The Role of Chronic Inflammation in Pediatric Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Chronic Inflammation and Genetic Instability
4. Chronic Inflammation and Epigenetic Dysregulation:
5. Chronic Inflammation and the Tumor Microenvironment
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ayub, M.; Jin, H.K.; Bae, J.-s. The blood cerebrospinal fluid barrier orchestrates immunosurveillance, immunoprotection, and immunopathology in the central nervous system. BMB Rep. 2021, 54, 196. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Willimann, K. Chemokines: Role in inflammation and immune surveillance. Ann. Rheum. Dis. 2004, 63 (Suppl. S2), ii84–ii89. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-mediated inflammation in skin wound healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Wolf, S.J.; Melvin, W.J.; Gallagher, K. Macrophage-mediated inflammation in diabetic wound repair. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [PubMed]
- Hou, J.; Karin, M.; Sun, B. Targeting cancer-promoting inflammation—Have anti-inflammatory therapies come of age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity 2018, 49, 666–677.e6. [Google Scholar] [CrossRef]
- Razi, S.; Yaghmoorian Khojini, J.; Kargarijam, F.; Panahi, S.; Tahershamsi, Z.; Tajbakhsh, A.; Gheibihayat, S.M. Macrophage efferocytosis in health and disease. Cell Biochem. Funct. 2023, 41, 152–165. [Google Scholar] [CrossRef]
- Afify, S.M.; Hassan, G.; Seno, A.; Seno, M. Cancer-inducing niche: The force of chronic inflammation. Br. J. Cancer 2022, 127, 193–201. [Google Scholar] [CrossRef]
- Ghorabi, S.; Esteghamati, A.; Azam, K.; Daneshzad, E.; Sadeghi, O.; Salari-Moghaddam, A.; Azadbakht, L.; Djafarian, K. Association between dietary inflammatory index and components of metabolic syndrome. J. Cardiovasc. Thorac. Res. 2020, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-R.; Song, J.-L.; Liu, H.-Q.; Chen, C. Metabolic syndrome and thyroid Cancer: Risk, prognosis, and mechanism. Discov. Oncol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Ruan, J.; Li, Z.; Tzeng, C.-M. Chronic inflammation links cancer and Parkinson’s disease. Front. Aging Neurosci. 2016, 8, 126. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis—No longer a theory. Clin. Chem. 2021, 67, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Alba, M.M.; Ebright, B.; Hua, B.; Slarve, I.; Zhou, Y.; Jia, Y.; Louie, S.G.; Stiles, B.L. Eicosanoids and other oxylipins in liver injury, inflammation and liver cancer development. Front. Physiol. 2023, 14, 1098467. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.G.; Tirosh, I.; Hovestadt, V.; Shaw, M.L.; Escalante, L.E.; Mathewson, N.D.; Neftel, C.; Frank, N.; Pelton, K.; Hebert, C.M. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science 2018, 360, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Langhans, S.A. In vivo and ex vivo pediatric brain tumor models: An overview. Front. Oncol. 2021, 11, 620831. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A. Geographic variation in pediatric cancer incidence—United States, 2003–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; King, J.B.; Lupo, P.J.; Durbin, E.B.; Tai, E.; Mills, K.; Van Dyne, E.; Buchanan Lunsford, N.; Henley, S.J.; Wilson, R.J. Counts, incidence rates, and trends of pediatric cancer in the United States, 2003-2019. JNCI J. Natl. Cancer Inst. 2023, 115, 1337–1354. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Richardson, L.C.; Henley, S.J.; Wilson, R.J.; Dowling, N.F.; Weir, H.K.; Tai, E.W.; Buchanan Lunsford, N. Pediatric cancer mortality and survival in the United States, 2001–2016. Cancer 2020, 126, 4379–4389. [Google Scholar] [CrossRef]
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.; Boyle, T.; Ahmed, M.; Lee, S.H.; Benyamin, B.; Hyppönen, E. Lifestyle, genetic risk and incidence of cancer: A prospective cohort study of 13 cancer types. Int. J. Epidemiol. 2023, 52, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and cervical cancer: A review of epidemiology and screening uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Brehm, C.U.; Gress, T.M.; Buchholz, M.; Alashkar Alhamwe, B.; Pogge von Strandmann, E.; Slater, E.P.; Bartsch, J.W.; Bauer, C.; Lauth, M. The immune microenvironment in pancreatic cancer. Int. J. Mol. Sci. 2020, 21, 7307. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, D.; Dörk, T. Genomic risk factors for cervical cancer. Cancers 2021, 13, 5137. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2021, 124, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Vargas-Rondón, N.; Villegas, V.E.; Rondón-Lagos, M. The role of chromosomal instability in cancer and therapeutic responses. Cancers 2017, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Bertacca, I.; Pegoraro, F.; Tondo, A.; Favre, C. Targeted treatment of solid tumors in pediatric precision oncology. Front. Oncol. 2023, 13, 1176790. [Google Scholar] [CrossRef]
- Kattner, P.; Strobel, H.; Khoshnevis, N.; Grunert, M.; Bartholomae, S.; Pruss, M.; Fitzel, R.; Halatsch, M.-E.; Schilberg, K.; Siegelin, M.D. Compare and contrast: Pediatric cancer versus adult malignancies. Cancer Metastasis Rev. 2019, 38, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.M.; Walton, M.A.; Carter, P.M. The major causes of death in children and adolescents in the United States. N. Engl. J. Med. 2018, 379, 2468–2475. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Thatikonda, V.; Islam, S.A.; Autry, R.J.; Jones, B.C.; Gröbner, S.N.; Warsow, G.; Hutter, B.; Huebschmann, D.; Fröhling, S.; Kool, M. Comprehensive analysis of mutational signatures reveals distinct patterns and molecular processes across 27 pediatric cancers. Nat. Cancer 2023, 4, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Derks, L.L.; van Boxtel, R. Stem cell mutations, associated cancer risk, and consequences for regenerative medicine. Cell Stem Cell 2023, 30, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Freire, N.H.; Jaeger, M.d.C.; de Farias, C.B.; Nör, C.; Souza, B.K.; Gregianin, L.; Brunetto, A.T.; Roesler, R. Targeting the epigenome of cancer stem cells in pediatric nervous system tumors. Mol. Cell. Biochem. 2023, 478, 2241–2255. [Google Scholar] [CrossRef] [PubMed]
- Sanalkumar, R.; Dong, R.; Lee, L.; Xing, Y.-H.; Iyer, S.; Letovanec, I.; La Rosa, S.; Finzi, G.; Musolino, E.; Papait, R. Highly connected 3D chromatin networks established by an oncogenic fusion protein shape tumor cell identity. Sci. Adv. 2023, 9, eabo3789. [Google Scholar] [CrossRef]
- Babaei, G.; Aziz, S.G.-G.; Jaghi, N.Z.Z. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother. 2021, 133, 110909. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.P.; Roesler, R.; Gregianin, L.; Brunetto, A.T.; da Cunha Jaeger, M.; Lunardi Brunetto, A.; de Farias, C.B. Cancer stem cells and chemoresistance in Ewing sarcoma. Curr. Stem Cell Res. Ther. 2023, 18, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol. Ther. 2016, 160, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Huether, R.; Dong, L.; Chen, X.; Wu, G.; Parker, M.; Wei, L.; Ma, J.; Edmonson, M.N.; Hedlund, E.K.; Rusch, M.C. The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nat. Commun. 2014, 5, 3630. [Google Scholar] [CrossRef] [PubMed]
- Cuella-Martin, R.; Hayward, S.B.; Fan, X.; Chen, X.; Huang, J.-W.; Taglialatela, A.; Leuzzi, G.; Zhao, J.; Rabadan, R.; Lu, C. Functional interrogation of DNA damage response variants with base editing screens. Cell 2021, 184, 1081–1097.e19. [Google Scholar] [CrossRef]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Klapp, V.; Álvarez-Abril, B.; Leuzzi, G.; Kroemer, G.; Ciccia, A.; Galluzzi, L. The DNA damage response and inflammation in cancer. Cancer Discov. 2023, 13, 1521–1545. [Google Scholar] [CrossRef] [PubMed]
- Dersh, D.; Hollý, J.; Yewdell, J.W. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat. Rev. Immunol. 2021, 21, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Linstra, R.; van Vugt, M.A. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2022, 1877, 188661. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Agustinus, A.S.; Li, J.; DiBona, M.; Bakhoum, S.F. Chromosomal instability as a driver of cancer progression. Nat. Rev. Genet. 2024, 26, 31–46. [Google Scholar] [CrossRef]
- Cinat, D.; Coppes, R.P.; Barazzuol, L. DNA damage-induced inflammatory microenvironment and adult stem cell response. Front. Cell Dev. Biol. 2021, 9, 729136. [Google Scholar] [CrossRef]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of resolution of inflammation? Pharmacol. Ther. 2021, 218, 107670. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.-A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.; Taunk, N.K. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Kumar, Y.; Bhatia, A. The link between chromosomal instability and immunity in cancer. In Handbook of Cancer and Immunology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–20. [Google Scholar]
- Orr, B.; Compton, D.A. A double-edged sword: How oncogenes and tumor suppressor genes can contribute to chromosomal instability. Front. Oncol. 2013, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Sansregret, L.; Vanhaesebroeck, B.; Swanton, C. Determinants and clinical implications of chromosomal instability in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Tijhuis, A.E.; Johnson, S.C.; McClelland, S.E. The emerging links between chromosomal instability (CIN), metastasis, inflammation and tumour immunity. Mol. Cytogenet. 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Albrecht, S.; Sollier, C.; Faury, D.; Sader, E.; Montpetit, A.; Serre, D.; Hauser, P.; Garami, M.; Bognár, L. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br. J. Cancer 2009, 101, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Panuciak, K.; Nowicka, E.; Mastalerczyk, A.; Zawitkowska, J.; Niedźwiecki, M.; Lejman, M. Overview on aneuploidy in childhood B-cell acute lymphoblastic leukemia. Int. J. Mol. Sci. 2023, 24, 8764. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Zaccaria, S.; Cannon, M.V.; Cam, M.; Gross, A.C.; Raphael, B.J.; Roberts, R.D. Structurally complex osteosarcoma genomes exhibit limited heterogeneity within individual tumors and across evolutionary time. Cancer Res. Commun. 2023, 3, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Tonini, G.P. Why Is Aneuploidy Associated with Favorable Outcome in Neuroblastoma? Biomolecules 2021, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Ceranski, A.K.; Carreño-Gonzalez, M.J.; Ehlers, A.C.; Colombo, M.V.; Cidre-Aranaz, F.; Grünewald, T.G. Hypoxia and HIFs in Ewing sarcoma: New perspectives on a multi-facetted relationship. Mol. Cancer 2023, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; McCord, M.; Jamshidi, P.; Sukhanova, M.; Lu, X. 6. The clinical implications of polyploidy in oligodendrogliomas. Cancer Genet. 2022, 268, 2–3. [Google Scholar] [CrossRef]
- Miguel-Fraile, P.S.; Carrillo-Gijón, R.; Rodriguez-Peralto, J.L.; Badiola, I.A. Prognostic significance of DNA ploidy and proliferative index (MIB-1 index) in childhood rhabdomyosarcoma. Am. J. Clin. Pathol. 2004, 121, 358–365. [Google Scholar] [CrossRef]
- Pinto, M.T.; Carcano, F.M.; Vieira, A.G.S.; Cabral, E.R.M.; Lopes, L.F. Molecular biology of pediatric and adult male germ cell tumors. Cancers 2021, 13, 2349. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.T.; Azad, T.D.; Breese, M.R.; Chabon, J.J.; Hamilton, E.G.; Straessler, K.; Kurtz, D.M.; Leung, S.G.; Spillinger, A.; Liu, H.-Y. A comprehensive circulating tumor DNA assay for detection of translocation and copy-number changes in pediatric sarcomas. Mol. Cancer Ther. 2021, 20, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Argani, P. Translocation carcinomas of the kidney. Genes Chromosomes Cancer 2022, 61, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Campregher, P.; Rosa, S.; Silveira, C.; Lima, L.; de Oliveira, J.B.; Pelegrino, K. 62. Molecular profile of patients with Acute Myeloid Leukemia at diagnosis. Cancer Genet. 2022, 268, 20. [Google Scholar] [CrossRef]
- van der Beek, J.N.; Geller, J.I.; de Krijger, R.R.; Graf, N.; Pritchard-Jones, K.; Drost, J.; Verschuur, A.C.; Murphy, D.; Ray, S.; Spreafico, F. Characteristics and outcome of children with renal cell carcinoma: A narrative review. Cancers 2020, 12, 1776. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.S.; Morsberger, L.; Hardy, M.; Ghabrial, J.; Stinnett, V.; Murry, J.B.; Long, P.; Kim, A.; Pratilas, C.A.; Llosa, N.J. Complex/cryptic EWSR1:: FLI1/ERG Gene Fusions and 1q Jumping Translocation in Pediatric Ewing Sarcomas. Genes 2023, 14, 1139. [Google Scholar] [CrossRef]
- Kenny, C.; O’Meara, E.; Ulaş, M.; Hokamp, K.; O’Sullivan, M.J. Global chromatin changes resulting from single-gene inactivation—The role of SMARCB1 in malignant rhabdoid tumor. Cancers 2021, 13, 2561. [Google Scholar] [CrossRef]
- Muscat, A.; Popovski, D.; Jayasekara, W.S.N.; Rossello, F.J.; Ferguson, M.; Marini, K.D.; Alamgeer, M.; Algar, E.M.; Downie, P.; Watkins, D.N. Low-dose histone deacetylase inhibitor treatment leads to tumor growth arrest and multi-lineage differentiation of malignant rhabdoid tumors. Clin. Cancer Res. 2016, 22, 3560–3570. [Google Scholar] [CrossRef] [PubMed]
- Navickas, S.M.; Giles, K.A.; Brettingham-Moore, K.H.; Taberlay, P.C. The role of chromatin remodeler SMARCA4/BRG1 in brain cancers: A potential therapeutic target. Oncogene 2023, 42, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Pastorczak, A.; Krajewska, K.; Urbanska, Z.; Szmyd, B.; Salacinska-Los, E.; Kobos, J.; Mlynarski, W.; Trelinska, J. Ovarian carcinoma in children with constitutional mutation of SMARCA4: Single-family report and literature review. Fam. Cancer 2021, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Byrjalsen, A.; Hansen, T.V.; Stoltze, U.K.; Mehrjouy, M.M.; Barnkob, N.M.; Hjalgrim, L.L.; Mathiasen, R.; Lautrup, C.K.; Gregersen, P.A.; Hasle, H. Nationwide germline whole genome sequencing of 198 consecutive pediatric cancer patients reveals a high incidence of cancer prone syndromes. PLoS Genet. 2020, 16, e1009231. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.; Nakitandwe, J.; Kesserwan, C.A.; Azzato, E.M.; Wheeler, D.A.; Rusch, M.; Shurtleff, S.; Hedges, D.J.; Hamilton, K.V.; Foy, S.G. Genomes for kids: The scope of pathogenic mutations in pediatric cancer revealed by comprehensive DNA and RNA sequencing. Cancer Discov. 2021, 11, 3008–3027. [Google Scholar] [CrossRef] [PubMed]
- Ney, G.M.; McKay, L.; Koschmann, C.; Mody, R.; Li, Q. The emerging role of Ras pathway signaling in pediatric cancer. Cancer Res. 2020, 80, 5155–5163. [Google Scholar] [CrossRef] [PubMed]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Savary, C.; Kim, A.; Lespagnol, A.; Gandemer, V.; Pellier, I.; Andrieu, C.; Pagès, G.; Galibert, M.-D.; Blum, Y.; de Tayrac, M. Depicting the genetic architecture of pediatric cancers through an integrative gene network approach. Sci. Rep. 2020, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.W.; Roy, A.; Yang, Y.; Wang, T.; Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Gutierrez, S.; Petersen, A.K.; Bavle, A. Diagnostic yield of clinical tumor and germline whole-exome sequencing for children with solid tumors. JAMA Oncol. 2016, 2, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A. Germline mutations in predisposition genes in pediatric cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef]
- Baumhoer, D.; Berthold, R.; Isfort, I.; Heinst, L.; Ameline, B.; Grünewald, I.; Thieringer, F.M.; Rudack, C.; Wardelmann, E.; Vieth, V. Recurrent CTNNB1 mutations in craniofacial osteomas. Mod. Pathol. 2022, 35, 489–494. [Google Scholar] [CrossRef]
- Capasso, M.; Montella, A.; Tirelli, M.; Maiorino, T.; Cantalupo, S.; Iolascon, A. Genetic predisposition to solid pediatric cancers. Front. Oncol. 2020, 10, 590033. [Google Scholar] [CrossRef]
- Feng, S.; Han, L.; Yue, M.; Zhong, D.; Cao, J.; Guo, Y.; Sun, Y.; Zhang, H.; Cao, Z.; Cui, X. Frequency detection of BRAF V600E mutation in a cohort of pediatric langerhans cell histiocytosis patients by next-generation sequencing. Orphanet J. Rare Dis. 2021, 16, 272. [Google Scholar] [CrossRef]
- He, J.; Zeng, Z.; Wang, Y.; Deng, J.; Tang, X.; Liu, F.; Huang, J.; Chen, H.; Liang, R.; Zan, X. Characterization of novel CTNNB1 mutation in Craniopharyngioma by whole-genome sequencing. Mol. Cancer 2021, 20, 168. [Google Scholar] [CrossRef] [PubMed]
- Khaiman, C.; Techavichit, P.; Poparn, H.; Chiengthong, K.; Lauhasurayotin, S.; Sosothikul, D.; Shotelersuk, K.; Nantavithya, C.; Jakchairoongruang, K.; Amornfa, J. KRAS mutation in pediatric intracranial germ cell tumors. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 3179. [Google Scholar] [CrossRef]
- Nobre, L.; Zapotocky, M.; Ramaswamy, V.; Ryall, S.; Bennett, J.; Alderete, D.; Balaguer Guill, J.; Baroni, L.; Bartels, U.; Bavle, A. Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis. Oncol. 2020, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Pondrom, M.; Bougeard, G.; Karanian, M.; Bonneau-Lagacherie, J.; Boulanger, C.; Boutroux, H.; Briandet, C.; Chevreau, C.; Corradini, N.; Coze, C. Rhabdomyosarcoma associated with germline TP53 alteration in children and adolescents: The French experience. Pediatr. Blood Cancer 2020, 67, e28486. [Google Scholar] [CrossRef]
- Simonin, M.; Andrieu, G.P.; Birsen, R.; Balsat, M.; Hypolite, G.; Courtois, L.; Graux, C.; Grardel, N.; Cayuela, J.-M.; Huguet, F. Prognostic value and oncogenic landscape of TP53 alterations in adult and pediatric T-ALL. Blood 2023, 141, 1353–1358. [Google Scholar] [CrossRef]
- Talloa, D.; Triarico, S.; Agresti, P.; Mastrangelo, S.; Attinà, G.; Romano, A.; Maurizi, P.; Ruggiero, A. BRAF and MEK targeted therapies in pediatric central nervous system tumors. Cancers 2022, 14, 4264. [Google Scholar] [CrossRef] [PubMed]
- Apfelbaum, A.A.; Wrenn, E.D.; Lawlor, E.R. The importance of fusion protein activity in Ewing sarcoma and the cell intrinsic and extrinsic factors that regulate it: A review. Front. Oncol. 2022, 12, 1044707. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, H.; Farrar, J.E.; Triche, T., Jr.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018, 24, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Erkizan, H.V.; Uversky, V.N.; Toretsky, J.A. Oncogenic partnerships: EWS-FLI1 protein interactions initiate key pathways of Ewing’s sarcoma. Clin. Cancer Res. 2010, 16, 4077–4083. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.K.; Qian, K.; Bisson, W.H.; Watanabe-Smith, K.; Huang, A.; Bottomly, D.; Traer, E.; Tyner, J.W.; McWeeney, S.K.; Davare, M.A. Discovery and characterization of targetable NTRK point mutations in hematologic neoplasms. Blood J. Am. Soc. Hematol. 2020, 135, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Raze, T.; Lapouble, E.; Lacour, B.; Guissou, S.; Defachelles, A.S.; Gaspar, N.; Delattre, O.; Pierron, G.; Desandes, E. PAX–FOXO1 fusion status in children and adolescents with alveolar rhabdomyosarcoma: Impact on clinical, pathological, and survival features. Pediatr. Blood Cancer 2023, 70, e30228. [Google Scholar] [CrossRef]
- Shiba, N.; Ichikawa, H.; Taki, T.; Park, M.J.; Jo, A.; Mitani, S.; Kobayashi, T.; Shimada, A.; Sotomatsu, M.; Arakawa, H. NUP98-NSD1 gene fusion and its related gene expression signature are strongly associated with a poor prognosis in pediatric acute myeloid leukemia. Genes Chromosomes Cancer 2013, 52, 683–693. [Google Scholar] [CrossRef]
- Sweet-Cordero, E.A.; Biegel, J.A. The genomic landscape of pediatric cancers: Implications for diagnosis and treatment. Science 2019, 363, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, F.M.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef] [PubMed]
- Lazow, M.A.; Hoffman, L.; Schafer, A.; Osorio, D.S.; Boué, D.R.; Rush, S.; Wright, E.; Lane, A.; DeWire-Schottmiller, M.D.; Smolarek, T. Characterizing temporal genomic heterogeneity in pediatric low-grade gliomas. Acta Neuropathol. Commun. 2020, 8, 182. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.S.; Clarke, S.; Veschi, V.; Thiele, C.J. Targeting MYCN in pediatric and adult cancers. Front. Oncol. 2021, 10, 623679. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Plant-Fox, A.S.; O’Halloran, K.; Goldman, S. Pediatric brain tumors: The era of molecular diagnostics, targeted and immune-based therapeutics, and a focus on long term neurologic sequelae. Curr. Probl. Cancer 2021, 45, 100777. [Google Scholar] [CrossRef] [PubMed]
- Aubin, R.G.; Troisi, E.C.; Montelongo, J.; Alghalith, A.N.; Nasrallah, M.P.; Santi, M.; Camara, P.G. Pro-inflammatory cytokines mediate the epithelial-to-mesenchymal-like transition of pediatric posterior fossa ependymoma. Nat. Commun. 2022, 13, 3936. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef] [PubMed]
- Panina, S.B.; Baran, N.; Brasil da Costa, F.H.; Konopleva, M.; Kirienko, N.V. A mechanism for increased sensitivity of acute myeloid leukemia to mitotoxic drugs. Cell Death Dis. 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Lotti, F.; Geronzi, U.; Guidoni, E.; Longini, M.; Buonocore, G. Oxidative Stress in Cancer-Prone Genetic Diseases in Pediatric Age: The Role of Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2016, 2016, 4782426. [Google Scholar] [CrossRef]
- Raber, M.; Wu, J.; Donnella, H.; Knouse, P.; Pise, M.; Munsell, M.; Liu, D.; Chandra, J. Cellular oxidative stress in pediatric leukemia and lymphoma patients undergoing treatment is associated with protein consumption. Nutrients 2019, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.A.M.; Al-Dolaimy, F.; Baymakov, S.; Ullah, M.I.; Khlewee, I.H.; Bisht, Y.S.; Alsaalamy, A.H. Oxidative stress affects the beginning of the growth of cancer cells through a variety of routes. Pathol.-Res. Pract. 2023, 249, 154664. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Q.; Pierrevelcin, M.; Messe, M.; Lhermitte, B.; Blandin, A.-F.; Papin, C.; Coca, A.; Dontenwill, M.; Entz-Werlé, N. Hypoxia inducible factors’ signaling in pediatric high-grade gliomas: Role, modelization and innovative targeted approaches. Cancers 2020, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Pierrevelcin, M.; Fuchs, Q.; Lhermitte, B.; Messé, M.; Guérin, E.; Weingertner, N.; Martin, S.; Lelong-Rebel, I.; Nazon, C.; Dontenwill, M. Focus on hypoxia-related pathways in pediatric osteosarcomas and their druggability. Cells 2020, 9, 1998. [Google Scholar] [CrossRef]
- Wang, M.Y.; Liow, P.; Guzman, M.I.T.; Qi, J. Exploring methods of targeting histone methyltransferases and their applications in cancer therapeutics. ACS Chem. Biol. 2022, 17, 744–755. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-l.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.C.; Alejo, A.L.; Samson, T.K.; Alejo, A.M.; Safadi, F.F. Epigenetic regulation of chondrocytes and subchondral bone in osteoarthritis. Life 2022, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, G.; DeMeo, D.L.; Glass, K.; Silverman, E.K.; Napoli, C. Epigenetics and pulmonary diseases in the horizon of precision medicine: A review. Eur. Respir. J. 2021, 57. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.K. Chromatin regulators in retinoblastoma: Biological roles and therapeutic applications. J. Cell. Physiol. 2021, 236, 2318–2332. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in health and disease. Epigenetics Allergy Autoimmun. 2020, 1253, 3–55. [Google Scholar]
- Morel, D.; Jeffery, D.; Aspeslagh, S.; Almouzni, G.; Postel-Vinay, S. Combining epigenetic drugs with other therapies for solid tumours—Past lessons and future promise. Nat. Rev. Clin. Oncol. 2020, 17, 91–107. [Google Scholar] [CrossRef]
- Mosaab, A.; El-Ayadi, M.; Khorshed, E.N.; Amer, N.; Refaat, A.; El-Beltagy, M.; Hassan, Z.; Soror, S.H.; Zaghloul, M.S.; El-Naggar, S. Histone H3K27M mutation overrides histological grading in pediatric gliomas. Sci. Rep. 2020, 10, 8368. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Peng, D.; Liu, L. Drug resistance mechanisms of acute myeloid leukemia stem cells. Front. Oncol. 2022, 12, 896426. [Google Scholar] [CrossRef] [PubMed]
- Rakotomalala, A.; Bailleul, Q.; Savary, C.; Arcicasa, M.; Hamadou, M.; Huchedé, P.; Hochart, A.; Restouin, A.; Castellano, R.; Collette, Y. H3. 3K27M mutation controls cell growth and resistance to therapies in pediatric glioma cell lines. Cancers 2021, 13, 5551. [Google Scholar] [CrossRef] [PubMed]
- Johann, P.-D. Invited Review: Dysregulation of chromatin remodellers in paediatric brain tumours–SMARCB1 and beyond. Neuropathol. Appl. Neurobiol. 2020, 46, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S. Crosstalk between Noncoding RNAs and the Epigenetics Machinery in Pediatric Tumors and Their Microenvironment. Cancers 2023, 15, 2833. [Google Scholar] [CrossRef]
- Perla, A.; Fratini, L.; Cardoso, P.S.; Nör, C.; Brunetto, A.T.; Brunetto, A.L.; de Farias, C.B.; Jaeger, M.; Roesler, R. Histone deacetylase inhibitors in pediatric brain cancers: Biological activities and therapeutic potential. Front. Cell Dev. Biol. 2020, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- French, R.; Pauklin, S. Epigenetic regulation of cancer stem cell formation and maintenance. Int. J. Cancer 2021, 148, 2884–2897. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Hüser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in development and cancer biology. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wu, S.; Tan, Y.; Li, F.; Han, Y.; Zhang, S.; Lin, X. CD44: A cancer stem cell marker and therapeutic target in leukemia treatment. Front. Immunol. 2024, 15, 1354992. [Google Scholar] [CrossRef] [PubMed]
- Celarain, N.; Tomas-Roig, J. Aberrant DNA methylation profile exacerbates inflammation and neurodegeneration in multiple sclerosis patients. J. Neuroinflammation 2020, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, M.; Payne, F.; Nayak, K.; Kraiczy, J.; Glemas, C.; Philip-McKenzie, Y.; Ross, A.; Edgar, R.D.; Zerbino, D.R.; Salvestrini, C. Transcription and DNA methylation patterns of blood-derived CD8+ T cells are associated with age and inflammatory bowel disease but do not predict prognosis. Gastroenterology 2021, 160, 232–244.e7. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.; Marzi, C.; Aslibekyan, S.; Mendelson, M.M.; Conneely, K.N.; Tanaka, T.; Colicino, E.; Waite, L.L.; Joehanes, R.; Guan, W. DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Myte, R.; Sundkvist, A.; Van Guelpen, B.; Harlid, S. Circulating levels of inflammatory markers and DNA methylation, an analysis of repeated samples from a population based cohort. Epigenetics 2019, 14, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Karimi, M.; Johansson, S.; Axelsson, J.; Suliman, M.; Lindholm, B.; Heimbürger, O.; Barany, P.; Alvestrand, A.; Nordfors, L. Impact of inflammation on epigenetic DNA methylation–a novel risk factor for cardiovascular disease? J. Intern. Med. 2007, 261, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Wang, T.; Qi, C.; Yan, Q.; Xu, C.-J.; Boutaoui, N.; Han, Y.-Y.; Weeks, D.E.; Jiang, Y.; Rosser, F. DNA methylation in nasal epithelium, atopy, and atopic asthma in children: A genome-wide study. Lancet Respir. Med. 2019, 7, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.J.; Kraiczy, J.; Nayak, K.M.; Gasparetto, M.; Ross, A.; Lee, C.; Mak, T.N.; Koo, B.-K.; Kumar, N.; Lawley, T. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology 2018, 154, 585–598. [Google Scholar] [CrossRef]
- Somineni, H.K.; Venkateswaran, S.; Kilaru, V.; Marigorta, U.M.; Mo, A.; Okou, D.T.; Kellermayer, R.; Mondal, K.; Cobb, D.; Walters, T.D. Blood-derived DNA methylation signatures of Crohn’s disease and severity of intestinal inflammation. Gastroenterology 2019, 156, 2254–2265.e3. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Forno, E.; Cardenas, A.; Qi, C.; Han, Y.Y.; Acosta-Pérez, E.; Kim, S.; Zhang, R.; Boutaoui, N.; Canino, G. Exposure to violence, chronic stress, nasal DNA methylation, and atopic asthma in children. Pediatr. Pulmonol. 2021, 56, 1896–1905. [Google Scholar] [CrossRef]
- Gujar, H.; Weisenberger, D.J.; Liang, G. The roles of human DNA methyltransferases and their isoforms in shaping the epigenome. Genes 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Loaeza-Loaeza, J.; Beltran, A.S.; Hernández-Sotelo, D. DNMTs and impact of CpG content, transcription factors, consensus motifs, lncRNAs, and histone marks on DNA methylation. Genes 2020, 11, 1336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y. Role of mammalian DNA methyltransferases in development. Annu. Rev. Biochem. 2020, 89, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Blackledge, N.P.; Klose, R. CpG island chromatin: A platform for gene regulation. Epigenetics 2011, 6, 147–152. [Google Scholar] [CrossRef]
- Angeloni, A.; Bogdanovic, O. Sequence determinants, function, and evolution of CpG islands. Biochem. Soc. Trans. 2021, 49, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.M.; Bird, A. CpG islands and the regulation of transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.H.; Lee, S.; Kim, J.-S.; Jung, H.-Y. Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab. Investig. 2003, 83, 635–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Liu, S.; Wang, H.; Mai, H.; Yuan, X.; Li, C.; Chen, X.; Wen, F. Methylation level of CpG islands in GGH gene promoter in pediatric acute leukemia. PLoS ONE 2017, 12, e0173472. [Google Scholar] [CrossRef] [PubMed]
- Lietz, C.E.; Newman, E.T.; Kelly, A.D.; Xiang, D.H.; Zhang, Z.; Luscko, C.A.; Lozano-Calderon, S.A.; Ebb, D.H.; Raskin, K.A.; Cote, G.M. Genome-wide DNA methylation patterns reveal clinically relevant predictive and prognostic subtypes in human osteosarcoma. Commun. Biol. 2022, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- Cristalli, C.; Scotlandi, K. Targeting DNA Methylation Machinery in Pediatric Solid Tumors. Cells 2024, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Herman, J.G. Generating mutations but providing chemosensitivity: The role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene 2004, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.C.; da Silva, R.M.; Stecchini, M.F.; Marrero-Gutiérrez, J.; e Silva, D.C.d.A.; Cardinalli, I.; Scrideli, C.A.; Junqueira, T.; Molina, C.A.; Ramalho, F.S. DNA methylation is a comprehensive marker for pediatric adrenocortical tumors. Endocr.-Relat. Cancer 2022, 29, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Krali, O.; Palle, J.; Bäcklin, C.L.; Abrahamsson, J.; Norén-Nyström, U.; Hasle, H.; Jahnukainen, K.; Jónsson, Ó.G.; Hovland, R.; Lausen, B. DNA methylation signatures predict cytogenetic subtype and outcome in pediatric acute myeloid leukemia (AML). Genes 2021, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Lalchungnunga, H.; Hao, W.; Maris, J.M.; Asgharzadeh, S.; Henrich, K.-O.; Westermann, F.; Tweddle, D.A.; Schwalbe, E.C.; Strathdee, G. Genome wide DNA methylation analysis identifies novel molecular subgroups and predicts survival in neuroblastoma. Br. J. Cancer 2022, 127, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Saint Fleur-Lominy, S.; Evensen, N.A.; Bhatla, T.; Sethia, G.; Narang, S.; Choi, J.H.; Ma, X.; Yang, J.J.; Kelly, S.; Raetz, E. Evolution of the epigenetic landscape in childhood B acute lymphoblastic leukemia and its role in drug resistance. Cancer Res. 2020, 80, 5189–5202. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.C.; Gray, L.-A.; White, C.L.; Maclean, F.M.; Grimison, P.; Ardakani, N.M.; Bonar, F.; Algar, E.M.; Cheah, A.L.; Russell, P. Genome wide methylation profiling of selected matched soft tissue sarcomas identifies methylation changes in metastatic and recurrent disease. Sci. Rep. 2021, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Aberuyi, N.; Rahgozar, S.; Ghodousi, E.S.; Ghaedi, K. Drug resistance biomarkers and their clinical applications in childhood acute lymphoblastic leukemia. Front. Oncol. 2020, 9, 1496. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA methyltransferase inhibitors combination therapy for the treatment of solid tumor: Mechanism and clinical application. Clin. Epigenetics 2021, 13, 166. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Prado-Garcia, H.; Carlos-Reyes, A. Role of DNA methylation in the resistance to therapy in solid tumors. Front. Oncol. 2020, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Fortuny, A.; Chansard, A.; Caron, P.; Chevallier, O.; Leroy, O.; Renaud, O.; Polo, S.E. Imaging the response to DNA damage in heterochromatin domains reveals core principles of heterochromatin maintenance. Nat. Commun. 2021, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Helin, K. Oncohistones: Drivers of pediatric cancers. Genes Dev. 2017, 31, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Zandian, M.; Gonzalez Salguero, N.; Shannon, M.D.; Purusottam, R.N.; Theint, T.; Poirier, M.G.; Jaroniec, C.P. Conformational dynamics of histone H3 tails in chromatin. J. Phys. Chem. Lett. 2021, 12, 6174–6181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of histone modification. Histone Mutat. Cancer 2021, 1283, 1–16. [Google Scholar]
- Gulati, R.; Fleifil, Y.; Jennings, K.; Bondoc, A.; Tiao, G.; Geller, J.; Timchenko, L.; Timchenko, N. Inhibition of histone deacetylase activity increases cisplatin efficacy to eliminate metastatic cells in pediatric liver cancers. Cancers 2024, 16, 2300. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Aliev, G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Rugo, H.S.; Jacobs, I.; Sharma, S.; Scappaticci, F.; Paul, T.A.; Jensen-Pergakes, K.; Malouf, G.G. The promise for histone methyltransferase inhibitors for epigenetic therapy in clinical oncology: A narrative review. Adv. Ther. 2020, 37, 3059–3082. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Bedford, M.T. Histone arginine methylation. FEBS Lett. 2011, 585, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Wakabayashi, S.; Soma, A.; Sato, S.; Nakatake, Y.; Oda, M.; Murakami, M.; Sakota, M.; Chikazawa-Nohtomi, N.; Ko, S.B. Transient ectopic expression of the histone demethylase JMJD3 accelerates the differentiation of human pluripotent stem cells. Development 2016, 143, 3674–3685. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yao, Y.; Gong, X.; Zhuo, Q.; Chen, J.; Tian, M.; Farzaneh, M. JMJD3: A critical epigenetic regulator in stem cell fate. Cell Commun. Signal. 2021, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Zhang, Y.; Zhao, Y.; Wang, T.; Zhang, J.; Yao, J.; Ma, N.; Liang, Z.; Huang, W.; Huang, K. JMJD3 and UTX determine fidelity and lineage specification of human neural progenitor cells. Nat. Commun. 2020, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef] [PubMed]
- De Santa, F.; Totaro, M.G.; Prosperini, E.; Notarbartolo, S.; Testa, G.; Natoli, G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 2007, 130, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Camarillo, J.M.; Huang, T.Y.-T.; Li, D.; Morris, J.A.; Zoltek, M.A.; Qi, J.; Behbahani, M.; Kambhampati, M.; Kelleher, N.L. Histone tail analysis reveals H3K36me2 and H4K16ac as epigenetic signatures of diffuse intrinsic pontine glioma. J. Exp. Clin. Cancer Res. 2020, 39, 261. [Google Scholar] [CrossRef]
- Badodi, S.; Marino, S. Epigenetic mechanisms in paediatric brain tumours: Regulators lose control. Biochem. Soc. Trans. 2022, 50, 167–185. [Google Scholar] [CrossRef]
- Klonou, A.; Korkolopoulou, P.; Gargalionis, A.N.; Kanakoglou, D.S.; Katifelis, H.; Gazouli, M.; Chlamydas, S.; Mitsios, A.; Kalamatianos, T.; Stranjalis, G. Histone mark profiling in pediatric astrocytomas reveals prognostic significance of H3K9 trimethylation and histone methyltransferase SUV39H1. Neurotherapeutics 2021, 18, 2073–2090. [Google Scholar] [CrossRef] [PubMed]
- Klonou, A.; Korkolopoulou, P.; Giannopoulou, A.-I.; Kanakoglou, D.S.; Pampalou, A.; Gargalionis, A.N.; Sarantis, P.; Mitsios, A.; Sgouros, S.; Papavassiliou, A.G. Histone H3K9 methyltransferase SETDB1 overexpression correlates with pediatric high-grade gliomas progression and prognosis. J. Mol. Med. 2023, 101, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Sepsa, A.; Levidou, G.; Gargalionis, A.; Adamopoulos, C.; Spyropoulou, A.; Dalagiorgou, G.; Thymara, I.; Boviatsis, E.; Themistocleous, M.S.; Petraki, K. Emerging role of linker histone variant H1x as a biomarker with prognostic value in astrocytic gliomas. A multivariate analysis including trimethylation of H3K9 and H4K20. PLoS ONE 2015, 10, e0115101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, Y.; Lin, S.; Chen, Y.; Qian, Y.; Zhao, Z.; Fan, H. H3K9me3, H3K36me3, and H4K20me3 expression correlates with patient outcome in esophageal squamous cell carcinoma as epigenetic markers. Dig. Dis. Sci. 2019, 64, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Agredo, A.; Kasinski, A.L. Histone 4 lysine 20 tri-methylation: A key epigenetic regulator in chromatin structure and disease. Front. Genet. 2023, 14, 1243395. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Burford, A.; Molinari, V.; Kessler, K.; Popov, S.; Clarke, M.; Taylor, K.R.; Pemberton, H.N.; Lord, C.J.; Gutteridge, A. Functional diversity and cooperativity between subclonal populations of pediatric glioblastoma and diffuse intrinsic pontine glioma cells. Nat. Med. 2018, 24, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wen, Y.; Jin, R.; Chen, H. Epigenetic modifications and targeted therapy in pediatric acute myeloid leukemia. Front. Pediatr. 2022, 10, 975819. [Google Scholar] [CrossRef]
- Dubuc, A.M.; Remke, M.; Korshunov, A.; Northcott, P.A.; Zhan, S.H.; Mendez-Lago, M.; Kool, M.; Jones, D.T.; Unterberger, A.; Morrissy, A.S. Aberrant patterns of H3K4 and H3K27 histone lysine methylation occur across subgroups in medulloblastoma. Acta Neuropathol. 2013, 125, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Li, Y.D.; Hoffman, L.; Hashizume, R.; Gravohac, G.; Rice, G.; Wadhwani, N.R.; Jie, C.; Pundy, T.; Mania-Farnell, B. Global reduction of H3K4me3 improves chemotherapeutic efficacy for pediatric ependymomas. Neoplasia 2019, 21, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.F.; Stripay, J.L. Epigenetic drivers in pediatric medulloblastoma. Cerebellum 2018, 17, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bressan, R.B.; Southgate, B.; Ferguson, K.M.; Blin, C.; Grant, V.; Alfazema, N.; Wills, J.C.; Marques-Torrejon, M.A.; Morrison, G.M.; Ashmore, J. Regional identity of human neural stem cells determines oncogenic responses to histone H3. 3 mutants. Cell Stem Cell 2021, 28, 877–893.e9. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, Y.; Zou, Z. Combining EZH2 inhibitors with other therapies for solid tumors: More choices for better effects. Epigenomics 2022, 14, 1449–1464. [Google Scholar] [CrossRef]
- Huang, T.Y.-T.; Piunti, A.; Qi, J.; Morgan, M.; Bartom, E.; Shilatifard, A.; Saratsis, A.M. Effects of H3. 3G34V mutation on genomic H3K36 and H3K27 methylation patterns in isogenic pediatric glioma cells. Acta Neuropathol. Commun. 2020, 8, 219. [Google Scholar] [CrossRef]
- Shi, L.; Shi, J.; Shi, X.; Li, W.; Wen, H. Histone H3. 3 G34 mutations alter histone H3K36 and H3K27 methylation in cis. J. Mol. Biol. 2018, 430, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.U.; Khazaei, S.; Marchione, D.M.; Lundgren, S.M.; Wang, X.; Weinberg, D.N.; Deshmukh, S.; Juretic, N.; Lu, C.; Allis, C.D. Histone H3. 3 G34 mutations promote aberrant PRC2 activity and drive tumor progression. Proc. Natl. Acad. Sci. USA 2020, 117, 27354–27364. [Google Scholar] [CrossRef] [PubMed]
- Moritz, L.E.; Trievel, R.C. Structure, mechanism, and regulation of polycomb-repressive complex 2. J. Biol. Chem. 2018, 293, 13805–13814. [Google Scholar] [CrossRef] [PubMed]
- Parreno, V.; Martinez, A.-M.; Cavalli, G. Mechanisms of Polycomb group protein function in cancer. Cell Res. 2022, 32, 231–253. [Google Scholar] [CrossRef]
- Becker, J.S.; Nicetto, D.; Zaret, K.S. H3K9me3-dependent heterochromatin: Barrier to cell fate changes. Trends Genet. 2016, 32, 29–41. [Google Scholar] [CrossRef]
- Béguelin, W.; Popovic, R.; Teater, M.; Jiang, Y.; Bunting, K.L.; Rosen, M.; Shen, H.; Yang, S.N.; Wang, L.; Ezponda, T. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013, 23, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Taube, J.H. Regulating methylation at H3K27: A trick or treat for cancer cell plasticity. Cancers 2020, 12, 2792. [Google Scholar] [CrossRef] [PubMed]
- Lund, K.; Adams, P.; Copland, M. EZH2 in normal and malignant hematopoiesis. Leukemia 2014, 28, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Nacev, B.A.; Jones, K.B.; Intlekofer, A.M.; Yu, J.S.; Allis, C.D.; Tap, W.D.; Ladanyi, M.; Nielsen, T.O. The epigenomics of sarcoma. Nat. Rev. Cancer 2020, 20, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M. Targeting H3K27me3 loss in pediatric brain tumors-a perspective on epigenetically guided cancer therapy. Oncotarget 2023, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Cress, W.D.; Seto, E. Histone deacetylases, transcriptional control, and cancer. J. Cell. Physiol. 2000, 184, 1–16. [Google Scholar] [CrossRef]
- Gallinari, P.; Marco, S.D.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, X.; Li, H. Beyond histone acetylation—Writing and erasing histone acylations. Curr. Opin. Struct. Biol. 2018, 53, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Pomeroy, S.L.; Pomeranz Krummel, D.A.; Sengupta, S. Epigenetics and survivorship in pediatric brain tumor patients. J. Neuro-Oncol. 2020, 150, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Brassesco, M.S.; Roberto, G.M.; Delsin, L.E.; Baldissera, G.C.; Medeiros, M.; Umezawa, K.; Tone, L.G. A foretaste for pediatric glioblastoma therapy: Targeting the NF-kB pathway with DHMEQ. Child’s Nerv. Syst. 2023, 39, 1519–1528. [Google Scholar] [CrossRef]
- Kumar, V.; Palermo, R.; Talora, C.; Campese, A.F.; Checquolo, S.; Bellavia, D.; Tottone, L.; Testa, G.; Miele, E.; Indraccolo, S. Notch and NF-kB signaling pathways regulate miR-223/FBXW7 axis in T-cell acute lymphoblastic leukemia. Leukemia 2014, 28, 2324–2335. [Google Scholar] [CrossRef]
- Medeiros, M.; Candido, M.F.; Valera, E.T.; Brassesco, M.S. The multifaceted NF-kB: Are there still prospects of its inhibition for clinical intervention in pediatric central nervous system tumors? Cell. Mol. Life Sci. 2021, 78, 6161–6200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Zhang, L.-Y.; Wen, R.; Yang, N.; Zhang, T.-N. Histone deacetylases and their inhibitors in inflammatory diseases. Biomed. Pharmacother. 2024, 179, 117295. [Google Scholar] [CrossRef]
- Ding, L.-W.; Sun, Q.-Y.; Tan, K.-T.; Chien, W.; Thippeswamy, A.M.; Eng Juh Yeoh, A.; Kawamata, N.; Nagata, Y.; Xiao, J.-F.; Loh, X.-Y. Mutational landscape of pediatric acute lymphoblastic leukemia. Cancer Res. 2017, 77, 390–400. [Google Scholar] [CrossRef]
- Ding, W.; Wang, D.; Cai, M.; Yan, Y.; Liu, S.; Liu, X.; Luo, A.; Deng, D.; Liu, X.; Jiang, H. PIWIL1 gene polymorphism and pediatric acute lymphoblastic leukemia relapse susceptibility among Chinese children: A five-center case–control study. Front. Oncol. 2023, 13, 1203002. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-y.; Ling, Z.-y.; Zhu, Y.-R.; Shi, C.; Wang, Y.; Zhang, X.-y.; Zhang, Z.-q.; Jiang, Q.; Chen, M.-B.; Yang, S. The histone acetyltransferase HBO1 functions as a novel oncogenic gene in osteosarcoma. Theranostics 2021, 11, 4599. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.J.; Gerbing, R.B.; Smith, J.L.; Ries, R.E.; Kolb, E.A.; Alonzo, T.A.; Meshinchi, S. Crebbp alterations are associated with a poor prognosis in de novo AML. Blood 2021, 138, 3451. [Google Scholar] [CrossRef]
- Milde, T.; Oehme, I.; Korshunov, A.; Kopp-Schneider, A.; Remke, M.; Northcott, P.; Deubzer, H.E.; Lodrini, M.; Taylor, M.D.; Von Deimling, A. HDAC5 and HDAC9 in medulloblastoma: Novel markers for risk stratification and role in tumor cell growth. Clin. Cancer Res. 2010, 16, 3240–3252. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Johnston II, M.E.; Gulati, R.; Kumbaji, M.; Aguiar, T.F.M.; Timchenko, L.; Krepischi, A.; Shin, S.; Bondoc, A.; Tiao, G. HDAC1-dependent repression of markers of hepatocytes and P21 is involved in development of pediatric liver cancer. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, B.; Usluer, S. p21 in cancer research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef]
- Charlab, R.; Racz, R. The expanding universe of NUTM1 fusions in pediatric cancer. Clin. Transl. Sci. 2023, 16, 1331–1339. [Google Scholar] [CrossRef]
- Kotekar, A.; Singh, A.K.; Devaiah, B.N. BRD4 and MYC: Power couple in transcription and disease. FEBS J. 2023, 290, 4820–4842. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Takahashi, Y.; Mizzen, C.A.; Cook, R.G.; Fujita, M.; Allis, C.D.; Frierson, H.F., Jr.; Fukusato, T.; Smith, M.M. Histone acetyltransferase Hbo1: Catalytic activity, cellular abundance, and links to primary cancers. Gene 2009, 436, 108–114. [Google Scholar] [CrossRef]
- Roussel, M.F.; Robinson, G.W. Role of MYC in Medulloblastoma. Cold Spring Harb. Perspect. Med. 2013, 3, a014308. [Google Scholar] [CrossRef]
- Schwalbe, E.C.; Lindsey, J.C.; Danilenko, M.; Hill, R.M.; Crosier, S.; Ryan, S.L.; Williamson, D.; Castle, J.; Hicks, D.; Kool, M. Molecular and clinical heterogeneity within MYC-family amplified medulloblastoma is associated with survival outcomes: A multicenter cohort study. Neuro-Oncology 2024, noae178. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.; Thatikonda, V.; Sigismondo, G.; Selt, F.; Valinciute, G.; Oehme, I.; Müller, C.; Buhl, J.L.; Ridinger, J.; Usta, D. Reduced chromatin binding of MYC is a key effect of HDAC inhibition in MYC amplified medulloblastoma. Neuro-Oncology 2021, 23, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Shofuda, T.; Kanemura, Y. HDACs and MYC in Medulloblastoma: How Do HDAC Inhibitors Control MYC-Amplified Tumors? Neuro-oncology 2021, 23, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Vlasevska, S.; Garcia-Ibanez, L.; Duval, R.; Holmes, A.B.; Jahan, R.; Cai, B.; Kim, A.; Mo, T.; Basso, K.; Soni, R.K. KMT2D acetylation by CREBBP reveals a cooperative functional interaction at enhancers in normal and malignant germinal center B cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2218330120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Z.; Li, Y.; Peng, H.; Liu, J.; Zhang, J.; Xiao, X. The role of CREBBP/EP300 and its therapeutic implications in hematological malignancies. Cancers 2023, 15, 1219. [Google Scholar] [CrossRef] [PubMed]
- Khodarev, N.N.; Roizman, B.; Weichselbaum, R.R. Molecular pathways: Interferon/stat1 pathway: Role in the tumor resistance to genotoxic stress and aggressive growth. Clin. Cancer Res. 2012, 18, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Wang, D.; Xiong, W.; Wang, X.; Huang, W.-h.; Wu, G.-h.; Liu, W.-z.; Wang, Q.; Chen, J.-s.; Kuai, Y.-y. Unveiling the role of HP1α-HDAC1-STAT1 axis as a therapeutic target for HP1α-positive intrahepatic cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 152. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.; Sun, G.; Wu, F.; Cheng, Y.; Sun, G.; Jiang, W.; Li, X.; Zhong, Y.; Wu, L.; Zhang, C. Epigenetics: Roles and therapeutic implications of non-coding RNA modifications in human cancers. Mol. Ther.-Nucleic Acids 2021, 25, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-W.; Huang, K.; Yang, C.; Kang, C.-S. Non-coding RNAs as regulators in epigenetics. Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Mostafavi, E.; Aref, A.R.; Sethi, G.; Wang, L.; Tergaonkar, V. Non-coding RNA-based regulation of inflammation. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Ginckels, P.; Holvoet, P. Focus: The science of stress: Oxidative stress and inflammation in cardiovascular diseases and cancer: Role of non-coding RNAs. Yale J. Biol. Med. 2022, 95, 129. [Google Scholar]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone modifications and non-coding RNAs: Mutual epigenetic regulation and role in pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Fayyaz, S.; Poltronieri, P.; Calin, G.; Mallardo, M. Epigenetic deregulation in cancer: Enzyme players and non-coding RNAs. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Morselli, M.; Dieci, G. Epigenetic regulation of human non-coding RNA gene transcription. Biochem. Soc. Trans. 2022, 50, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Della Bella, E.; Koch, J.; Baerenfaller, K. Translation and emerging functions of non-coding RNAs in inflammation and immunity. Allergy 2022, 77, 2025–2037. [Google Scholar] [CrossRef]
- Green, J.; Ansari, M.; Ball, H.; Haqqi, T. tRNA-derived fragments (tRFs) regulate post-transcriptional gene expression via AGO-dependent mechanism in IL-1β stimulated chondrocytes. Osteoarthr. Cartil. 2020, 28, 1102–1110. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Tsagakis, I.; Douka, K.; Birds, I.; Aspden, J.L. Long non-coding RNAs in development and disease: Conservation to mechanisms. J. Pathol. 2020, 250, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Eldesouki, S.; Samara, K.A.; Qadri, R.; Obaideen, A.A.; Otour, A.H.; Habbal, O.; Ahmed, S.B. XIST in brain cancer. Clin. Chim. Acta 2022, 531, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, T.B.; Wei, G.; Coker, H.; Pintacuda, G.; Bowness, J.S.; Zhang, T.; Almeida, M.; Bloechl, B.; Moindrot, B.; Carter, E.J. Systematic allelic analysis defines the interplay of key pathways in X chromosome inactivation. Nat. Commun. 2019, 10, 3129. [Google Scholar] [CrossRef]
- Sahakyan, A.; Yang, Y.; Plath, K. The role of Xist in X-chromosome dosage compensation. Trends Cell Biol. 2018, 28, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Agur, A.; Scheithauer, B.W.; Kovacs, K.; Lloyd, R.V.; Cusimano, M. Biomarkers of pituitary neoplasms: A review (Part II). Neurosurgery 2010, 67, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, X.; Zhao, Y.; Sun, G.; Zhang, J.; Gao, Y.; Liu, Q.; Zhang, W.; Zhu, H. Downregulation of lncRNA XIST represses tumor growth and boosts radiosensitivity of neuroblastoma via modulation of the miR-375/L1CAM Axis. Neurochem. Res. 2020, 45, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Akpa, M.M.; Iglesias, D.; Chu, L.; Thiébaut, A.; Jentoft, I.; Hammond, L.; Torban, E.; Goodyer, P.R. Wilms tumor suppressor, WT1, cooperates with microRNA-26a and microRNA-101 to suppress translation of the polycomb protein, EZH2, in mesenchymal stem cells. J. Biol. Chem. 2016, 291, 3785–3795. [Google Scholar] [CrossRef] [PubMed]
- Illarregi, U.; Telleria, J.; Bilbao-Aldaiturriaga, N.; Lopez-Lopez, E.; Ballesteros, J.; Martin-Guerrero, I.; Gutierrez-Camino, A. lncRNA deregulation in childhood acute lymphoblastic leukemia: A systematic review. Int. J. Oncol. 2022, 60, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiong, Q.-W.; Wang, J.-H.; Peng, W.-X. Roles of lncRNAs in childhood cancer: Current landscape and future perspectives. Front. Oncol. 2023, 13, 1060107. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Hu, Y.; Zhong, J.; Jiang, C.; Zhang, H. LncRNA CCAT1 sponges miR-218-5p to promote EMT, cellular migration and invasion of retinoblastoma by targeting MTF2. Cell. Signal. 2021, 86, 110088. [Google Scholar] [CrossRef] [PubMed]
- Rea, J.; Carissimo, A.; Trisciuoglio, D.; Illi, B.; Picard, D.; Remke, M.; Laneve, P.; Caffarelli, E. Identification and functional characterization of novel MYC-regulated long noncoding RNAs in group 3 medulloblastoma. Cancers 2021, 13, 3853. [Google Scholar] [CrossRef]
- Carvalho de Oliveira, J.; Molinari Roberto, G.; Baroni, M.; Bezerra Salomão, K.; Alejandra Pezuk, J.; Sol Brassesco, M. MiRNA dysregulation in childhood hematological cancer. Int. J. Mol. Sci. 2018, 19, 2688. [Google Scholar] [CrossRef] [PubMed]
- Deffenbacher, K.E.; Iqbal, J.; Sanger, W.; Shen, Y.; Lachel, C.; Liu, Z.; Liu, Y.; Lim, M.S.; Perkins, S.L.; Fu, K. Molecular distinctions between pediatric and adult mature B-cell non-Hodgkin lymphomas identified through genomic profiling. Blood J. Am. Soc. Hematol. 2012, 119, 3757–3766. [Google Scholar] [CrossRef] [PubMed]
- Galardi, A.; Colletti, M.; Di Paolo, V.; Vitullo, P.; Antonetti, L.; Russo, I.; Di Giannatale, A. Exosomal MiRNAs in pediatric cancers. Int. J. Mol. Sci. 2019, 20, 4600. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.; Mobark, N.; Al-Rashed, M.; AlHarbi, M. Epigenomics and immunotherapeutic advances in pediatric brain tumors. NPJ Precis. Oncol. 2021, 5, 34. [Google Scholar] [CrossRef]
- Gruszka, R.; Zakrzewski, J.; Nowosławska, E.; Grajkowska, W.; Zakrzewska, M. Identification and validation of miRNA-target genes network in pediatric brain tumors. Sci. Rep. 2024, 14, 17922. [Google Scholar] [CrossRef] [PubMed]
- Hovestadt, V.; Smith, K.S.; Bihannic, L.; Filbin, M.G.; Shaw, M.L.; Baumgartner, A.; DeWitt, J.C.; Groves, A.; Mayr, L.; Weisman, H.R. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 2019, 572, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Misiak, D.; Hagemann, S.; Bell, J.L.; Busch, B.; Lederer, M.; Bley, N.; Schulte, J.H.; Hüttelmaier, S. The MicroRNA landscape of MYCN-amplified neuroblastoma. Front. Oncol. 2021, 11, 647737. [Google Scholar] [CrossRef] [PubMed]
- Parodi, F.; Carosio, R.; Ragusa, M.; Di Pietro, C.; Maugeri, M.; Barbagallo, D.; Sallustio, F.; Allemanni, G.; Pistillo, M.P.; Casciano, I. Epigenetic dysregulation in neuroblastoma: A tale of miRNAs and DNA methylation. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2016, 1859, 1502–1514. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan, V.P.; Jayaraman, S.; Rengasamy, G.; Mony, U.; Ganapathy, D.M.; Geetha, R.V.; Sekar, D. Deciphering the role of microRNAs in neuroblastoma. Molecules 2021, 27, 99. [Google Scholar] [CrossRef] [PubMed]
- Anelli, L.; Zagaria, A.; Specchia, G.; Musto, P.; Albano, F. Dysregulation of miRNA in leukemia: Exploiting miRNA expression profiles as biomarkers. Int. J. Mol. Sci. 2021, 22, 7156. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, E.; Gaffo, E.; Niedermayer, A.; Boer, J.M.; Zimmermann, M.; Weichenhan, D.; Claus, R.; Münch, V.; Sun, Q.; Enzenmüller, S. MicroRNA-497/195 is tumor suppressive and cooperates with CDKN2A/B in pediatric acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2021, 138, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Camino, A.; Garcia-Obregon, S.; Lopez-Lopez, E.; Astigarraga, I.; Garcia-Orad, A. miRNA deregulation in childhood acute lymphoblastic leukemia: A systematic review. Epigenomics 2020, 12, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Wang, Y.; Li, Y.; Wurpel, J.N.; Huang, Z.; Chen, Z.-S. The battlefield of chemotherapy in pediatric cancers. Cancers 2023, 15, 1963. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Beylerli, O.; Liang, Y.; Xiang, H.; Liu, C.; Xu, X.; Yuan, C.; Ahmad, A.; Yang, G. The role of MicroRNAs in therapeutic resistance of malignant primary brain tumors. Front. Cell Dev. Biol. 2021, 9, 740303. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lu, Y.; Chen, Y.; Zhang, J.; Chen, Z.; Wang, Q. Research progress of MicroRNA in chemotherapy resistance of osteosarcoma. Technol. Cancer Res. Treat. 2021, 20, 15330338211034262. [Google Scholar] [CrossRef] [PubMed]
- Messiaen, J.; Jacobs, S.A.; De Smet, F. The tumor micro-environment in pediatric glioma: Friend or foe? Front. Immunol. 2023, 14, 1227126. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, N.A.; DeGolier, K.; Kovar, H.M.; Davis, A.; Hoglund, V.; Stevens, J.; Winter, C.; Deutsch, G.; Furlan, S.N.; Vitanza, N.A. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: Implications for development of immunotherapy. Neuro-Oncology 2019, 21, 83–94. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Geurts, M.; French, P.J.; Smits, M.; Capper, D.; Bromberg, J.E.; Chang, S.M. Primary brain tumours in adults. Lancet 2023, 402, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Ramkissoon, S.H.; Ross, J.; Weintraub, L. Tumor mutational burden and driver mutations: Characterizing the genomic landscape of pediatric brain tumors. Pediatr. Blood Cancer 2020, 67, e28338. [Google Scholar] [CrossRef]
- Roux, A.; Pallud, J.; Saffroy, R.; Edjlali-Goujon, M.; Debily, M.-A.; Boddaert, N.; Sanson, M.; Puget, S.; Knafo, S.; Adam, C. High-grade gliomas in adolescents and young adults highlight histomolecular differences from their adult and pediatric counterparts. Neuro-Oncology 2020, 22, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Baumgarth, N. The shaping of a B cell pool maximally responsive to infections. Annu. Rev. Immunol. 2021, 39, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.H.; Smith, F.L.; Baumgarth, N. B cell activation and response regulation during viral infections. Viral Immunol. 2020, 33, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Scharping, N.E.; Goldrath, A.W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 2021, 21, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yoshimoto, M. Multiple waves of fetal-derived immune cells constitute adult immune system. Immunol. Rev. 2023, 315, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.P.; Moreno, B.; Meraviglia-Crivelli, D.; Nonatelli, F.; Villanueva, H.; Barainka, M.; Zheleva, A.; Van Santen, H.M.; Pastor, F. Modulating T cell responses by targeting CD3. Cancers 2023, 15, 1189. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.; Pina-Vaz, C.; Baltazar, F. Microbes and cancer: Friends or faux? Int. J. Mol. Sci. 2020, 21, 3115. [Google Scholar] [CrossRef] [PubMed]
- Abad, E.; Graifer, D.; Lyakhovich, A. DNA damage response and resistance of cancer stem cells. Cancer Lett. 2020, 474, 106–117. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, T.; Sun, T.; Dilimulati, D.; Xiao, Y. The oncomicrobiome: New insights into microorganisms in cancer. Microb. Pathog. 2024, 197, 107091. [Google Scholar] [CrossRef] [PubMed]
- Bert, S.; Ward, E.J.; Nadkarni, S. Neutrophils in pregnancy: New insights into innate and adaptive immune regulation. Immunology 2021, 164, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Ginhoux, F.; Yona, S. Monocytes, macrophages, dendritic cells and neutrophils: An update on lifespan kinetics in health and disease. Immunology 2021, 163, 250–261. [Google Scholar] [CrossRef] [PubMed]
- True, H.; Blanton, M.; Sureshchandra, S.; Messaoudi, I. Monocytes and macrophages in pregnancy: The good, the bad, and the ugly. Immunol. Rev. 2022, 308, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hirschi, K.K. Tissue-resident macrophage development and function. Front. Cell Dev. Biol. 2021, 8, 617879. [Google Scholar] [CrossRef] [PubMed]
- Pieren, D.K.; Boer, M.C.; de Wit, J. The adaptive immune system in early life: The shift makes it count. Front. Immunol. 2022, 13, 1031924. [Google Scholar] [CrossRef] [PubMed]
- Semmes, E.C.; Chen, J.-L.; Goswami, R.; Burt, T.D.; Permar, S.R.; Fouda, G.G. Understanding early-life adaptive immunity to guide interventions for pediatric health. Front. Immunol. 2021, 11, 595297. [Google Scholar] [CrossRef] [PubMed]
- Hau, P.M.; Lung, H.L.; Wu, M.; Tsang, C.M.; Wong, K.-L.; Mak, N.K.; Lo, K.W. Targeting Epstein-Barr virus in nasopharyngeal carcinoma. Front. Oncol. 2020, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, O.; Colli, S.; Garcia Lombardi, M.; Preciado, M.V.; De Matteo, E.; Chabay, P. Epstein–Barr virus recruits PDL1-positive cells at the microenvironment in pediatric Hodgkin lymphoma. Cancer Immunol. Immunother. 2021, 70, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein–Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Frappier, L. Epstein–Barr Virus is an Agent of Genomic Instability; Nature Publishing Group UK London: London, UK, 2023. [Google Scholar]
- Harper, K.L.; Harrington, E.M.; Hayward, C.; Anene, C.A.; Wongwiwat, W.; White, R.E.; Whitehouse, A. Virus-modified paraspeckle-like condensates are hubs for viral RNA processing and their formation drives genomic instability. Nat. Commun. 2024, 15, 10240. [Google Scholar] [CrossRef]
- Ahye, N.; Bellizzi, A.; May, D.; Wollebo, H.S. The role of the JC virus in central nervous system tumorigenesis. Int. J. Mol. Sci. 2020, 21, 6236. [Google Scholar] [CrossRef] [PubMed]
- Mayr, L.; Steinmaurer, T.; Weseslindtner, L.; Madlener, S.; Strassl, R.; Gojo, J.; Azizi, A.A.; Slavc, I.; Peyrl, A. Viral infections in pediatric brain tumor patients treated with targeted therapies. Pediatr. Blood Cancer 2023, 70, e30065. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Gordon, J.; Enam, S.; Delbue, S.; Croul, S.; Abraham, S.; Radhakrishnan, S.; Assimakopoulou, M.; Katsetos, C.D.; Khalili, K. Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J. Natl. Cancer Inst. 2002, 94, 267–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Clermont, G.; Vodovotz, Y.; Chow, C.C. The dynamics of acute inflammation. J. Theor. Biol. 2004, 230, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, S.; Aranda-Uribe, I.S.; Pérez-Amado, C.J.; Ramírez-Bello, J.; Hidalgo-Miranda, A. Mechanisms of immunosuppressive tumor evasion: Focus on acute lymphoblastic leukemia. Front. Immunol. 2021, 12, 737340. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, D.A.; Anderson, J. Inflammation: What role in pediatric cancer? Pediatr. Blood Cancer 2012, 58, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Giridharan, T.; Suzuki, S.; Khan, A.N.H.; Zsiros, E.; Emmons, T.R.; Yaffe, M.B.; Gankema, A.A.; Hoogeboom, M.; Goetschalckx, I. Neutrophil interactions with T cells, platelets, endothelial cells, and of course tumor cells. Immunol. Rev. 2023, 314, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.P.; Li, W. Tumor necrosis: A synergistic consequence of metabolic stress and inflammation. Bioessays 2021, 43, 2100029. [Google Scholar] [CrossRef] [PubMed]
- Bockmayr, M.; Klauschen, F.; Maire, C.L.; Rutkowski, S.; Westphal, M.; Lamszus, K.; Schüller, U.; Mohme, M. Immunologic profiling of mutational and transcriptional subgroups in pediatric and adult high-grade gliomas. Cancer Immunol. Res. 2019, 7, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Bockmayr, M.; Mohme, M.; Klauschen, F.; Winkler, B.; Budczies, J.; Rutkowski, S.; Schüller, U. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology 2018, 7, e1462430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hambardzumyan, D. Immune microenvironment in glioblastoma subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Grabovska, Y.; Mackay, A.; O’Hare, P.; Crosier, S.; Finetti, M.; Schwalbe, E.C.; Pickles, J.C.; Fairchild, A.R.; Avery, A.; Cockle, J. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 2020, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Güç, E.; Pollard, J.W. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity 2021, 54, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Johnson, K.C.C.; Gatti-Mays, M.E.; Li, Z. Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy. J. Hematol. Oncol. 2022, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Doak, G.R.; Schwertfeger, K.L.; Wood, D.K. Distant relations: Macrophage functions in the metastatic niche. Trends Cancer 2018, 4, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Frederico, S.C.; Sharma, N.; Darling, C.; Taori, S.; Dubinsky, A.C.; Zhang, X.; Raphael, I.; Kohanbash, G. Myeloid cells as potential targets for immunotherapy in pediatric gliomas. Front. Pediatr. 2024, 12, 1346493. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, A.C.; Zea, A.H.; Hernandez, C.; Rodriguez, P.C. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 721s–726s. [Google Scholar] [CrossRef] [PubMed]
- Kailayangiri, S.; Altvater, B.; Urban, K.; Meltzer, J.; Greune, L.; Farwick, N.; Jamitzky, S.; Rossig, C. Evaluation of anti-Gr1 antibody for depletion of MDSC in preclinical NSG mouse models of pediatric sarcoma. Cancer Res. 2020, 80, 4999. [Google Scholar] [CrossRef]
- Perzolli, A.; Koedijk, J.B.; Zwaan, C.M.; Heidenreich, O. Targeting the innate immune system in pediatric and adult AML. Leukemia 2024, 38, 1191–1201. [Google Scholar] [CrossRef]
- Thakur, M.D.; Franz, C.J.; Brennan, L.; Brouwer-Visser, J.; Tam, R.; Korski, K.; Koeppen, H.; Ziai, J.; Babitzki, G.; Ranchere-Vince, D. Immune contexture of paediatric cancers. Eur. J. Cancer 2022, 170, 179–193. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.P.; Gilroy, D.W. Macrophage development and polarization in chronic inflammation. Semin Immunol 2015, 27, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lavin, Y.; Mortha, A.; Rahman, A.; Merad, M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 2015, 15, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Feng, X.; Herting, C.J.; Garcia, V.A.; Nie, K.; Pong, W.W.; Rasmussen, R.; Dwivedi, B.; Seby, S.; Wolf, S.A. Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 2017, 77, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Sidibe, A.; Ropraz, P.; Jemelin, S.; Emre, Y.; Poittevin, M.; Pocard, M.; Bradfield, P.F.; Imhof, B.A. Angiogenic factor-driven inflammation promotes extravasation of human proangiogenic monocytes to tumours. Nat. Commun. 2018, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Ishina, I.A.; Zakharova, M.Y.; Kurbatskaia, I.N.; Mamedov, A.E.; Belogurov, A.A., Jr.; Gabibov, A.G. MHC class II presentation in autoimmunity. Cells 2023, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, A.; Ortiz, L.A. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front. Immunol. 2022, 13, 936167. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Mora, J.; Namgaladze, D.; Weigert, A.; Brüne, B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr. Opin. Pharmacol. 2017, 35, 12–19. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, X.; Wang, Y.; Liu, J.; Li, Z.; Li, S.; Liu, Y.; Gong, X.; Sun, Y.; Wu, W. Tumor-associated macrophages correlate with prognosis in medulloblastoma. Front. Oncol. 2022, 12, 893132. [Google Scholar] [CrossRef]
- Lin, H.; Wei, S.; Hurt, E.M.; Green, M.D.; Zhao, L.; Vatan, L.; Szeliga, W.; Herbst, R.; Harms, P.W.; Fecher, L.A. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade–mediated tumor regression. J. Clin. Investig. 2018, 128, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Dai, R.; Lapalombella, R.; Baiocchi, R.A.; Benson, D.M.; Li, Z.; Huang, X.; Yang, Y. Hedgehog-induced PD-L1 on tumor-associated macrophages is critical for suppression of tumor-infiltrating CD8+ T cell function. JCI Insight 2021, 6, e146707. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Hino, M.; Koh, K.; Kyushiki, M.; Kishimoto, H.; Arakawa, Y.; Hanada, R.; Kawashima, H.; Kurihara, J.; Shimojo, N. Low frequency of programmed death ligand 1 expression in pediatric cancers. Pediatr. Blood Cancer 2016, 63, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.; Park, J.R.; Murphy, E.; Yearley, J.; McClanahan, T.; Annamalai, L.; Hawkins, D.S.; Rudzinski, E.R. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr. Blood Cancer 2017, 64, e26613. [Google Scholar] [CrossRef]
- van Dam, L.S.; de Zwart, V.M.; Meyer-Wentrup, F.A. The role of programmed cell death-1 (PD-1) and its ligands in pediatric cancer. Pediatr. Blood Cancer 2015, 62, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-associated macrophages in osteosarcoma: From mechanisms to therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef] [PubMed]
- Dumars, C.; Ngyuen, J.-M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.-F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343. [Google Scholar] [CrossRef]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019, 440, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.X.; Joshi, S. “Re-educating” tumor associated macrophages as a novel immunotherapy strategy for neuroblastoma. Front. Immunol. 2020, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic cells revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Wesa, A.K.; Galy, A. IL-1β induces dendritic cells to produce IL-12. Int. Immunol. 2001, 13, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Dutertre, C.-A.; Scott, C.L.; McGovern, N.; Sichien, D.; Chakarov, S.; Van Gassen, S.; Chen, J.; Poidinger, M.; De Prijck, S. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 2016, 45, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Segura, E. Human dendritic cell subsets: An updated view of their ontogeny and functional specialization. Eur. J. Immunol. 2022, 52, 1759–1767. [Google Scholar] [CrossRef]
- Albert, M.L.; Sauter, B.; Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998, 392, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Musella, M.; Galassi, C.; Manduca, N.; Sistigu, A. The yin and yang of type I IFNs in cancer promotion and immune activation. Biology 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Reizis, B. Plasmacytoid dendritic cells: Development, regulation, and function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Vakkila, J.; Jaffe, R.; Michelow, M.; Lotze, M.T. Pediatric cancers are infiltrated predominantly by macrophages and contain a paucity of dendritic cells: A major nosologic difference with adult tumors. Clin. Cancer Res. 2006, 12, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, H.; Schneider, K.; Evdokimova, V.; Ruzanov, P.; Schober, S.J.; Xue, B.; von Heyking, K.; Thiede, M.; Richter, G.H.; Pfaffl, M.W. Ewing sarcoma-derived extracellular vesicles impair dendritic cell maturation and function. Cells 2021, 10, 2081. [Google Scholar] [CrossRef] [PubMed]
- Melcher, V.; Kerl, K. The growing relevance of immunoregulation in pediatric brain tumors. Cancers 2021, 13, 5601. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.E.; Lynn, G.M.; Valdes, P.A.; Cerecedo Lopez, C.D.; Ishizuka, A.S.; Arnaout, O.; Bi, W.L.; Peruzzi, P.P.; Chiocca, E.A.; Friedman, G.K. Therapeutic cancer vaccines for pediatric malignancies: Advances, challenges, and emerging technologies. Neuro-Oncol. Adv. 2021, 3, vdab027. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, M.; Lo Presti, V.; de Haar, C.G.; Dünnebach, E.; Madrigal, A.; Lindemans, C.A.; Boelens, J.J.; Nierkens, S. Clinical grade production of wilms’ tumor-1 loaded cord blood-derived dendritic cells to prevent relapse in pediatric AML after cord blood transplantation. Front. Immunol. 2020, 11, 559152. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, S.; Anguille, S.; Verlooy, J.; Smits, E.L.; van Tendeloo, V.F.; De Laere, M.; Norga, K.; Berneman, Z.N.; Lion, E. Dendritic cell-based and other vaccination strategies for pediatric cancer. Cancers 2019, 11, 1396. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Anguille, S.; Van Tendeloo, V.F.; Lion, E. Empowering gamma delta T cells with antitumor immunity by dendritic cell-based immunotherapy. Oncoimmunology 2015, 4, e1021538. [Google Scholar] [CrossRef]
- Alzumaili, B.; Xu, B.; Spanheimer, P.M.; Tuttle, R.M.; Sherman, E.; Katabi, N.; Dogan, S.; Ganly, I.; Untch, B.R.; Ghossein, R.A. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod. Pathol. 2020, 33, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.W.; Kim, J.J.; Jeong, S.C.; Kim, Y.H.; Han, J.W. Clinical significance of tumor necrosis and viability in non-small cell lung cancer. J. Thorac. Dis. 2022, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Karsch-Bluman, A.; Benny, O. Necrosis in the tumor microenvironment and its role in cancer recurrence. Tumor Microenviron. Recent Adv. 2020, 1225, 89–98. [Google Scholar]
- Land, W.G. The role of damage-associated molecular patterns (DAMPs) in human diseases: Part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ. Med. J. 2015, 15, e157. [Google Scholar] [PubMed]
- Chirra, M.; Newton, H.S.; Gawali, V.S.; Wise-Draper, T.M.; Chimote, A.A.; Conforti, L. How the potassium channel response of T lymphocytes to the tumor microenvironment shapes antitumor immunity. Cancers 2022, 14, 3564. [Google Scholar] [CrossRef]
- Eil, R.; Vodnala, S.K.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Pan, J.H.; Palmer, D.C.; Gros, A.; Yamamoto, T.N.; Patel, S.J. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016, 537, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Bomken, S.; Davies, B.; Chong, L.; Cole, M.; Wood, K.M.; McDermott, M.; Tweddle, D.A. Percentage tumor necrosis following chemotherapy in neuroblastoma correlates with MYCN status but not survival. Pediatr. Hematol. Oncol. 2011, 28, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hanif, I.; Mahmoud, H.; Pui, C.H. Avascular femoral head necrosis in pediatric cancer patients. Med. Pediatr. Oncol. 1993, 21, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Mersfelder, R.B.; Lwin, C.; Malik, S.; Badgett, T.C.; Chenard, S.W.; Rekulapelli, A.; Blette, B.S.; Lawrenz, J.M.; Borinstein, S.C. Chemotherapy–Surgery Interval Effects on Tumor Necrosis and Outcome in Children and Young Adults With Osteosarcoma. Pediatr. Blood Cancer 2024, e31466. [Google Scholar] [CrossRef] [PubMed]
- Fiala, E.M.; Jayakumaran, G.; Mauguen, A.; Kennedy, J.A.; Bouvier, N.; Kemel, Y.; Fleischut, M.H.; Maio, A.; Salo-Mullen, E.E.; Sheehan, M. Prospective pan-cancer germline testing using MSK-IMPACT informs clinical translation in 751 patients with pediatric solid tumors. Nat. Cancer 2021, 2, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.I.; Sayour, E.J.; Flores, C.T.; Grant, G.; Wechsler-Reya, R.; Hoang-Minh, L.B.; Kieran, M.W.; Salcido, J.; Prins, R.M.; Figg, J.W. The current landscape of immunotherapy for pediatric brain tumors. Nat. Cancer 2022, 3, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.J.; Kearney, C.J.; Silke, J.; Oliaro, J. Unleashing TNF cytotoxicity to enhance cancer immunotherapy. Trends Immunol. 2021, 42, 1128–1142. [Google Scholar] [CrossRef]
- Mori, T.; Sato, Y.; Miyamoto, K.; Kobayashi, T.; Shimizu, T.; Kanagawa, H.; Katsuyama, E.; Fujie, A.; Hao, W.; Tando, T. TNFα promotes osteosarcoma progression by maintaining tumor cells in an undifferentiated state. Oncogene 2014, 33, 4236–4241. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Hayashi, M.; Verneris, M.R.; Lee-Sherick, A.B. Targeting tumor-associated macrophages in the pediatric sarcoma tumor microenvironment. Front. Oncol. 2020, 10, 581107. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Molnarfi, N.; Gruaz, L.; Dayer, J.-M. Differential induction of IL-1β and TNF by CD40 ligand or cellular contact with stimulated T cells depends on the maturation stage of human monocytes. J. Immunol. 2004, 173, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, M.; Dupaul-Chicoine, J.; Douglas, T.; Champagne, C.; Morizot, A.; Saleh, M. The Interleukin (IL)-1R1 pathway is a critical negative regulator of PyMT-mediated mammary tumorigenesis and pulmonary metastasis. Oncoimmunology 2017, 6, e1287247. [Google Scholar] [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Borcherding, N.; Kolb, R. IL-1 signaling in tumor microenvironment. Tumor Microenviron. Role Interleukins–Part A 2020, 1240, 1–23. [Google Scholar]
- Landuzzi, L.; Ruzzi, F.; Pellegrini, E.; Lollini, P.-L.; Scotlandi, K.; Manara, M.C. IL-1 Family Members in Bone Sarcomas. Cells 2024, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Soker, M.; Çolpan, L.; Ece, A.; Devecioğlu, C.; Haspolat, K. Serum levels of IL-1 beta, sIL-2R, IL-6, IL-8, and TNF-alpha in febrile children with cancer and neutropenia. Med. Oncol. 2001, 18, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Rašková, M.; Lacina, L.; Kejík, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K., Jr.; Brábek, J. The role of IL-6 in cancer cell invasiveness and metastasis—Overview and therapeutic opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, S.; Fang, T.; Qiu, X.; Wang, X.; Zhou, X.; Morse, M.A.; Hobeika, A.; Wu, W.; Yang, H. Changes in peripheral blood regulatory T cells and IL-6 and IL-10 levels predict response of pediatric medulloblastoma and germ cell tumors with residual or disseminated disease to craniospinal irradiation. Int. J. Radiat. Oncol. * Biol. * Phys. 2021, 111, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.M.; Miller, J.M.; Munoz, J.O.; Gaikwad, A.S.; Redell, M.S. Interleukin-6 levels predict event-free survival in pediatric AML and suggest a mechanism of chemotherapy resistance. Blood Adv. 2017, 1, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A. Influences of the IL-6 cytokine family on bone structure and function. Cytokine 2021, 146, 155655. [Google Scholar] [CrossRef]
- Chua, L.L.; Rajasuriar, R.; Azanan, M.S.; Abdullah, N.K.; Tang, M.S.; Lee, S.C.; Woo, Y.L.; Lim, Y.A.L.; Ariffin, H.; Loke, P.n. Reduced microbial diversity in adult survivors of childhood acute lymphoblastic leukemia and microbial associations with increased immune activation. Microbiome 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Vahabnezhad, E.; Mochon, A.B.; Wozniak, L.J.; Ziring, D.A. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J. Clin. Gastroenterol. 2013, 47, 437–439. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Sun, Y.; Zhang, J.; Yao, Y.; Shen, Z.; Xiang, D.; He, A. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci. Rep. 2016, 6, 39862. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Geoerger, B.; Kang, H.J.; Yalon-Oren, M.; Marshall, L.V.; Vezina, C.; Pappo, A.; Laetsch, T.W.; Petrilli, A.S.; Ebinger, M.; Toporski, J. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): Interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020, 21, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Roemer, M.G.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Long, A.H.; Morgenstern, D.A.; Leruste, A.; Bourdeaut, F.; Davis, K.L. Checkpoint Immunotherapy in Pediatrics: Here, Gone, and Back Again. In American Society of Clinical Oncology Educational book 2022, 42, 1–14. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Giovannoni, R.; Fruci, D.; Gemignani, F. News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Tabori, U.; Sambira Nahum, L.C.; Collins, N.B.; Deyell, R.; Dvir, R.; Faure-Conter, C.; Hassall, T.E.; Minturn, J.E.; Edwards, M.; et al. Efficacy of Nivolumab in Pediatric Cancers with High Mutation Burden and Mismatch Repair Deficiency. Clin. Cancer Res. 2023, 29, 4770–4783. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, I.J.; Doz, F.; Foreman, N.K.; Hargrave, D.; Lassaletta, A.; Andre, N.; Hansford, J.R.; Hassall, T.; Eyrich, M.; Gururangan, S.; et al. Nivolumab with or without ipilimumab in pediatric patients with high-grade CNS malignancies: Safety, efficacy, biomarker, and pharmacokinetics-CheckMate 908. Neuro-Oncology 2023, 25, 1530–1545. [Google Scholar] [CrossRef]
- Kaneda, D.; Iehara, T.; Kikuchi, K.; Sugimoto, Y.; Nakagawa, N.; Yagyu, S.; Miyachi, M.; Konishi, E.; Sakai, T.; Hosoi, H. The histone deacetylase inhibitor OBP-801 has in vitro/in vivo anti-neuroblastoma activity. Pediatr. Int. 2022, 64, e15159. [Google Scholar] [CrossRef] [PubMed]
- Nawar, N.; Bukhari, S.; Adile, A.A.; Suk, Y.; Manaswiyoungkul, P.; Toutah, K.; Olaoye, O.O.; Raouf, Y.S.; Sedighi, A.; Garcha, H.K. Discovery of HDAC6-selective inhibitor NN-390 with in vitro efficacy in group 3 medulloblastoma. J. Med. Chem. 2022, 65, 3193–3217. [Google Scholar] [CrossRef] [PubMed]
- Chilamakuri, R.; Agarwal, S. Dual targeting of PI3K and HDAC by CUDC-907 inhibits pediatric neuroblastoma growth. Cancers 2022, 14, 1067. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.I.; Honeyman, J.N.; Zhang, J.; Lin, C.; Sharma, A.; Zhou, L.; Oliveira, J.; Tapinos, N.; Lulla, R.R.; Prabhu, V.V.; et al. Novel combination of imipridones and histone deacetylase inhibitors demonstrate cytotoxic effect through integrated stress response in pediatric solid tumors. Am. J. Cancer Res. 2023, 13, 6241–6255. [Google Scholar] [PubMed]
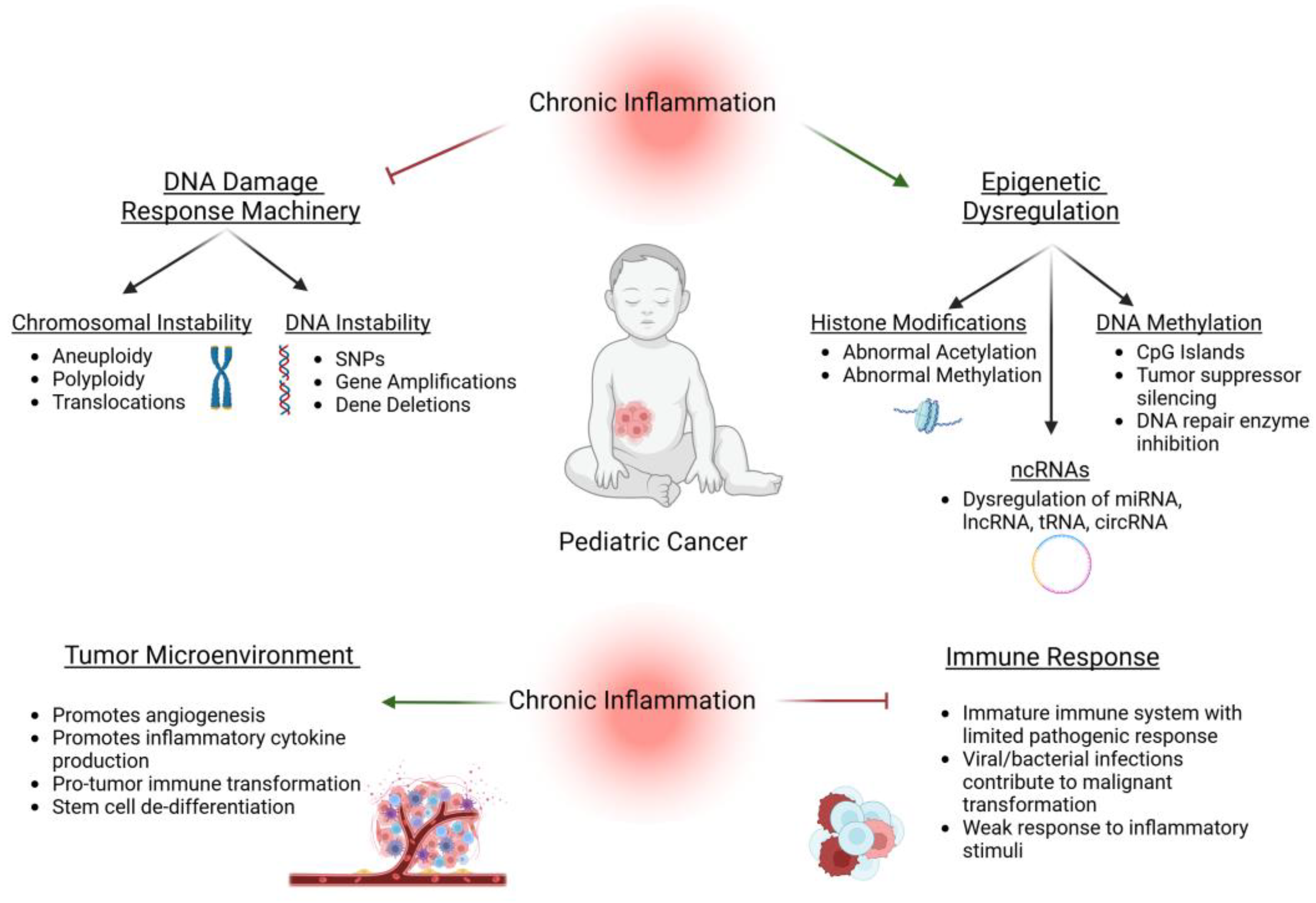

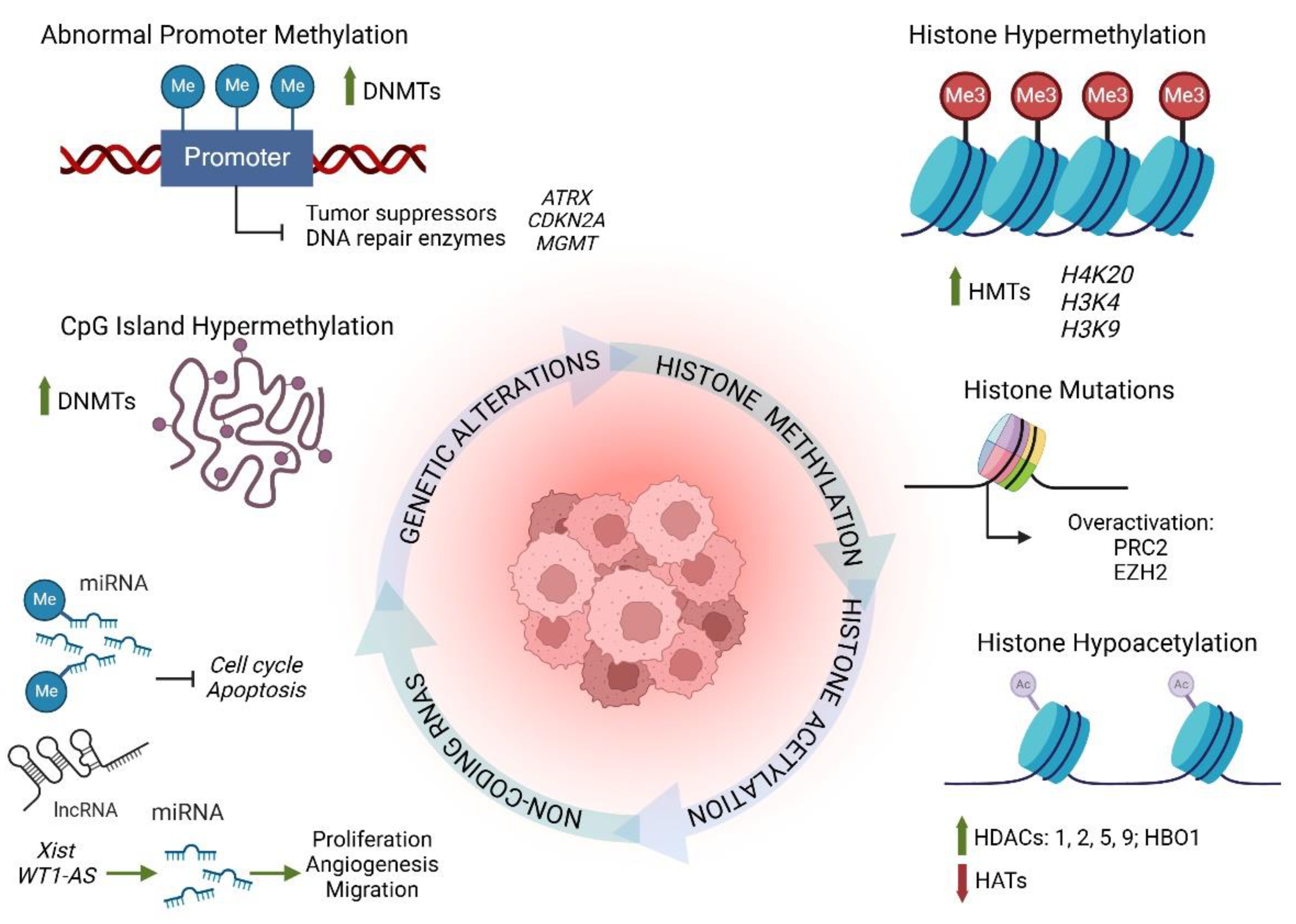

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mella, C.; Tsarouhas, P.; Brockwell, M.; Ball, H.C. The Role of Chronic Inflammation in Pediatric Cancer. Cancers 2025, 17, 154. https://doi.org/10.3390/cancers17010154
Mella C, Tsarouhas P, Brockwell M, Ball HC. The Role of Chronic Inflammation in Pediatric Cancer. Cancers. 2025; 17(1):154. https://doi.org/10.3390/cancers17010154
Chicago/Turabian StyleMella, Christine, Panogiotis Tsarouhas, Maximillian Brockwell, and Hope C. Ball. 2025. "The Role of Chronic Inflammation in Pediatric Cancer" Cancers 17, no. 1: 154. https://doi.org/10.3390/cancers17010154
APA StyleMella, C., Tsarouhas, P., Brockwell, M., & Ball, H. C. (2025). The Role of Chronic Inflammation in Pediatric Cancer. Cancers, 17(1), 154. https://doi.org/10.3390/cancers17010154



