DWI in the Differentiation of Malignant and Benign Breast Lesions Presenting with Non-Mass Enhancement on CE-MRI
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
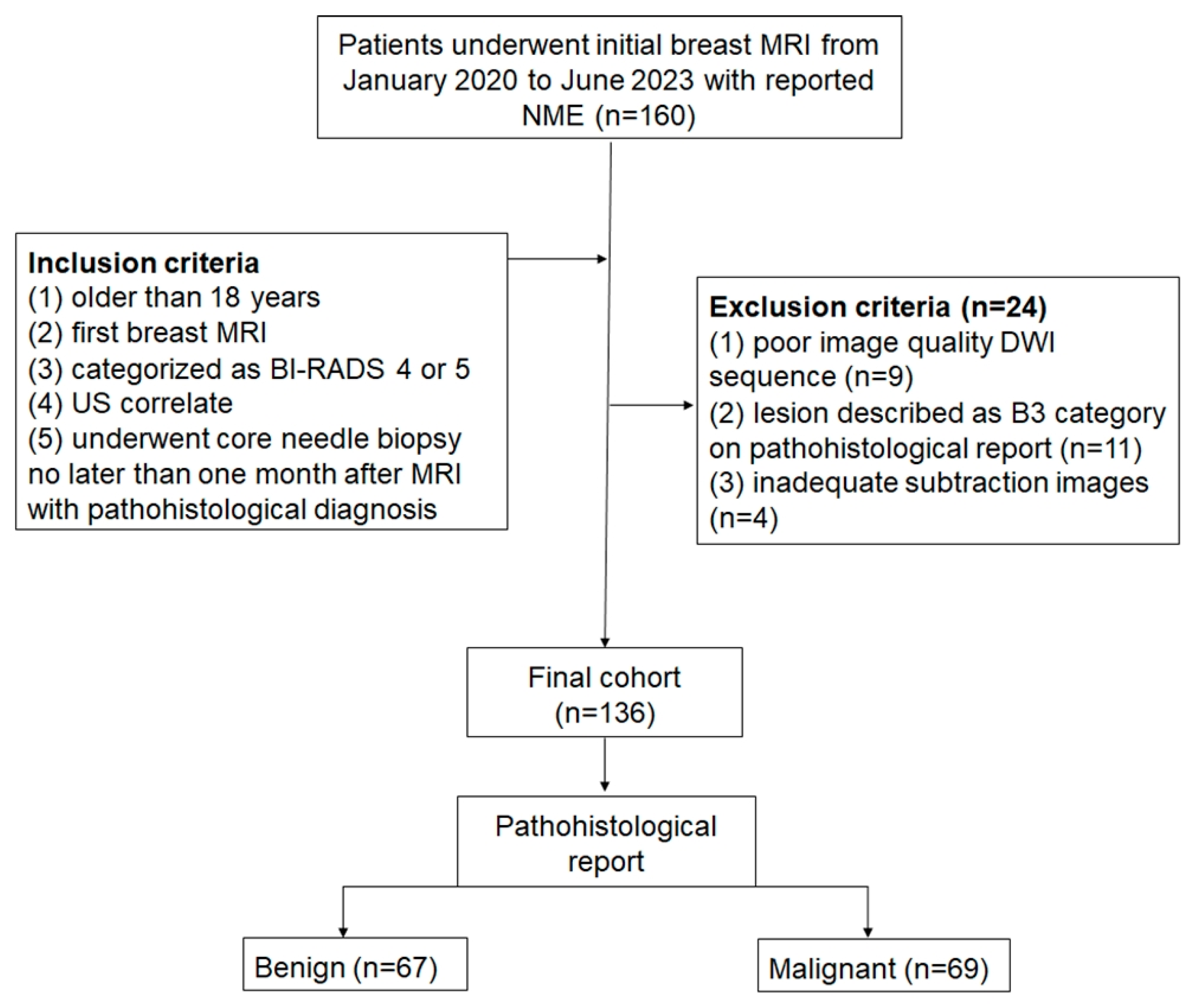
2.1. Patients
2.2. MR Scanning Protocol
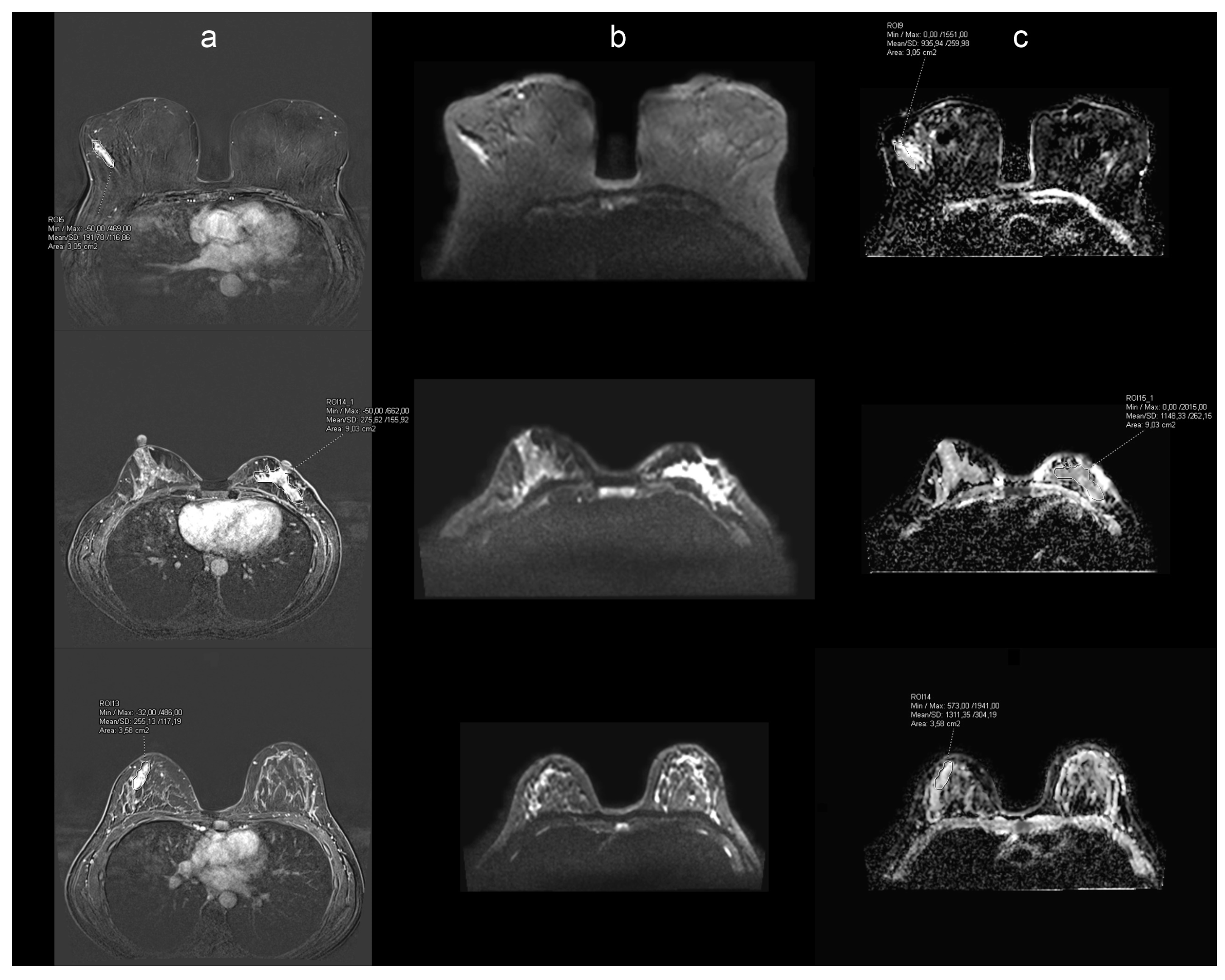
2.3. Image Evaluation and Histopathology
2.4. Statistical Analysis
3. Results
3.1. Patients and Lesion Characteristics
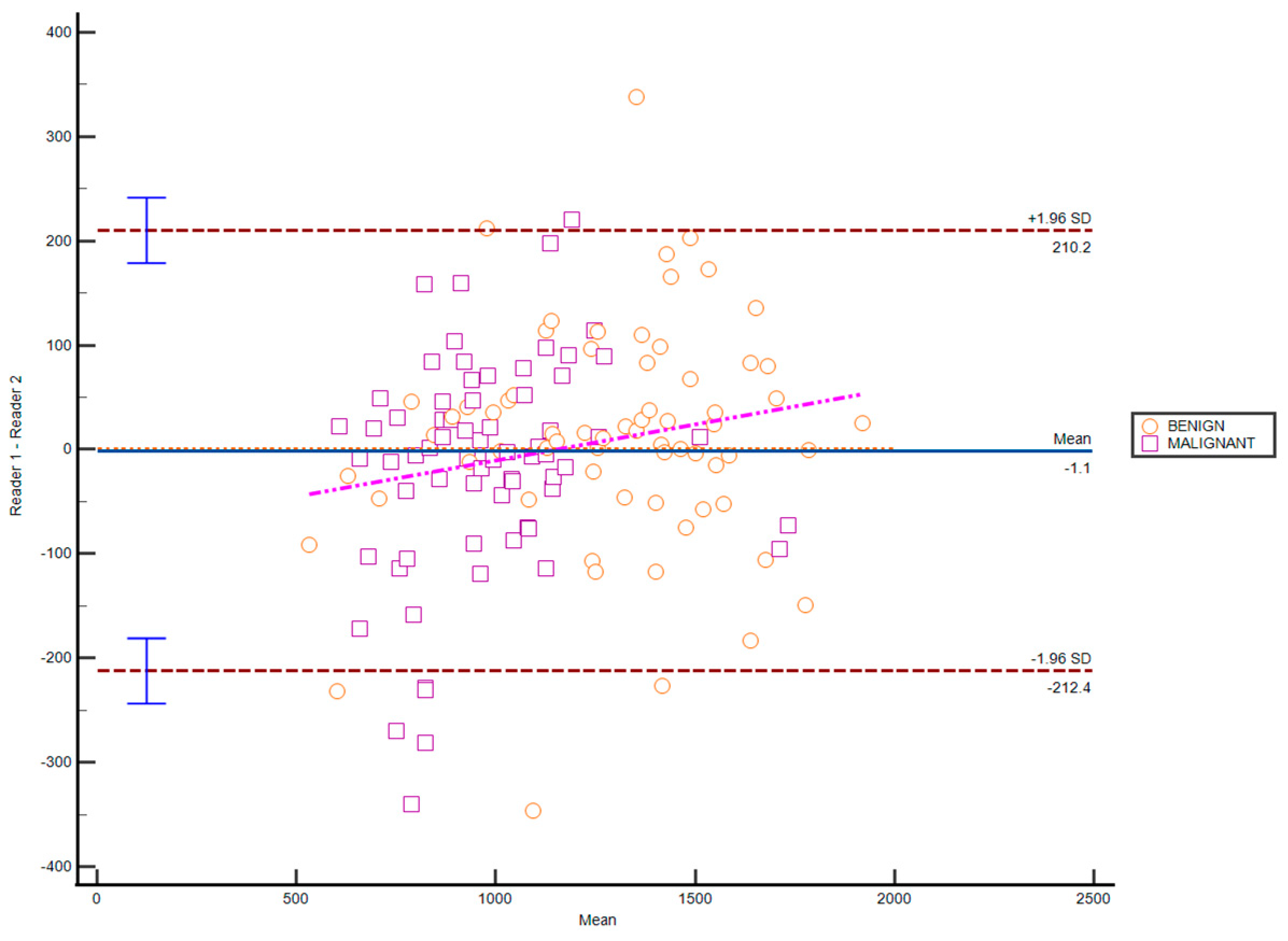
3.2. Inter-Observer Agreement
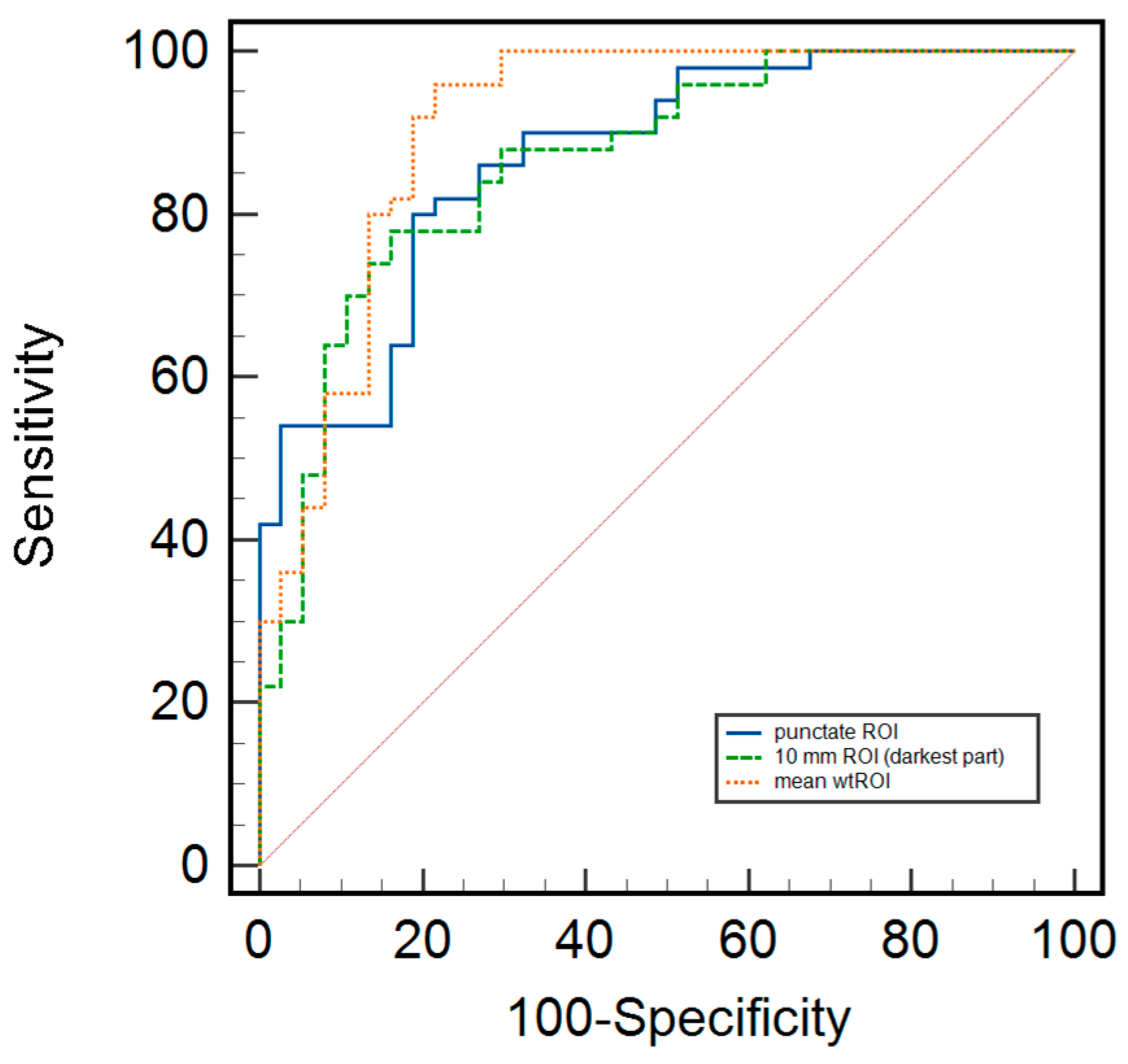
3.3. Differentiation of Benign and Malignant Breast Lesions, Including Differences Related to Breast Composition Using Different Measurement Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.E.; Clauser, P.; Gilbert, F.J.; Martincich, L.; Partridge, S.C.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast—A consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2020, 30, 1436–1450. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, A.; Dietzel, M.; Kaiser, C.G.; Baltzer, P.A. Combined reading of Contrast Enhanced and Diffusion Weighted Magnetic Resonance Imaging by using a simple sum score. Eur. Radiol. 2016, 26, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Pinker, K.; Bickel, H.; Helbich, T.H.; Gruber, S.; Dubsky, P.; Pluschnig, U.; Rudas, M.; Bago-Horvath, Z.; Weber, M.; Trattnig, S.; et al. Combined contrast-enhanced magnetic resonance and diffusion-weighted imaging reading adapted to the “Breast Imaging Reporting and Data System” for multiparametric 3-T imaging of breast lesions. Eur. Radiol. 2013, 23, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, R.; Matsunaga, K.; Iwabuchi, K.; Kan, S.; Hata, H.; Kuranami, M.; Watanabe, M.; Hayakawa, K. Diffusion-weighted imaging of malignant breast tumors: The usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J. Comput. Assist. Tomogr. 2005, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Kul, S.; Eyuboglu, I.; Cansu, A.; Alhan, E. Diagnostic efficacy of the diffusion weighted imaging in the characterization of different types of breast lesions. J. Magn. Reson. Imaging 2014, 40, 1158–1164. [Google Scholar] [CrossRef]
- Tsvetkova, S.; Doykova, K.; Vasilska, A.; Sapunarova, K.; Doykov, D.; Andonov, V.; Uchikov, P. Differentiation of Benign and Malignant Breast Lesions Using ADC Values and ADC Ratio in Breast MRI. Diagnostics 2022, 12, 332. [Google Scholar] [CrossRef]
- Avendano, D.; Marino, M.A.; Leithner, D.; Thakur, S.; Bernard-Davila, B.; Martinez, D.F.; Helbich, T.H.; Morris, E.A.; Jochelson, M.S.; Baltzer, P.A.T.; et al. Limited role of DWI with apparent diffusion coefficient mapping in breast lesions presenting as non-mass enhancement on dynamic contrast-enhanced MRI. Breast Cancer Res. 2019, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.A.; Avendano, D.; Sevilimedu, V.; Thakur, S.; Martinez, D.; Lo Gullo, R.; Horvat, J.V.; Helbich, T.H.; Baltzer, P.A.T.; Pinker, K. Limited value of multiparametric MRI with dynamic contrast-enhanced and diffusion-weighted imaging in non-mass enhancing breast tumors. Eur. J. Radiol. 2022, 156, 110523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Yang, Z.; Fan, C.; Qin, Y.; Tang, C.; Yin, T.; Ai, T.; Xia, L. Contrasts Between Diffusion-Weighted Imaging and Dynamic Contrast-Enhanced MR in Diagnosing Malignancies of Breast Nonmass Enhancement Lesions Based on Morphologic Assessment. J. Magn. Reson. Imaging 2023, 58, 963–974. [Google Scholar] [CrossRef]
- Wielema, M.; Dorrius, M.D.; Pijnappel, R.M.; De Bock, G.H.; Baltzer, P.A.T.; Oudkerk, M.; Sijens, P.E. Diagnostic performance of breast tumor tissue selection in diffusion weighted imaging: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232856. [Google Scholar] [CrossRef]
- Gity, M.; Moradi, B.; Arami, R.; Arabkheradmand, A.; Kazemi, M.A. Two Different Methods of Region-of-Interest Placement for Differentiation of Benign and Malignant Breast Lesions by Apparent Diffusion Coefficient Value. Asian Pac. J. Cancer Prev. 2018, 19, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Gullo, R.L.; Partridge, S.C.; Shin, H.J.; Thakur, S.B.; Pinker, K. Update on DWI for Breast Cancer Diagnosis and Treatment Monitoring. AJR Am. J. Roentgenol. 2024, 222, e2329933. [Google Scholar] [CrossRef] [PubMed]
- Duran, B.; Ucpinar, B.A. Four Different Apparent Diffusion Coefficient Measurement Methods in Breast Masses. J. Coll. Physicians Surg. Pak. 2021, 31, 1024–1029. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, B.; Liu, H.; Wang, D.; Gu, Y. Feasibility study of dual parametric 2D histogram analysis of breast lesions with dynamic contrast-enhanced and diffusion-weighted MRI. J. Transl. Med. 2018, 16, 325. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Singer, L.; Sun, R.; Wilmes, L.J.; Klifa, C.S.; Lehman, C.D.; Hylton, N.M. Diffusion-weighted MRI: Influence of intravoxel fat signal and breast density on breast tumor conspicuity and apparent diffusion coefficient measurements. Magn. Reson. Imaging 2011, 29, 1215–1221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, G.; Li, Y.; Chen, S.L.; Chen, Q. Non-mass enhancement breast lesions: MRI findings and associations with malignancy. Ann Transl. Med. 2022, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Iacconi, C.; Thakur, S.B.; Dershaw, D.D.; Brooks, J.; Fry, C.W.; Morris, E.A. Impact of fibroglandular tissue and background parenchymal enhancement on diffusion weighted imaging of breast lesions. Eur. J. Radiol. 2014, 83, 2137–2143. [Google Scholar] [CrossRef]
- Wielema, M.; Sijens, P.E.; Pijnappel, R.M.; De Bock, G.H.; Zorgdrager, M.; Kok, M.G.J.; Rainer, E.; Varga, R.; Clauser, P.; Oudkerk, M.; et al. Image quality of DWI at breast MRI depends on the amount of fibroglandular tissue: Implications for unenhanced screening. Eur. Radiol. 2024, 34, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.H.; Lim, A.S.; Thike, A.A.; Tien, S.L.; Tan, P.H. Implications of the Updated 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations on Human Epidermal Growth Factor Receptor 2 Gene Testing Using Immunohistochemistry and Fluorescence In Situ Hybridization for Breast Cancer. Arch. Pathol. Lab. Med. 2016, 140, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Overcast, W.B.; Zhang, J.; Zynger, D.L.; Tozbikian, G.H. Impact of the 2013 ASCO/CAP HER2 revised guidelines on HER2 results in breast core biopsies with invasive breast carcinoma: A retrospective study. Virchows Arch. 2016, 469, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Marija, C.; Kresimir, D.; Ognjen, B.; Iva, P.; Nenad, K.; Matija, B. Estimation of colon cancer grade and metastatic lymph node involvement using DWI/ADC sequences. Acta Radiol. 2023, 64, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Lee, C.Y.; Lui, C.Y.; Fong, C.Y.; Lau, K.C. Apparent diffusion coefficient in differentiation between malignant and benign breast masses: Does size matter? Clin. Radiol. 2016, 71, 170–177. [Google Scholar] [CrossRef]
- Fanariotis, M.; Tsougos, I.; Vlychou, M.; Fezoulidis, I.; Vassiou, K. Contrast-enhanced and unenhanced diffusion-weighted imaging of the breast at 3 T. Clin. Radiol. 2018, 73, 928–935. [Google Scholar] [CrossRef]
- Marini, C.; Iacconi, C.; Giannelli, M.; Cilotti, A.; Moretti, M.; Bartolozzi, C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur. Radiol. 2007, 17, 2646–2655. [Google Scholar] [CrossRef]
- Baltzer, P.A.T.; Kapetas, P.; Marino, M.A.; Clauser, P. New diagnostic tools for breast cancer. Memo 2017, 10, 175–180. [Google Scholar] [CrossRef]
- Arponen, O.; Sudah, M.; Masarwah, A.; Taina, M.; Rautiainen, S.; Kononen, M.; Sironen, R.; Kosma, V.M.; Sutela, A.; Hakumaki, J.; et al. Diffusion-Weighted Imaging in 3.0 Tesla Breast MRI: Diagnostic Performance and Tumor Characterization Using Small Subregions vs. Whole Tumor Regions of Interest. PLoS ONE 2015, 10, e0138702. [Google Scholar] [CrossRef]
- An, Y.Y.; Kim, S.H.; Kang, B.J. Differentiation of malignant and benign breast lesions: Added value of the qualitative analysis of breast lesions on diffusion-weighted imaging (DWI) using readout-segmented echo-planar imaging at 3.0 T. PLoS ONE 2017, 12, e0174681. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Y.Q.; Cai, Z.L.; Gao, Y.G.; An, N.Y.; Ma, L.; Mahankali, S.; Gao, J.H. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J. Magn. Reson. Imaging 2002, 16, 172–178. [Google Scholar] [CrossRef]
- Spick, C.; Bickel, H.; Pinker, K.; Bernathova, M.; Kapetas, P.; Woitek, R.; Clauser, P.; Polanec, S.H.; Rudas, M.; Bartsch, R.; et al. Diffusion-weighted MRI of breast lesions: A prospective clinical investigation of the quantitative imaging biomarker characteristics of reproducibility, repeatability, and diagnostic accuracy. NMR Biomed. 2016, 29, 1445–1453. [Google Scholar] [CrossRef]
| Malignant (n = 69) (%) | Benign (n = 67) (%) | p-Value | |
|---|---|---|---|
| Distribution | |||
| Focal | 21 (31) | 35 (52) | 0.049 |
| Linear | 9 (13) | 13 (19) | 0.37 |
| Segmental | 12 (17) | 4 (6) | 0.06 |
| Regional | 16 (23) | 8 (12) | 0.12 |
| Multiple regions | 7 (10) | 2 (3) | 0.12 |
| Diffuse | 4 (6) | 5 (8) | 0.72 |
| Internal enhancement patterns | |||
| Homogeneous | 6 (9) | 8 (12) | 0.57 |
| Heterogeneous | 47 (68) | 55 (82) | 0.35 |
| Clumped | 10 (14) | 3 (5) | 0.06 |
| Clustered-ring | 6 (9) | 1 (1) | 0.08 |
| ADC Value (×10−3 mm2/s) | Mean ± SD | p-Value | |
|---|---|---|---|
| Malignant | Benign | ||
| Punctate ROI b | |||
| All included | 0.618 ± 0.282 | 0.985 ± 0.311 | <0.01 |
| Group A | 0.526 ± 0.235 | 0.826 ± 0.316 | <0.01 |
| Group B | 0.687 ± 0.292 | 1.081 ± 0.271 | <0.01 |
| 10 mm ROI | |||
| All included | 0.928 ± 0.237 | 1.357 ± 0.418 | <0.01 |
| Group A | 0.868 ± 0.232 | 1.425 ± 0.702 | 0.03 |
| Group B | 0.984 ± 0.234 | 1.323 ± 0.259 | <0.01 |
| Whole tumor ROI | |||
| All included | 0.985 ± 0.213 | 1.294 ± 0.297 | <0.01 |
| Group A | 0.912 ± 0.156 | 1.140 ± 0.342 | 0.004 |
| Group B | 1.038 ± 0.234 | 1.385 ± 0.225 | <0.01 |
| Malignant (n = 69) (%) | Benign (n = 67) (%) | p Value | |
|---|---|---|---|
| Breast composition | |||
| Group A (ACR a and b) | 29 (42) | 25 (37) | 0.67 |
| Group B (ACR c and d) | 40 (58) | 42 (63) | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perić, I.; Brkljačić, B.; Tadić, T.; Jerković, K.; Dolić, K.; Borić, M.; Ćavar, M. DWI in the Differentiation of Malignant and Benign Breast Lesions Presenting with Non-Mass Enhancement on CE-MRI. Cancers 2025, 17, 31. https://doi.org/10.3390/cancers17010031
Perić I, Brkljačić B, Tadić T, Jerković K, Dolić K, Borić M, Ćavar M. DWI in the Differentiation of Malignant and Benign Breast Lesions Presenting with Non-Mass Enhancement on CE-MRI. Cancers. 2025; 17(1):31. https://doi.org/10.3390/cancers17010031
Chicago/Turabian StylePerić, Iva, Boris Brkljačić, Tade Tadić, Kristian Jerković, Krešimir Dolić, Matija Borić, and Marija Ćavar. 2025. "DWI in the Differentiation of Malignant and Benign Breast Lesions Presenting with Non-Mass Enhancement on CE-MRI" Cancers 17, no. 1: 31. https://doi.org/10.3390/cancers17010031
APA StylePerić, I., Brkljačić, B., Tadić, T., Jerković, K., Dolić, K., Borić, M., & Ćavar, M. (2025). DWI in the Differentiation of Malignant and Benign Breast Lesions Presenting with Non-Mass Enhancement on CE-MRI. Cancers, 17(1), 31. https://doi.org/10.3390/cancers17010031








