The Current Achievements of Multi-Gene Panel Tests in Clinical Settings for Patients with Non-Small-Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Sampling Methods and FFPE Sample Preparation
2.3. Outcomes
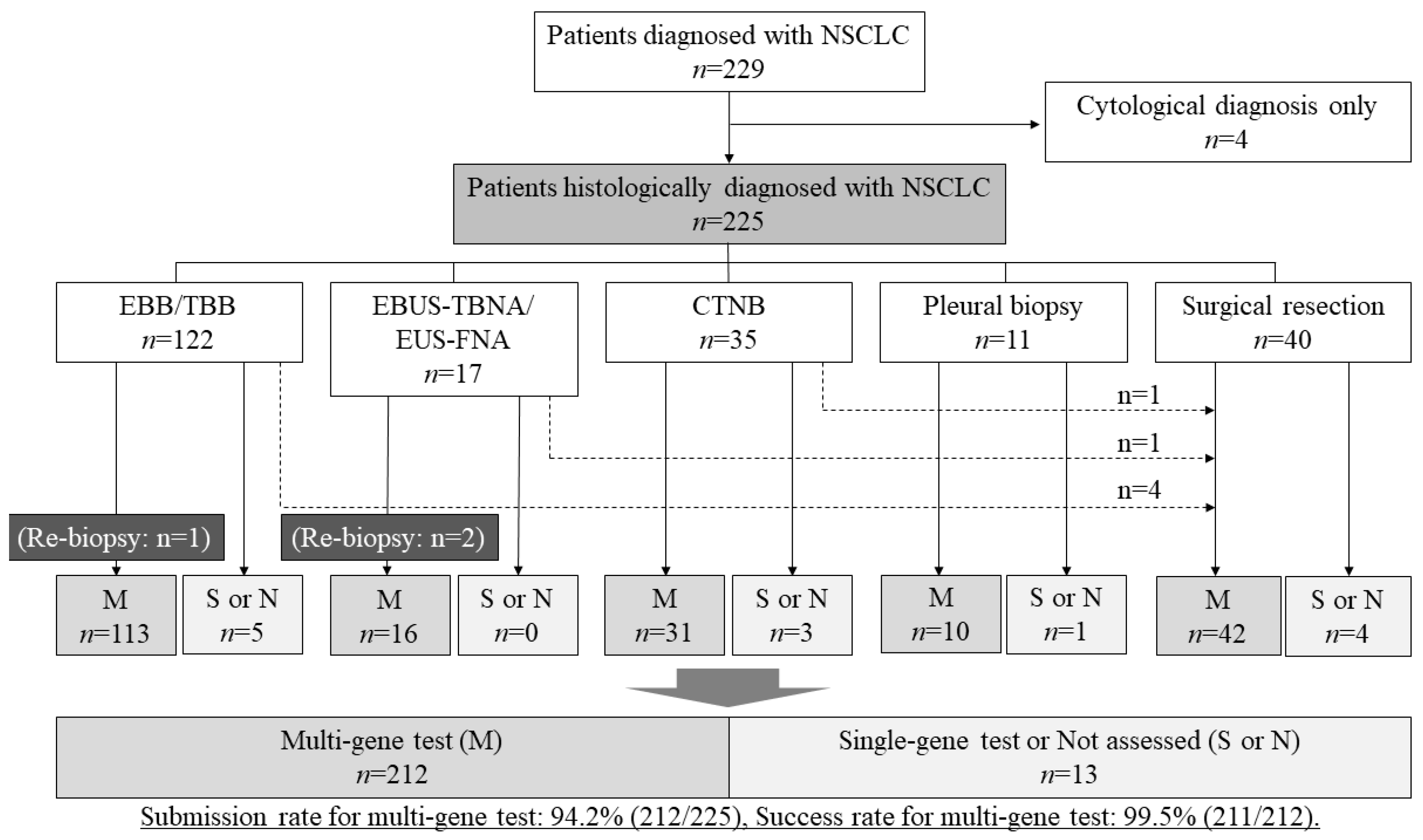
3. Results
3.1. Sample Characteristics
3.2. The Submission Rate and the Success Rate of Multi-Gene Panel Tests
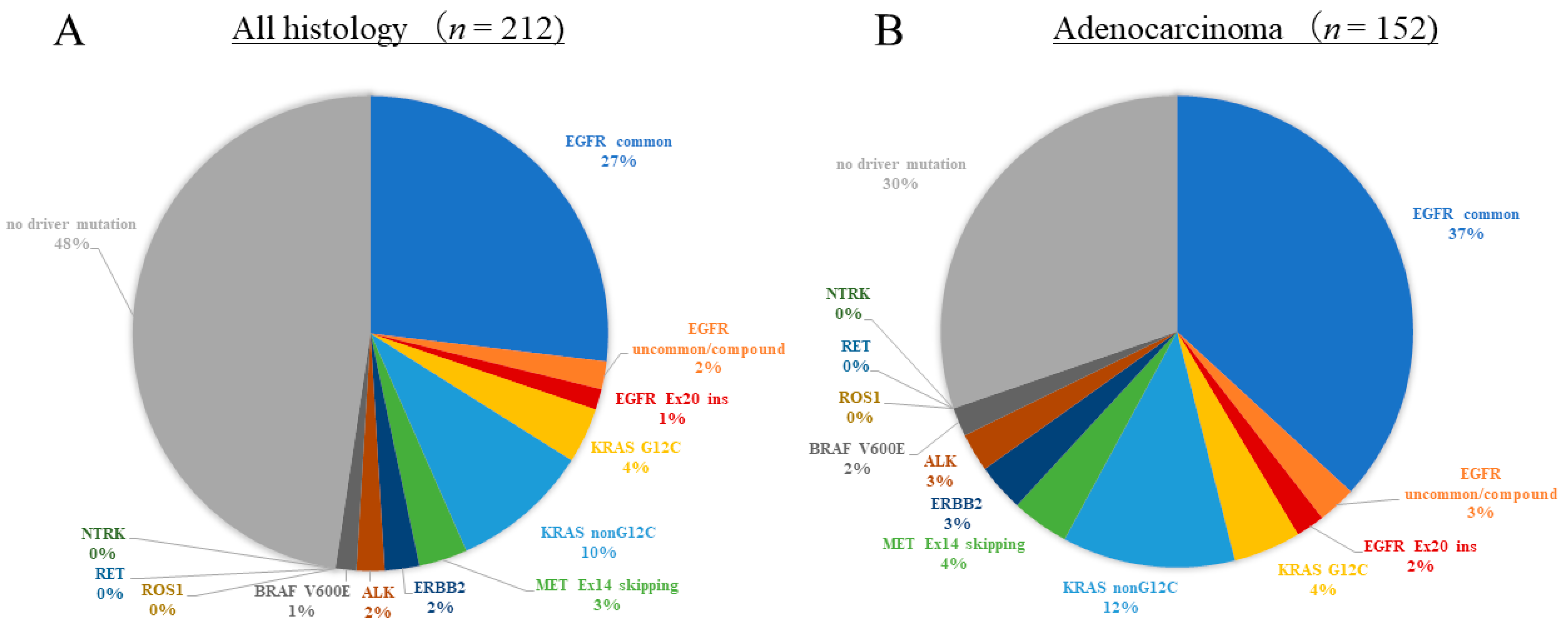
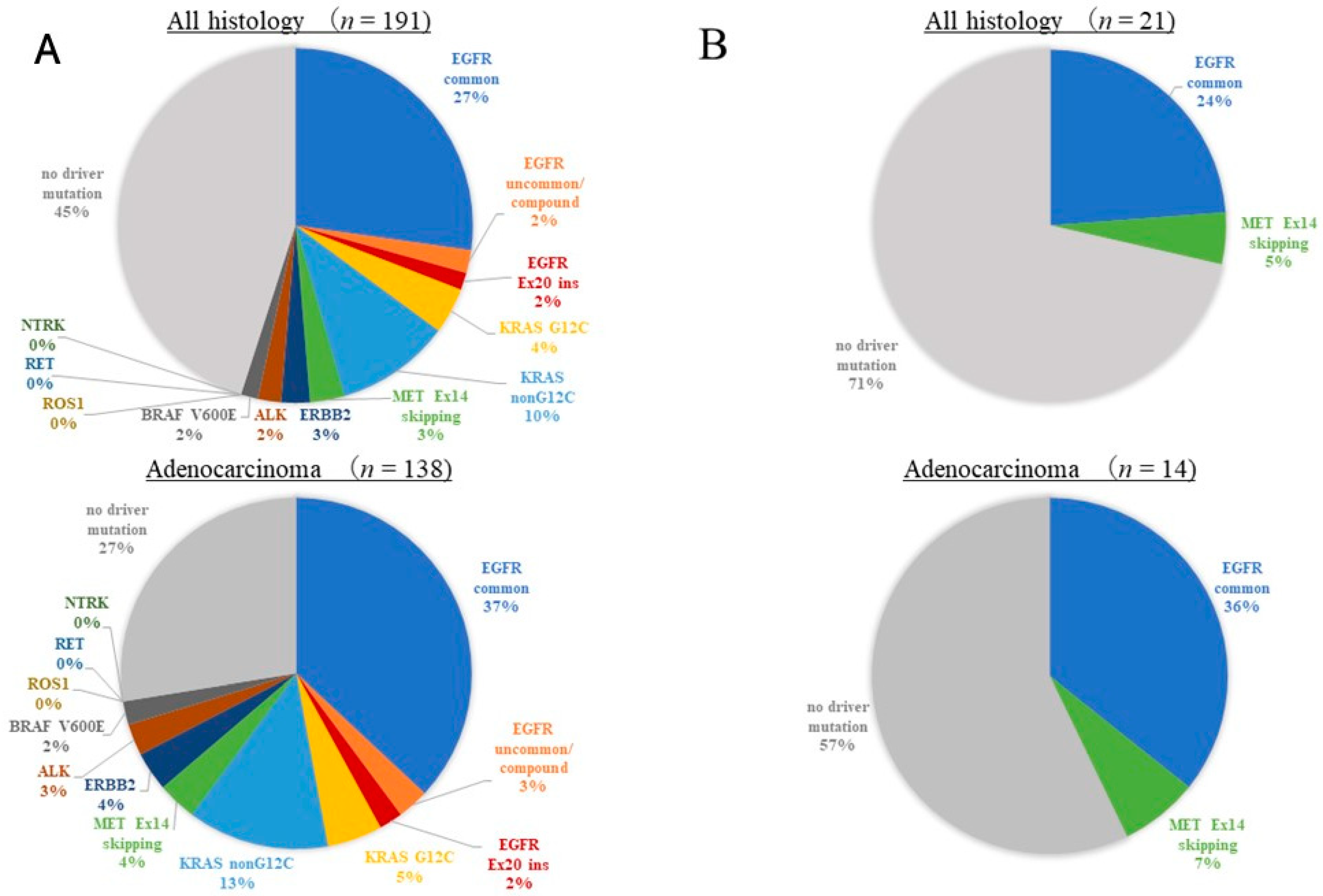
3.3. The Results of Multi-Gene Panel Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | Multi-Gene Test | ODxTT | AmoyDx-Multi | |||
|---|---|---|---|---|---|---|
| n = 212 | (%) | n = 191 | (%) | n = 21 | (%) | |
| Sampling method | ||||||
| EBB/TBB | 112 | 52.8% | 103 | 53.9% | 9 | 42.9% |
| Surgical resection | 43 | 20.3% | 41 | 21.5% | 2 | 9.5% |
| CTNB | 31 | 14.6% | 24 | 12.6% | 7 | 33.3% |
| EBUS-TBNA/EUS-FNA | 16 | 7.5% | 14 | 7.3% | 2 | 9.5% |
| Pleural biopsy | 10 | 4.7% | 9 | 4.7% | 1 | 4.8% |
| Histology | ||||||
| ADC | 152 | 71.7% | 138 | 72.3% | 14 | 66.7% |
| Sq | 46 | 21.7% | 43 | 22.5% | 3 | 14.3% |
| NSCC NOS | 14 | 6.6% | 10 | 5.2% | 4 | 19.0% |
| Stage (UICC-8) | ||||||
| 0/IA-IB/IIA-IIB/IIIIA-C | 129 | 60.8% | 116 | 60.7% | 13 | 61.9% |
| Rec/IVA-B | 78 | 36.8% | 71 | 37.2% | 7 | 33.3% |
| NA | 5 | 2.4% | 4 | 2.1% | 1 | 4.8% |
References
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F.; et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef]
- Takeda, M.; Sakai, K.; Terashima, M.; Kaneda, H.; Hayashi, H.; Tanaka, K.; Okamoto, K.; Takahama, T.; Yoshida, T.; Iwasa, T.; et al. Clinical application of amplicon-based next-generation sequencing to therapeutic decision making in lung cancer. Ann. Oncol. 2015, 26, 2477–2482. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Conci, N.; Rossi, G.; Fiorentino, M.; De Giglio, A.; Grilli, G.; Altimari, A.; Gruppioni, E.; Filippini, D.M.; Di Federico, A.; et al. Comparison of Sequential Testing and Next Generation Sequencing in advanced Lung Adenocarcinoma patients—A single centre experience. Lung Cancer 2020, 149, 5–9. [Google Scholar] [CrossRef]
- Yu, T.M.; Morrison, C.; Gold, E.J.; Tradonsky, A.; Layton, A.J. Multiple biomarker testing tissue consumption and completion rates with single-gene tests and investigational use of Oncomine Dx target test for advanced non-small-cell lung cancer: A single-center analysis. Clin. Lung Cancer 2018, 20, 20–29. [Google Scholar] [CrossRef]
- Kanasaki, H.; Ozawa, Y.; Nakamura, N.; Nagasaki, K.; Matsuyama, W.; Akahori, D.; Niwa, M.; Ogasawara, T.; Sato, J. Upfront Multiplex Gene Test Helps Prolong Survival in Advanced Non-small Cell Lung Cancer. Anticancer Res. 2024, 44, 723–730. [Google Scholar] [CrossRef]
- Yatabe, Y.; Sunami, K.; Goto, K.; Nishio, K.; Aragane, N.; Ikeda, S.; Inoue, A.; Kinoshita, I.; Kimura, H.; Sakamoto, T.; et al. Multiplex gene-panel testing for lung cancer patients. Pathol. Int. 2020, 70, 921–931. [Google Scholar] [CrossRef]
- Murakami, S.; Yokose, T.; Nemoto, D.; Suzuki, M.; Usui, R.; Nakahara, Y.; Kondo, T.; Kato, T.; Saito, H. Suitability of bronchoscopic biopsy tissue samples for next-generation sequencing. Diagnostics 2021, 11, 391. [Google Scholar] [CrossRef]
- Kunimasa, K.; Matsumoto, S.; Nishino, K.; Nakamura, H.; Kuhara, H.; Tamiya, M.; Inoue, T.; Kawamura, T.; Kawachi, H.; Kuno, K.; et al. Improvement strategies for successful next-generation sequencing analysis of lung cancer. Future Oncol. 2020, 16, 1597–1606. [Google Scholar] [CrossRef]
- Sakata, S.; Otsubo, K.; Yoshida, H.; Ito, K.; Nakamura, A.; Teraoka, S.; Matsumoto, N.; Shiraishi, Y.; Haratani, K.; Tamiya, M.; et al. Real-world data on NGS using the Oncomine DxTT for detecting genetic alterations in non-small-cell lung cancer: WJOG13019L. Cancer Sci. 2022, 113, 221–228. [Google Scholar] [CrossRef]
- Takahashi, T.; Nishio, M.; Nishino, K.; Yoshiki, Y.; Shiraiwa, N.; Emir, B.; Iadeluca, L.; Yatabe, Y.; Nishio, K. Real-world study of next-generation sequencing diagnostic biomarker testing for patients with lung cancer in Japan. Cancer Sci. 2023, 114, 2524–2533. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Kinoshita, I.; Amemiya, K.; Dosaka-Akita, H. Predictive Biomarker Testing for Lung Cancer: Past and Future Perspectives. JJLC 2022, 62, 15–25. [Google Scholar] [CrossRef]
- Uchimura, K.; Yanase, K.; Imabayashi, T.; Takeyasu, Y.; Furuse, H.; Tanaka, M.; Matsumoto, Y.; Sasada, S.; Tsuchida, T. The Impact of Core Tissues on Successful Next-Generation Sequencing Analysis of Specimens Obtained through Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Cancers 2021, 13, 5879. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Iketani, A.; Furuhashi, K.; Nakamura, Y.; Suzuki, Y.; Ito, K.; Fujiwara, K.; Nishii, Y.; Katsuta, K.; Taguchi, O.; et al. A method to improve genetic analysis of lung cancer samples. Respirology 2021, 26, 887–890. [Google Scholar] [CrossRef]
- Food and Drug Administration. Summary of Safety and Effectiveness Data. 2017. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160045B.pdf (accessed on 8 April 2018).
- Sakamoto, T.; Matsubara, T.; Takahama, T.; Yokoyama, T.; Nakamura, A.; Tokito, T.; Okamoto, T.; Akamatsu, H.; Oki, M.; Sato, Y.; et al. Biomarker testing in patients with unresectable advanced or recurrent non-small cell lung cancer. JAMA Netw. Open 2023, 6, e2347700. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nishii, Y.; Iketani, A.; Esumi, S.; Esumi, M.; Furuhashi, K.; Nakamura, Y.; Suzuki, Y.; Ito, K.; Fujiwara, K.; et al. Comparison of the analytical performance of the Oncomine dx target test focusing on bronchoscopic biopsy forceps size in non-small cell lung cancer. Thorac. Cancer 2022, 13, 1449–1456. [Google Scholar] [CrossRef]
- Takeyasu, Y.; Yoshida, T.; Motoi, N.; Teishikata, T.; Tanaka, M.; Matsumoto, Y.; Shinno, Y.; Okuma, Y.; Goto, Y.; Horinouchi, H.; et al. Feasibility of next-generation sequencing (Oncomine™ DX Target Test) for the screening of oncogenic mutations in advanced non-small-cell lung cancer patients. Jpn. J. Clin. Oncol. 2021, 51, 1114–1122. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Kuwata, T.; Morii, E.; Kanai, Y.; Ichikawa, H.; Kubo, T.; Hatanaka, K.C.; Sakai, K.; Nishio, K.; Fujii, S.; et al. The Japanese Society of Pathology Practical Guidelines on the handling of pathological tissue samples for cancer genomic medicine. Pathol. Int. 2021, 71, 725–740. [Google Scholar] [CrossRef]
- Murakami, S.; Yokose, T.; Shinada, K.; Isaka, T.; Katakura, K.; Ushio, R.; Kondo, T.; Kato, T.; Ito, H.; Saito, H. Comparison of next-generation sequencing and cobas EGFR mutation test v2 in detecting EGFR mutations. Thorac. Cancer 2022, 13, 3217–3224. [Google Scholar] [CrossRef]
- Kanaoka, K.; Tamiya, A.; Inagaki, Y.; Taniguchi, Y.; Nakao, K.; Takeda, M.; Matsuda, Y.; Okishio, K.; Shimizu, S. Possible False Results with cobas® EGFR Mutation Test v2 and Oncomine Dx Target Test for EGFR Mutation. Anticancer Res. 2023, 43, 2771–2776. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Iketani, A.; Esumi, S.; Esumi, M.; Suzuki, Y.; Ito, K.; Fujiwara, K.; Nishii, Y.; Katsuta, K.; Yasui, H.; et al. Clinical importance of the range of detectable variants between the Oncomine Dx target test and a conventional single-gene test for EGFR mutation. Sci. Rep. 2023, 13, 13759. [Google Scholar] [CrossRef]
- Sakamoto, T.; Furukawa, T.; Pham, H.H.N.; Kuroda, K.; Tabata, K.; Kashima, Y.; Okoshi, E.N.; Morimoto, S.; Bychkov, A.; Fukuoka, J. A collaborative workflow between pathologists and deep learning for the evaluation of tumour cellularity in lung adenocarcinoma. Histopathology 2022, 81, 758–769. [Google Scholar] [CrossRef]
- Mikubo, M.; Seto, K.; Kitamura, A.; Nakaguro, M.; Hattori, Y.; Maeda, N.; Miyazaki, T.; Watanabe, K.; Murakami, H.; Tsukamoto, T.; et al. Calculating the Tumor Nuclei Content for Comprehensive Cancer Panel Testing. J. Thorac. Oncol. 2020, 15, 130–137. [Google Scholar] [CrossRef]
- Smits, A.J.; Kummer, J.A.; de Bruin, P.C.; Bol, M.; van den Tweel, J.G.; Seldenrijk, K.A.; Willems, S.M.; Offerhaus, G.J.; de Weger, R.A.; van Diest, P.J.; et al. The estimation of tumor cell percentage for molecular testing by pathologists is not accurate. Mod. Pathol. 2014, 27, 168–174. [Google Scholar] [CrossRef]
- Viray, H.; Li, K.; Long, T.A.; Vasalos, P.; Bridge, J.A.; Jennings, L.J.; Halling, K.C.; Hameed, M.; Rimm, D.L. A prospective, multi-institutional diagnostic trial to determine pathologist accuracy in estimation of percentage of malignant cells. Arch. Pathol. Lab. Med. 2013, 137, 1545–1549. [Google Scholar] [CrossRef]
- Kunimasa, K.; Matsumoto, S.; Kawamura, T.; Inoue, T.; Tamiya, M.; Kanzaki, R.; Maniwa, T.; Okami, J.; Honma, K.; Goto, K.; et al. Clinical application of the AMOY 9-in-1 panel to lung cancer patients. Lung Cancer 2023, 179, 107190. [Google Scholar] [CrossRef]
- Morikawa, K.; Kida, H.; Handa, H.; Inoue, T.; Saji, H.; Koike, J.; Nakamura, S.; Sato, Y.; Ueda, Y.; Suzuki, F.; et al. A Prospective Validation Study of Lung Cancer Gene Panel Testing Using Cytological Specimens. Cancers 2022, 14, 3784. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.H.; Gao, S.; Chen, K.N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
| Tumor Cell Count | Number of Submitted Slides | Selection of Multi-Gene Panel Test |
|---|---|---|
| (Cells/Slide) | (8 µm Thickness) | |
| ≤100 | Inappropriate for multi-gene testing | |
| 100~200 | 20 slides | mainly submitted to AmoyDx-multi |
| 200~300 | 20 slides | mainly submitted to ODxTT (considering AmoyDx-multi in case with low tumor content or rich necrosis) |
| 300~400 | 15 slides | |
| 400~ | 10 slides | |
| Surgical specimens | 5–10 slides | |
| Characteristics | Total | Multi-Gene Test | Single-Gene Test or NA | |||
|---|---|---|---|---|---|---|
| n = 225 | (%) | n = 212 | (%) | n = 13 | (%) | |
| Sampling method | ||||||
| EBB/TBB | 117 | 52.0% | 112 | 52.8% | 5 | 38.5% |
| Surgical resection | 47 | 20.9% | 43 | 20.3% | 4 | 30.8% |
| CTNB | 34 | 15.1% | 31 | 14.6% | 3 | 23.1% |
| EBUS-TBNA/EUS-FNA | 16 | 7.1% | 16 | 7.5% | 0 | 0.0% |
| Pleural biopsy | 11 | 4.9% | 10 | 4.7% | 1 | 7.7% |
| Histology | ||||||
| ADC | 158 | 70.2% | 152 | 71.7% | 6 | 46.2% |
| Sq | 51 | 22.7% | 46 | 21.7% | 5 | 38.5% |
| NSCC NOS | 16 | 7.1% | 14 | 6.6% | 2 | 15.4% |
| Stage (UICC-8) | ||||||
| 0/IA-IB/IIA-IIB/IIIIA-C | 137 | 60.9% | 129 | 60.8% | 8 | 61.5% |
| Rec/IVA-B | 83 | 36.9% | 78 | 36.8% | 5 | 38.5% |
| NA | 5 | 2.2% | 5 | 2.4% | 0 | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, T.; Iketani, A.; Esumi, S.; Esumi, M.; Suzuki, Y.; Ito, K.; Fujiwara, K.; Nishii, Y.; Katsuta, K.; Yasui, H.; et al. The Current Achievements of Multi-Gene Panel Tests in Clinical Settings for Patients with Non-Small-Cell Lung Cancer. Cancers 2024, 16, 1670. https://doi.org/10.3390/cancers16091670
Sakaguchi T, Iketani A, Esumi S, Esumi M, Suzuki Y, Ito K, Fujiwara K, Nishii Y, Katsuta K, Yasui H, et al. The Current Achievements of Multi-Gene Panel Tests in Clinical Settings for Patients with Non-Small-Cell Lung Cancer. Cancers. 2024; 16(9):1670. https://doi.org/10.3390/cancers16091670
Chicago/Turabian StyleSakaguchi, Tadashi, Akemi Iketani, Seiya Esumi, Maki Esumi, Yuta Suzuki, Kentaro Ito, Kentaro Fujiwara, Yoichi Nishii, Koji Katsuta, Hiroki Yasui, and et al. 2024. "The Current Achievements of Multi-Gene Panel Tests in Clinical Settings for Patients with Non-Small-Cell Lung Cancer" Cancers 16, no. 9: 1670. https://doi.org/10.3390/cancers16091670
APA StyleSakaguchi, T., Iketani, A., Esumi, S., Esumi, M., Suzuki, Y., Ito, K., Fujiwara, K., Nishii, Y., Katsuta, K., Yasui, H., Taguchi, O., & Hataji, O. (2024). The Current Achievements of Multi-Gene Panel Tests in Clinical Settings for Patients with Non-Small-Cell Lung Cancer. Cancers, 16(9), 1670. https://doi.org/10.3390/cancers16091670




