.6998 |
| 3a | 4f | 7a | OAc | OAc | 73 | C55H81N3O16 | 1062.5515 | 1062.5526 |
| 3a | 4g | 7b | OAc | OAc | 69 | C56H83N3O16 | 1076.5671 | 1076.5715 |
| 3b | 4f | 7c | OH | OAc | 59 | C53H79N3O15 | 1020.5409 | 1020.5486 |
| 3b | 4g | 7d | OH | OAc | 70 | C54H81N3O15 | 1034.5565 | 1034.5626 |
| 3a | 4h | 8a | OAc | OAc | 12 | C53H75N3O15 | 1018.5252 | 1018.5356 |
| 3a | 2-azidoethanol | 9a | OAc | - | 32 | C41H63N3O7 | 732.4564 | 732.4573 |
| Compound | HCT 116 | MCF-7 | NHDF-Neo | |||
|---|---|---|---|---|---|---|
| IC50 [µM] b | IC50 [µM] c | SI | IC50 [µM] d | SI | IC50 [µM] b | |
| BN | 34.88 ± 4.26 | 25.96 ± 5.40 | 1.3 | 17.89 ± 1.06 | 1.9 | 34.90 ± 8.36 |
| BN + Cu2+ e | - | 20.76 ± 2.71 | 1.6 | 13.91 ± 2.35 | 2.4 | 33.34 ± 5.97 |
| 5a | >200 | >200 | - | 163.12 ± 7.65 | >1.2 | >200 |
| 5a + Cu2+ e | - | >200 | - | 108.51 ± 5.85 | >1.8 | >200 |
| 5b | >200 | >200 | - | 167.61 ± 11.66 | >1.2 | >200 |
| 5c | >200 | >200 | - | 128.07 ± 1.35 | >1.6 | >200 |
| 5c + Cu2+ e | - | >200 | - | 70.71 ± 5.44 | >2.8 | >200 |
| 6a | >200 | >200 | - | 122.61 ± 6.84 | >1.6 | >200 |
| 6a + Cu2+ e | - | >200 | - | 59.81 ± 1.85 | >3.3 | >200 |
| 6b | >200 | >200 | - | 184.13 ± 7.59 | >1.1 | >200 |
| 7a | >200 | >200 | -- | 144.12 ± 4.70 | >1.4 | >200 |
| 7a + Cu2+ e | - | 150.08 ± 8.01 | >1.3 | 135.01 ± 10.23 | >1.5 | >200 |
| 7b | >200 | >200 | 152.23 ± 2.91 | >1.3 | >200 | |
| 7b + Cu2+ e | - | 112.48 ± 0.57 | >1.8 | 124.29 ± 1.64 | >1.6 | >200 |
| 7c | >200 | >200 | - | 134.92 ± 7.92 | >1.5 | >200 |
| 7c + Cu2+ e | - | >200 | - | 117.94 ± 1.42 | >1.7 | >200 |
| 7d | >200 | 131.16 ± 8.76 | >1.5 | 165.02 ± 8.32 | >1.2 | >200 |
| 7d + Cu2+ e | - | 60.40 ± 6.95 | >3.3 | 147.02 ± 2.32 | >1.4 | >200 |
| 8a | >200 | >200 | - | 152.63 ± 1.98 | >1.3 | >200 |
| 8a + Cu2+ e | - | >200 | - | 88.33 ± 3.31 | >2.3 | >200 |
| 9a | >200 | >200 | 147.56 ± 8.71 | >1.4 | >200 | |
| 9a + Cu2+ e | >200 | >200 | 138.8 ± 6.21 | >1,4 | >200 | |
| Doxorubicin | 13.17 ± 0.58 | 1.89 ± 0.19 | >10.6 | 0.29 ± 0.04 | >70 | >20 |
| Structure | pLogP | TPSA (Å2) | MW (g/mol) | Vol (Å3) | nON | nOHNH | nrotb |
|---|---|---|---|---|---|---|---|
| 5a | 7.96 | 224.09 | 996.21 | 930.36 | 18 | 0 | 22 |
| 5b | 9.94 | 155.80 | 1188.56 | 1141.03 | 14 | 0 | 26 |
| 5c | 7.25 | 218.01 | 954.17 | 893.85 | 17 | 1 | 20 |
| 6a | 7.96 | 224.09 | 996.21 | 930.36 | 18 | 0 | 22 |
| 6b | 9.94 | 155.80 | 1188.56 | 1141.03 | 14 | 0 | 26 |
| 7a | 7.94 | 233.32 | 1040.26 | 972.95 | 19 | 0 | 25 |
| 7b | 8.19 | 233.32 | 1054.29 | 989.75 | 19 | 0 | 26 |
| 7c | 7.24 | 227.24 | 998.22 | 936.44 | 18 | 1 | 23 |
| 7d | 7.51 | 227.24 | 1012.25 | 953.24 | 18 | 1 | 24 |
| 8a | 7.96 | 224.09 | 996.21 | 930.36 | 18 | 0 | 22 |
| 9a | 7.27 | 129.86 | 709.97 | 694.78 | 10 | 1 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szreder, J.; Woźniak, K.; Erfurt, K.; Grymel, M.; Pastuch-Gawołek, G. Synthesis and Preliminary Cytotoxicity Evaluation of 3-Lup-20(29)-Ene-3β,28-Diol Glycoconjugates Containing a Succinic Linker and a 1,2,3-Triazole Ring. Cancers 2025, 17, 1737. https://doi.org/10.3390/cancers17111737
Szreder J, Woźniak K, Erfurt K, Grymel M, Pastuch-Gawołek G. Synthesis and Preliminary Cytotoxicity Evaluation of 3-Lup-20(29)-Ene-3β,28-Diol Glycoconjugates Containing a Succinic Linker and a 1,2,3-Triazole Ring. Cancers. 2025; 17(11):1737. https://doi.org/10.3390/cancers17111737
Chicago/Turabian StyleSzreder, Julia, Klaudia Woźniak, Karol Erfurt, Mirosława Grymel, and Gabriela Pastuch-Gawołek. 2025. "Synthesis and Preliminary Cytotoxicity Evaluation of 3-Lup-20(29)-Ene-3β,28-Diol Glycoconjugates Containing a Succinic Linker and a 1,2,3-Triazole Ring" Cancers 17, no. 11: 1737. https://doi.org/10.3390/cancers17111737
APA StyleSzreder, J., Woźniak, K., Erfurt, K., Grymel, M., & Pastuch-Gawołek, G. (2025). Synthesis and Preliminary Cytotoxicity Evaluation of 3-Lup-20(29)-Ene-3β,28-Diol Glycoconjugates Containing a Succinic Linker and a 1,2,3-Triazole Ring. Cancers, 17(11), 1737. https://doi.org/10.3390/cancers17111737








