Interventional Treatments of Colorectal Liver Metastases Using Thermal Ablation and Transarterial Chemoembolization: A Single-Center Experience over 26 Years
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patient Cohort
2.3. Statistics
3. Results
3.1. Treatments
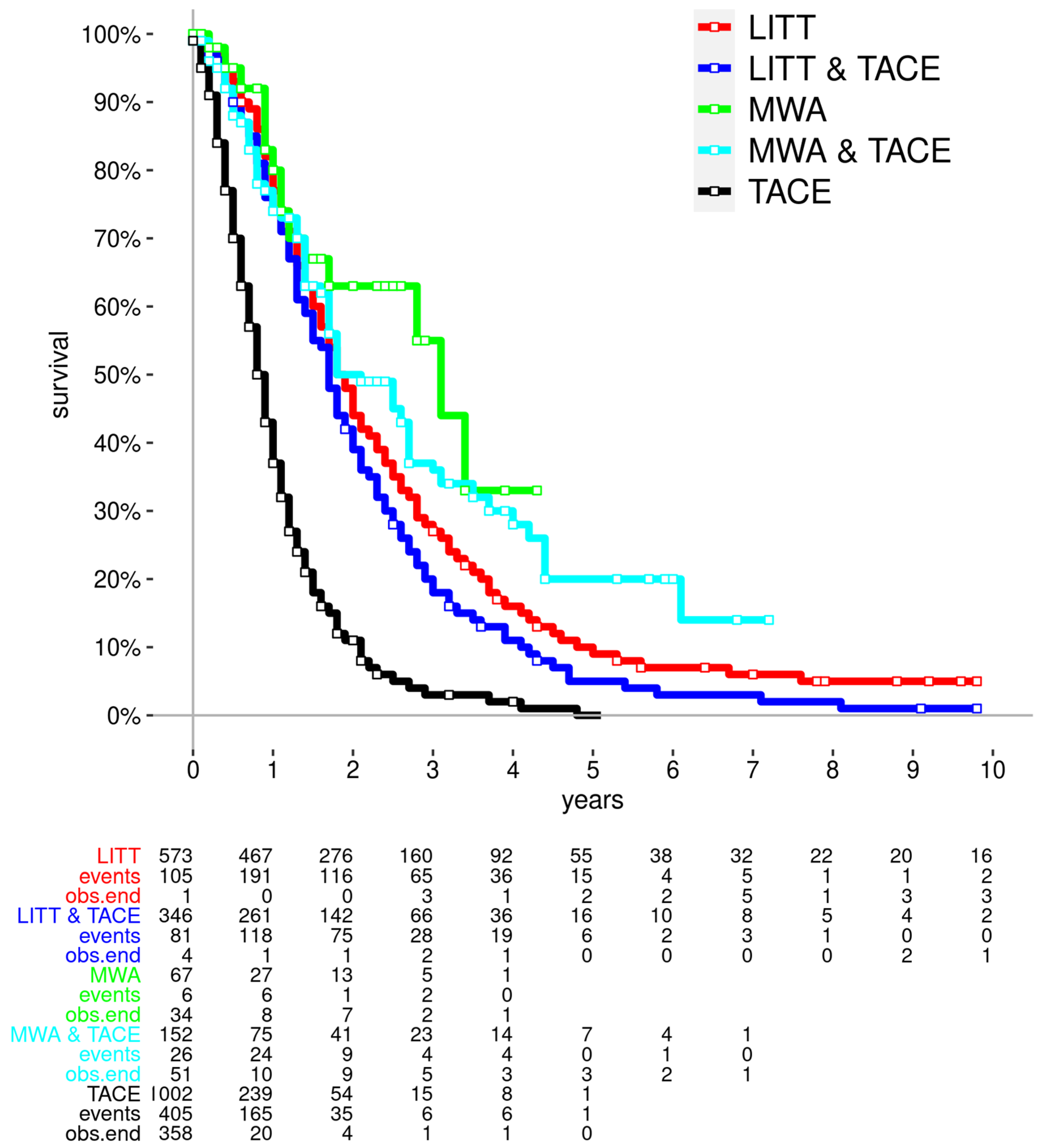
3.2. Survival
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef] [PubMed]
- Parnaby, C.N.; Bailey, W.; Balasingam, A.; Beckert, L.; Eglinton, T.; Fife, J.; Frizelle, F.A.; Jeffery, M.; Watson, A.J. Pulmonary staging in colorectal cancer: A review. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2012, 14, 660–670. [Google Scholar] [CrossRef]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef] [PubMed]
- Finotti, M.; D’Amico, F.E.; Romano, M.; Brizzolari, M.; Scopelliti, M.; Zanus, G. Colorectal Liver Metastases: A Literature Review of Viable Surgical Options with a Special Focus on Microwave Liver Thermal Ablation and Mini-Invasive Approach. J. Pers. Med. 2022, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Vinet, E. Regional treatment of metastasis: Surgery of colorectal liver metastases. Ann. Oncol. 2004, 15 (Suppl. S4), iv103–iv106. [Google Scholar] [CrossRef] [PubMed]
- Kallini, J.R.; Gabr, A.; Abouchaleh, N.; Ali, R.; Riaz, A.; Lewandowski, R.J.; Salem, R. New Developments in Interventional Oncology: Liver Metastases from Colorectal Cancer. Cancer J. 2016, 22, 373–380. [Google Scholar] [CrossRef]
- O’Leary, C.; Soulen, M.C.; Shamimi-Noori, S. Interventional Oncology Approach to Hepatic Metastases. Semin. Interv. Radiol. 2020, 37, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Berber, E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg. Nutr. 2020, 9, 49–58. [Google Scholar] [CrossRef]
- Mensel, B.; Weigel, C.; Heidecke, C.D.; Stier, A.; Hosten, N. Laserinduzierte Thermotherapie (LITT) von Lebertumoren in zentraler Lokalisation: Ergebnisse und Komplikationen [Laser-induced thermotherapy (LITT) of tumors of the liver in central location: Results and complications]. RoFo Fortschritte Auf Dem Geb. Rontgenstrahlen Nukl. 2005, 177, 1267–1275. [Google Scholar] [CrossRef]
- Massmann, A.; Rodt, T.; Marquardt, S.; Seidel, R.; Thomas, K.; Wacker, F.; Richter, G.M.; Kauczor, H.U.; Bücker, A.; Pereira, P.L.; et al. Transarterial chemoembolization (TACE) for colorectal liver metastases—Current status and critical review. Langenbeck’s Arch. Surg. 2015, 400, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Tombesi, P.; Vece, F.D.; Sartori, S. Radiofrequency, microwave, and laser ablation of liver tumors: Time to move toward a tailored ablation technique? Hepatoma. Res. 2015, 1, 52–57. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Ng, C.K. Image-guided thermal ablation with MR-based thermometry. Quant. Imaging Med. Surg. 2017, 7, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Choi, J.W.; Kwon, H.; Kim, K.Y.; Lee, B.C.; Chu, H.H.; Lee, D.H.; Lee, H.A.; Kim, G.M.; Oh, J.S.; et al. Transarterial Chemoembolization for Hepatocellular Carcinoma: 2023 Expert Consensus-Based Practical Recommendations of the Korean Liver Cancer Association. Korean J. Radiol. 2023, 24, 606–625. [Google Scholar] [CrossRef]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Kolorektales Karzinom, Langversion 2.1, 2019, AWMF Registrierungsnummer: 021/007OL. Available online: https://www.leitlinienprogrammonkologie.de/leitlinien/kolorektales-karzinom (accessed on 30 March 2024).
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Freichel, J.; Gruber-Rouh, T.; Nour-Eldin Abdelrehim, N.E.; Bechstein, W.O.; Zeuzem, S.; Naguib, N.N.N.; Stefenelli, U. Interventional oncological treatment of hepatocellular carcinoma (HCC)—A single-center long-term evaluation of thermoablation techniques like LITT, MWA, and TACE in a multimodal application over 26 years. Heliyon 2023, 9, e14646. [Google Scholar] [CrossRef]
- Vogl, T.J.; Freichel, J.; Gruber-Rouh, T.; Nour Eldin, N.E.; Becker, S.; Solbach, C.; Stefenelli, U.; Naguib, N.N.N. Interventional oncological treatment of breast cancer liver metastasis (BCLM): Single center long-term evaluation over 26 years using thermoablation techniques like LITT, MWA, and TACE in a multimodal application. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group 2023, 40, 2200582. [Google Scholar] [CrossRef]
- Filoni, E.; Musci, V.; Di Rito, A.; Inchingolo, R.; Memeo, R.; Mannavola, F. Multimodal Management of Colorectal Liver Metastases: State of the Art. Oncol. Rev. 2024, 17, 11799. [Google Scholar] [CrossRef]
- Groeschl, R.T.; Pilgrim, C.H.C.; Hanna, E.M.; Simo, K.A.; Swan, R.Z.; Sindram, D.; Martinie, J.B.; Iannitti, D.A.; Bloomston, M.; Schmidt, C.; et al. Microwave ablation for hepatic malignancies: A multiinstitutional analysis. Ann. Surg. 2014, 259, 1195–1200. [Google Scholar] [CrossRef]
- Lee, H.; Heo, J.S.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; Choi, S.H.; Choi, D.W. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: A propensity score analysis. World J. Gastroenterol. 2015, 21, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Puls, R.; Langner, S.; Rosenberg, C.; Hegenscheid, K.; Kuehn, J.P.; Noeckler, K.; Hosten, N. Laser ablation of liver metastases from colorectal cancer with MR thermometry: 5-year survival. J. Vasc. Interv. Radiol. 2009, 20, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pacella, C.M.; Valle, D.; Bizzarri, G.; Pacella, S.; Brunetti, M.; Maritati, R.; Osborn, J.; Stasi, R. Percutaneous laser ablation in patients with isolated unresectable liver metastases from colorectal cancer: Results of a phase II study. Acta Oncol. 2006, 45, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.S.; Timmer, F.E.; Geboers, B.; Schouten, E.A.; Opperman, J.; Scheffer, H.J.; de Vries, J.J.; Swijnenburg, R.-J.; et al. Thermal Ablation Compared to Partial Hepatectomy for Recurrent Colorectal Liver Metastases: An Amsterdam Colorectal Liver Met Registry (AmCORE) Based Study. Cancers 2021, 13, 2769. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, M.; van der Lei, S.; Puijk, R.S.; Schulz, H.H.; Vos, D.J.W.; Timmer, F.E.F.; Scheffer, H.J.; Buffart, T.E.; van den Tol, M.P.; Lissenberg-Witte, B.I.; et al. Efficacy of Thermal Ablation for Small-Size (0–3 cm) versus Intermediate-Size (3–5 cm) Colorectal Liver Metastases: Results from the Amsterdam Colorectal Liver Met Registry (AmCORE). Cancers 2023, 15, 4346. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Kiefer, M.V.; Sun, W.; Haller, D.; Fraker, D.L.; Tuite, C.M.; Stavropoulos, S.W.; Mondschein, J.I.; Soulen, M.C. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer 2011, 117, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; McBride, J.D.; Georgiades, C.S.; Reyes, D.K.; Herman, J.M.; Kamel, I.R.; Geschwind, J.F. Salvage therapy for liver-dominant colorectal metastatic adenocarcinoma: Comparison between transcatheter arterial chemoembolization versus yttrium-90 radioembolization. J. Vasc. Interv. Radiol. 2009, 20, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Faiella, E.; Calabrese, A.; Santucci, D.; de Felice, C.; Pusceddu, C.; Fior, D.; Fontana, F.; Piacentino, F.; Moramarco, L.P.; Muraca, R.M.; et al. Combined Trans-Arterial Embolization and Ablation for the Treatment of Large (>3 cm) Liver Metastases: Review of the Literature. J. Clin. Med. 2022, 11, 5576. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.B.; Si, Z.M.; Qian, S.; Liu, L.X.; Qu, X.D.; Zhou, B.; Zhang, W.; Wang, G.Z.; Liu, R.; Wang, J.H. Percutaneous microwave ablation combined with synchronous transcatheter arterial chemoembolization for the treatment of colorectal liver metastases: Results from a follow-up cohort. OncoTargets Ther. 2016, 9, 3783–3789. [Google Scholar] [CrossRef]
- Yamakado, K.; Inaba, Y.; Sato, Y.; Yasumoto, T.; Hayashi, S.; Yamanaka, T.; Nobata, K.; Takaki, H.; Nakatsuka, A. Radiofrequency Ablation Combined with Hepatic Arterial Chemoembolization Using Degradable Starch Microsphere Mixed with Mitomycin C for the Treatment of Liver Metastasis from Colorectal Cancer: A Prospective Multicenter Study. Cardiovasc. Interv. Radiol. 2017, 40, 560–567. [Google Scholar] [CrossRef]
| Variable | LITT | TACE + LITT | MWA | TACE + MWA | TACE | TOTALS |
|---|---|---|---|---|---|---|
| number of patients | 573 | 346 | 67 | 152 | 1002 | 2140 |
| Men | 385 | 231 | 37 | 93 | 618 | 1364 |
| Women | 188 | 115 | 30 | 59 | 384 | 776 |
| Number of ablations treatments | 1363 | 839 | 103 | 402 | 2707 | |
| Average number of ablation treatments per patient | 2.4 | 2.4 | 1.5 | 2.6 | ||
| Number of TACE cycles | 1979 | 1035 | 4321 | 7335 | ||
| Average number of TACE sessions per patient | 5.7 | 6.8 | 4.3 | |||
| Complication rate (%) | 14.1 | 17.4 | 2.9 | 0.7 | N/A | |
| Median age (years) | 62 | 62 | 59 | 65 | 62 |
| Variable | LITT | TACE + LITT | MWA | TACE + MWA | TACE | TOTALS | |
|---|---|---|---|---|---|---|---|
| number of patients | 573 | 346 | 67 | 152 | 1002 | 2140 | |
| events | 544 | 334 | 15 | 68 | 618 | 1579 | |
| mean survival time (years) | 2.9 | 2.1 | 7.6 | 4.5 | 1.0 | 2.1 | |
| median survival time (years) | 1.9 | 1.7 | 3.1 | 2.1 | 0.8 | 1.3 | |
| median lower CI | 1.7 | 1.6 | 1.7 | 1.7 | 0.8 | 1.3 | |
| median upper CI | 2.0 | 1.8 | 2.7 | 0.9 | 1.4 | ||
| 1-year survival rate | 77 | 74 | 80 | 74 | 37 | 61 | |
| 1-year 95% lower CI | 74 | 69 | 67 | 66 | 33 | 59 | |
| 1-year 95% upper CI | 81 | 78 | 94 | 82 | 41 | 63 | |
| 3-year survival rate | 27 | 18 | 55 | 36 | 3 | 17 | |
| 3-year 95% lower CI | 23 | 14 | 34 | 25 | 2 | 15 | |
| 3-year 95% upper CI | 30 | 22 | 76 | 46 | 5 | 19 | |
| 5-year survival rate | 9 | 5 | 33 | 20 | 0 | 6 | |
| 5-year 95% lower CI | 7 | 2 | 6 | 10 | 0 | 5 | |
| 5-year 95% upper CI | 12 | 7 | 60 | 31 | 1 | 7 | |
| # | Method 1 | Method 2 | p-Value | n (Total) | n1 | n2 | |
|---|---|---|---|---|---|---|---|
| a | LITT | vs. | TACE + LITT | p = 0.001 | n = 919 | 573 | 346 |
| b | LITT | vs. | MWA | p = 0.063 | n = 640 | 573 | 67 |
| c | LITT | vs. | TACE + MWA | p = 0.077 | n = 725 | 573 | 152 |
| d | LITT | vs. | TACE | p < 0.001 | n = 1575 | 573 | 1002 |
| e | TACE + LITT | vs. | MWA | p = 0.008 | n = 413 | 346 | 67 |
| f | TACE + LITT | vs. | TACE + MWA | p = 0.001 | n = 498 | 346 | 152 |
| g | TACE + LITT | vs. | TACE | p < 0.001 | n = 1348 | 346 | 1002 |
| h | MWA | vs. | TACE + MWA | p = 0.293 | n = 219 | 67 | 152 |
| i | MWA | vs. | TACE | p < 0.001 | n = 1069 | 67 | 1002 |
| j | TACE + MWA | vs. | TACE | p < 0.001 | n = 1154 | 152 | 1002 |
| Predictor | p-Value | Hazard Ratio | Lower 95%-CI | Upper 95%-CI |
|---|---|---|---|---|
| Method | 0.000 | 1.289 | 1.250 | 1.330 |
| Age | 0.668 | 0.999 | 0.994 | 1.004 |
| Female | 0.712 | 0.980 | 0.883 | 1.089 |
| Predictor | p-Value | Hazard Ratio | Lower 95%-CI | Upper 95%-CI |
|---|---|---|---|---|
| LITT | 0.000 | 0.587 | 0.528 | 0.653 |
| TACE + LITT | 0.016 | 0.862 | 0.764 | 0.973 |
| MWA | 0.001 | 0.413 | 0.248 | 0.687 |
| TACE + MWA | 0.000 | 0.551 | 0.432 | 0.702 |
| TACE | 0.000 | 3.007 | 2.693 | 3.359 |
| p-Value | Hazard Ratio | Lower 95%-CI | Upper 95%-CI | |
|---|---|---|---|---|
| LITT | 0.000 | 0.319 | 0.282 | 0.362 |
| TACE + LITT | 0.000 | 0.396 | 0.345 | 0.455 |
| MWA | 0.000 | 0.194 | 0.116 | 0.324 |
| TACE + MWA | 0.000 | 0.254 | 0.197 | 0.327 |
| Age | 0.977 | 1.000 | 0.995 | 1.005 |
| Female | 0.954 | 0.997 | 0.898 | 1.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogl, T.J.; Freichel, J.; Gruber-Rouh, T.; Nour-Eldin, N.-E.A.; Bechstein, W.-O.; Zeuzem, S.; Naguib, N.N.N.; Stefenelli, U.; Adwan, H. Interventional Treatments of Colorectal Liver Metastases Using Thermal Ablation and Transarterial Chemoembolization: A Single-Center Experience over 26 Years. Cancers 2024, 16, 1756. https://doi.org/10.3390/cancers16091756
Vogl TJ, Freichel J, Gruber-Rouh T, Nour-Eldin N-EA, Bechstein W-O, Zeuzem S, Naguib NNN, Stefenelli U, Adwan H. Interventional Treatments of Colorectal Liver Metastases Using Thermal Ablation and Transarterial Chemoembolization: A Single-Center Experience over 26 Years. Cancers. 2024; 16(9):1756. https://doi.org/10.3390/cancers16091756
Chicago/Turabian StyleVogl, Thomas J., Jason Freichel, Tatjana Gruber-Rouh, Nour-Eldin Abdelrehim Nour-Eldin, Wolf-Otto Bechstein, Stefan Zeuzem, Nagy N. N. Naguib, Ulrich Stefenelli, and Hamzah Adwan. 2024. "Interventional Treatments of Colorectal Liver Metastases Using Thermal Ablation and Transarterial Chemoembolization: A Single-Center Experience over 26 Years" Cancers 16, no. 9: 1756. https://doi.org/10.3390/cancers16091756
APA StyleVogl, T. J., Freichel, J., Gruber-Rouh, T., Nour-Eldin, N.-E. A., Bechstein, W.-O., Zeuzem, S., Naguib, N. N. N., Stefenelli, U., & Adwan, H. (2024). Interventional Treatments of Colorectal Liver Metastases Using Thermal Ablation and Transarterial Chemoembolization: A Single-Center Experience over 26 Years. Cancers, 16(9), 1756. https://doi.org/10.3390/cancers16091756








