A Two-Step Protocol for Isolation and Maintenance of Lung Cancer Primary 3D Cultures
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Enrollment
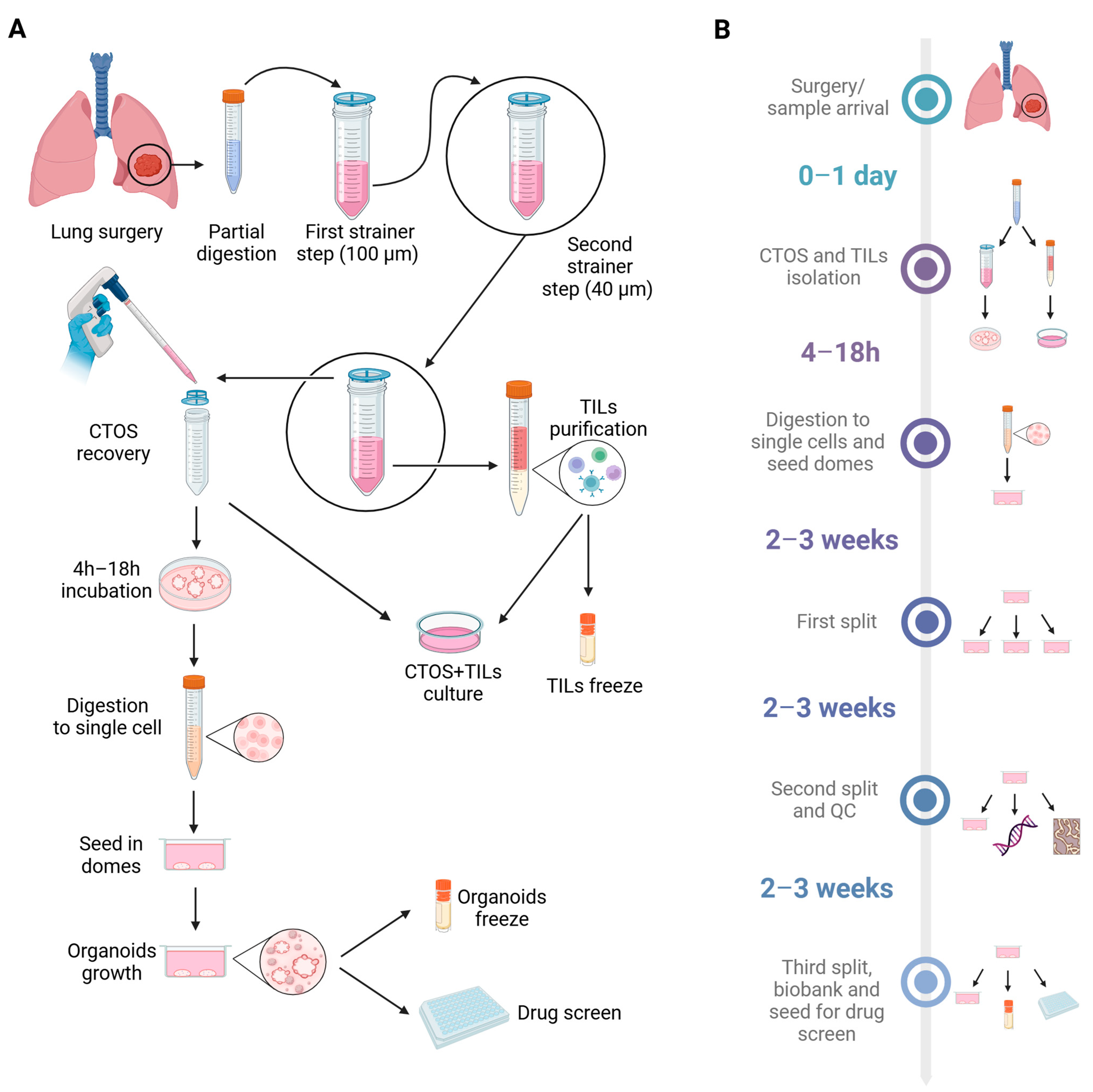
2.2. Isolation of CTOSs from Fresh Biopsies
2.3. Concomitant Isolation of TILs from Fresh Biopsies and Co-Cultures with CTOSs
2.4. Flow Cytometry Analysis of TILs
2.5. CTOS Processing to Start PDO Cultures
2.6. Organoids Splitting
2.7. Organoids Freezing and Thawing
2.8. Organoids Paraffin Inclusion and Staining
2.9. TP53 Sequencing
2.10. Mutational Analysis
2.11. Organoid Growth Curves and Drug Screening
2.12. Statistical Analysis
3. Results
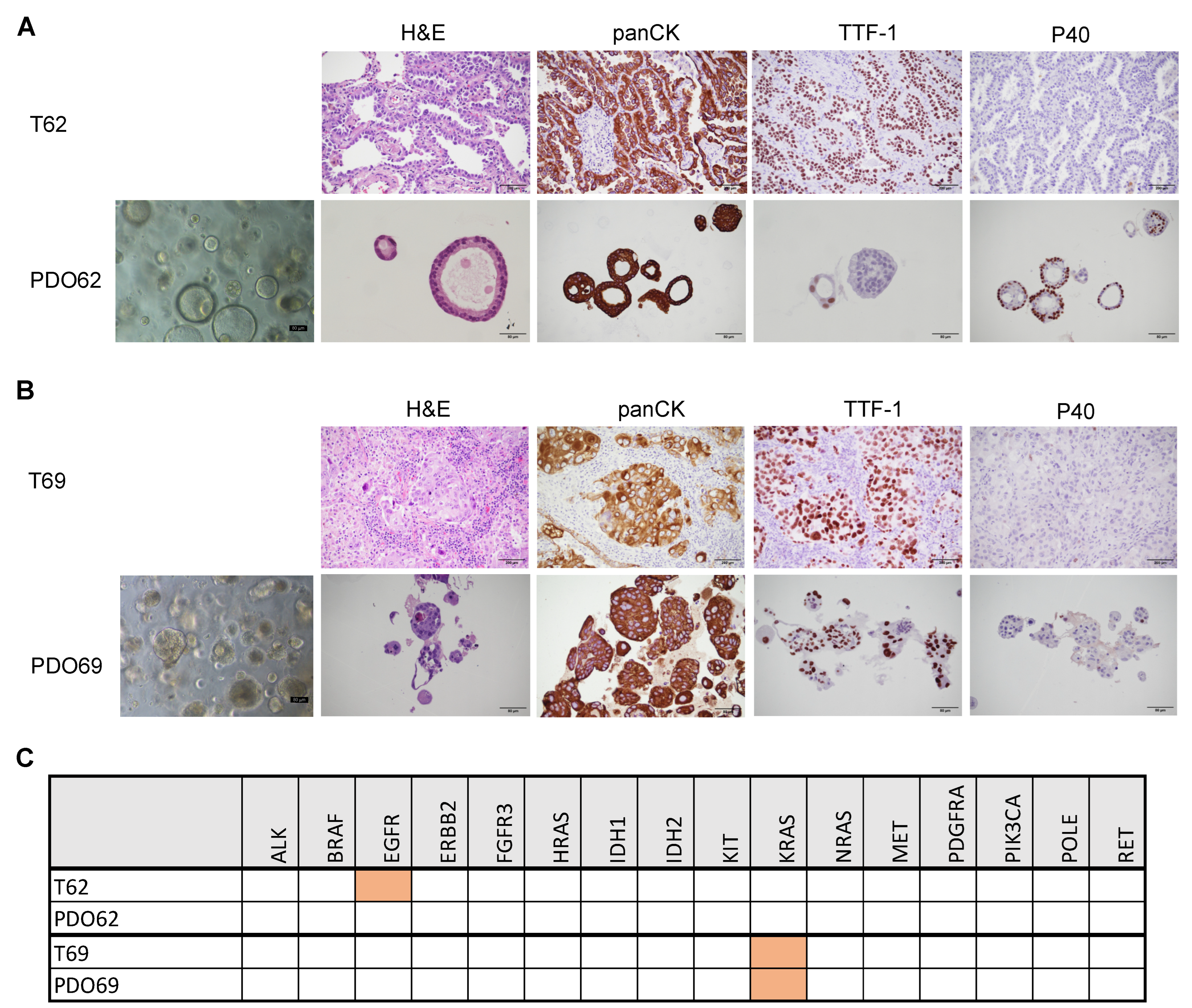
3.1. Establishment of CTOS as Short-Term Primary 3D Cultures
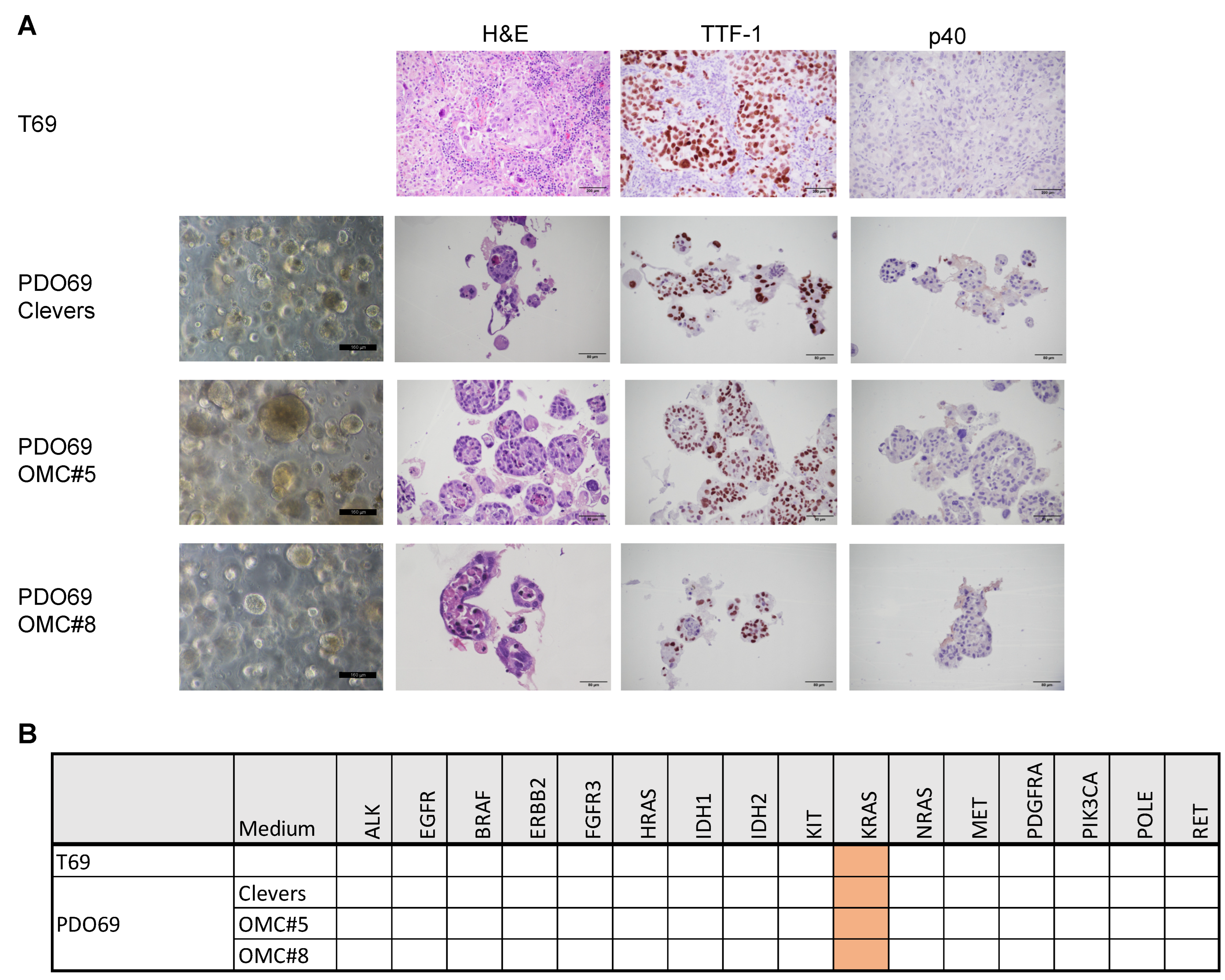
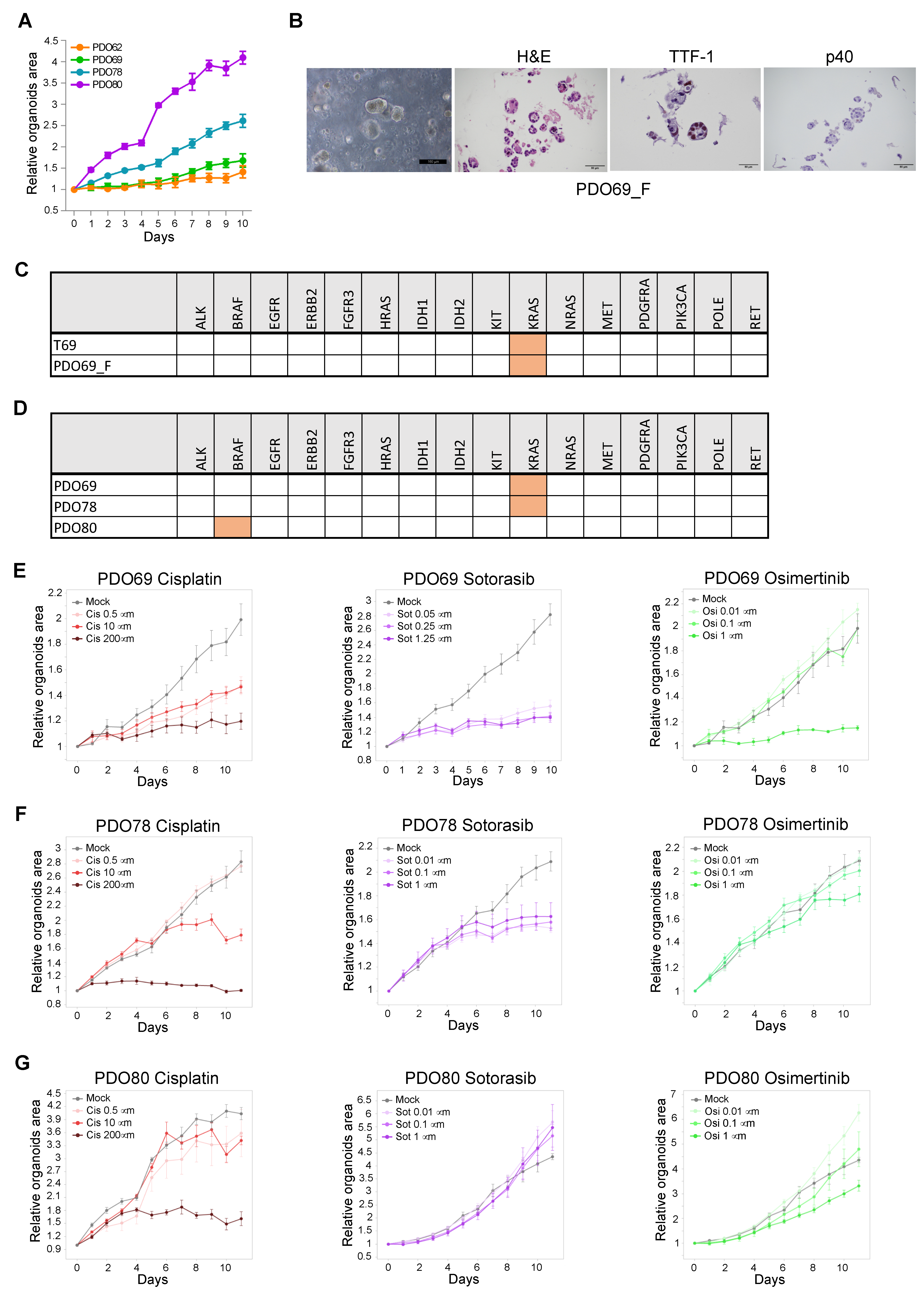
3.2. Defining the Optimal Medium for Long-Term Organoids Cultures
3.3. Establishment of PDO for Long-Term Primary 3D Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward Personalized Treatment Approaches for Non-Small-Cell Lung Cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef]
- Weng, G.; Tao, J.; Liu, Y.; Qiu, J.; Su, D.; Wang, R.; Luo, W.; Zhang, T. Organoid: Bridging the Gap Between Basic Research and Clinical Practice. Cancer Lett. 2023, 572, 216353. [Google Scholar] [CrossRef]
- Huang, W.; Navarro-Serer, B.; Jeong, Y.J.; Chianchiano, P.; Xia, L.; Luchini, C.; Veronese, N.; Dowiak, C.; Ng, T.; Trujillo, M.A.; et al. Pattern of Invasion in Human Pancreatic Cancer Organoids Is Associated with Loss of SMAD4 and Clinical Outcome. Cancer Res. 2020, 80, 2804–2817. [Google Scholar] [CrossRef]
- Ringel, T.; Frey, N.; Ringnalda, F.; Janjuha, S.; Cherkaoui, S.; Butz, S.; Srivatsa, S.; Pirkl, M.; Russo, G.; Villiger, L.; et al. Genome-Scale CRISPR Screening in Human Intestinal Organoids Identifies Drivers of TGF-β Resistance. Cell Stem Cell 2020, 26, 431–440.e8. [Google Scholar] [CrossRef] [PubMed]
- VanderVorst, K.; Dreyer, C.A.; Hatakeyama, J.; Bell, G.R.R.; Learn, J.A.; Berg, A.L.; Hernandez, M.; Lee, H.; Collins, S.R.; Carraway, K.L. 3rd Vangl-Dependent Wnt/Planar Cell Polarity Signaling Mediates Collective Breast Carcinoma Motility and Distant Metastasis. Breast Cancer Res. 2023, 25, 52. [Google Scholar] [CrossRef]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschênes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.-H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, V.; Wright, J.A.; Churchill, M.; Wang, T.; Rosati, R.; Lannagan, T.R.M.; Vrbanac, L.; Richardson, A.B.; Kobayashi, H.; Price, T.; et al. Medium-Throughput Drug Screening of Patient-Derived Organoids from Colorectal Peritoneal Metastases to Direct Personalized Therapy. Clin. Cancer Res. 2020, 26, 3662–3670. [Google Scholar] [CrossRef]
- Wang, T.; Pan, W.; Zheng, H.; Zheng, H.; Wang, Z.; Li, J.J.; Deng, C.; Yan, J. Accuracy of Using a Patient-Derived Tumor Organoid Culture Model to Predict the Response to Chemotherapy Regimens In Stage IV Colorectal Cancer: A Blinded Study. Dis. Colon Rectum 2021, 64, 833–850. [Google Scholar] [CrossRef]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-Term Expanding Human Airway Organoids for Disease Modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.-J.; Chun, S.-M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-Derived Lung Cancer Organoids as In Vitro Cancer Models for Therapeutic Screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Shi, R.; Radulovich, N.; Ng, C.; Liu, N.; Notsuda, H.; Cabanero, M.; Martins-Filho, S.N.; Raghavan, V.; Li, Q.; Mer, A.S.; et al. Organoid Cultures as Preclinical Models of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1162–1174. [Google Scholar] [CrossRef]
- Li, Z.; Qian, Y.; Li, W.; Liu, L.; Yu, L.; Liu, X.; Wu, G.; Wang, Y.; Luo, W.; Fang, F.; et al. Human Lung Adenocarcinoma-Derived Organoid Models for Drug Screening. iScience 2020, 23, 101411. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Monkhorst, K.; Schipper, L.J.; Hartemink, K.J.; Smit, E.F.; Kaing, S.; de Groot, R.; Wolkers, M.C.; Clevers, H.; Cuppen, E.; et al. Challenges in Establishing Pure Lung Cancer Organoids Limit Their Utility for Personalized Medicine. Cell Rep. 2020, 31, 107588. [Google Scholar] [CrossRef] [PubMed]
- Yokota, E.; Iwai, M.; Yukawa, T.; Yoshida, M.; Naomoto, Y.; Haisa, M.; Monobe, Y.; Takigawa, N.; Guo, M.; Maeda, Y.; et al. Clinical Application of a Lung Cancer Organoid (Tumoroid) Culture System. NPJ Precis. Oncol. 2021, 5, 29. [Google Scholar] [CrossRef]
- Hu, Y.; Sui, X.; Song, F.; Li, Y.; Li, K.; Chen, Z.; Yang, F.; Chen, X.; Zhang, Y.; Wang, X.; et al. Lung Cancer Organoids Analyzed on Microwell Arrays Predict Drug Responses of Patients within a Week. Nat. Commun. 2021, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, A.R.; Silwal-Pandit, L.; Meza-Zepeda, L.A.; Vodak, D.; Vu, P.; Sagerup, C.; Hovig, E.; Myklebost, O.; Børresen-Dale, A.-L.; Brustugun, O.T.; et al. TP53 Mutation Spectrum in Smokers and Never Smoking Lung Cancer Patients. Front. Genet. 2016, 7, 85. [Google Scholar] [CrossRef]
- Reggiani, F.; Talarico, G.; Gobbi, G.; Sauta, E.; Torricelli, F.; Manicardi, V.; Zanetti, E.; Orecchioni, S.; Falvo, P.; Piana, S.; et al. BET Inhibitors Drive Natural Killer Activation in Non-Small Cell Lung Cancer via BRD4 and SMAD3. Nat. Commun. 2024, 15, 2567. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Campbell, J.D.; Alexandrov, A.; Kim, J.; Wala, J.; Berger, A.H.; Pedamallu, C.S.; Shukla, S.A.; Guo, G.; Brooks, A.N.; et al. Distinct Patterns of Somatic Genome Alterations in Lung Adenocarcinomas and Squamous Cell Carcinomas. Nat. Genet. 2016, 48, 607–616. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Herberg, S.; Petty, W.J.; Marini, F.; Miller, L.; Kucera, G.; Dukes, D.K.; Ruiz, J.; Skardal, A.; et al. Pleural Effusion Aspirate for Use in 3D Lung Cancer Modeling and Chemotherapy Screening. ACS Biomater. Sci. Eng. 2019, 5, 1937–1943. [Google Scholar] [CrossRef]
- Surina; Tanggis; Suzuki, T.; Hisata, S.; Fujita, K.; Fujiwara, S.; Liu, F.; Fukushima, N.; Suzuki, T.; Mato, N.; et al. Patient-Derived Spheroids and Patient-Derived Organoids Simulate Evolutions of Lung Cancer. Heliyon 2023, 9, e13829. [Google Scholar] [CrossRef]



| Patient # | Histology | TP53 | Medium | Growth | Split # | Quality Checks | |
|---|---|---|---|---|---|---|---|
| IHC | NGS | ||||||
| 62 | ADK | n/a | Clevers | Y | 3 | X | X |
| 63 | ADK | n/a | Clevers | N | 1 | n/a | V |
| 64 | ADK | n/a | Clevers | Y | 1 | X | X |
| OMC#2 | Y | 1 | X | X | |||
| OMC#3 | Y | 1 | X | X | |||
| OMC#4 | Y | 1 | X | X | |||
| OMC#5 | Y | 1 | V | V | |||
| OMC#6 | Y | 1 | X | X | |||
| OMC#7 | N | 1 | n/a | n/a | |||
| 68 | ADK | n/a | OMC#5 | N | 2 | n/a | n/a |
| 69 | ADK | n/a | Clevers | Y | 3 | V | V |
| OMC#5 | Y | 21 | V | V | |||
| OMC#8 | Y | 3 | V | V | |||
| 70 | ADK | n/a | Clevers | N | 0 | n/a | n/a |
| OMC#5 | N | 0 | n/a | n/a | |||
| OMC#8 | N | 0 | n/a | n/a | |||
| 75 | ADK | n/a | Clevers | Y | 5 | X | X |
| 76 | ADK | n/a | Clevers | Y | 5 | X | X |
| OMC#5 | Y | 2 | X | X | |||
| 78 | ADK | MUT | Clevers | Y | 3 | X | X |
| Clevers + nutlin-3a | Y | 9 | V | V | |||
| 79 | ADK | WT | Clevers | N | 0 | n/a | n/a |
| 80 | ADK | MUT | Clevers | Y | 7 | V | V |
| Clevers + nutlin-3a | Y | 7 | V | V | |||
| 81 | ADK | WT | Clevers | Y | 10 | X | V |
| Clevers + nutlin-3a | N | 1 | n/a | n/a | |||
| OMC#5 | Y | 4 | V | V | |||
| 82 | ADK | WT | Clevers | N | 1 | n/a | n/a |
| Clevers + nutlin-3a | N | 1 | n/a | n/a | |||
| 83 | ADK | WT | Clevers | Y | 7 | X | V |
| Clevers + nutlin-3a | N | 0 | n/a | n/a | |||
| OMC#5 | Y | 3 | V | V | |||
| 85 | ADK | WT | Clevers | Y | 3 | X | X |
| Clevers + nutlin-3a | N | 0 | n/a | n/a | |||
| OMC#5 | N | 1 | n/a | n/a | |||
| 86 | ADK | WT | OMC#5 | N | 1 | n/a | n/a |
| 87 | ADK | MUT | OMC#5 | N | 1 | n/a | n/a |
| Clevers + nutlin-3a | N | 1 | n/a | n/a | |||
| 88 | ADK | WT | OMC#5 | Y | 2 | V | V |
| Medium | Total PDO | Growth + PDO | % Growth + PDO | QC Pass PDO | % QC on Total PDO | % QC on Growth + PDO |
|---|---|---|---|---|---|---|
| Clevers | 14 | 10 | 71% | 2 | 14% | 20% |
| Clevers + nutlin-3a | 7 | 2 | 29% | 2 | 29% | 100% |
| OMC#5 | 11 | 6 | 55% | 5 | 45% | 84% |
| Any medium | 18 | 11 | 61% | 7 | 38% | 63% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strocchi, S.; Santandrea, G.; Zanetti, E.; Verna, G.; Cusenza, V.Y.; Nicoli, D.; Fantini, V.; Grieco, A.; Paci, M.; Ciarrocchi, A.; et al. A Two-Step Protocol for Isolation and Maintenance of Lung Cancer Primary 3D Cultures. Cancers 2025, 17, 27. https://doi.org/10.3390/cancers17010027
Strocchi S, Santandrea G, Zanetti E, Verna G, Cusenza VY, Nicoli D, Fantini V, Grieco A, Paci M, Ciarrocchi A, et al. A Two-Step Protocol for Isolation and Maintenance of Lung Cancer Primary 3D Cultures. Cancers. 2025; 17(1):27. https://doi.org/10.3390/cancers17010027
Chicago/Turabian StyleStrocchi, Silvia, Giacomo Santandrea, Eleonora Zanetti, Giulio Verna, Vincenza Ylenia Cusenza, Davide Nicoli, Valentina Fantini, Alessandra Grieco, Massimiliano Paci, Alessia Ciarrocchi, and et al. 2025. "A Two-Step Protocol for Isolation and Maintenance of Lung Cancer Primary 3D Cultures" Cancers 17, no. 1: 27. https://doi.org/10.3390/cancers17010027
APA StyleStrocchi, S., Santandrea, G., Zanetti, E., Verna, G., Cusenza, V. Y., Nicoli, D., Fantini, V., Grieco, A., Paci, M., Ciarrocchi, A., & Sancisi, V. (2025). A Two-Step Protocol for Isolation and Maintenance of Lung Cancer Primary 3D Cultures. Cancers, 17(1), 27. https://doi.org/10.3390/cancers17010027







