Controversies in Endoscopic Ultrasound-Guided Biliary Drainage
Abstract
Simple Summary
Abstract
1. Introduction
2. History of EUS-Guided Biliary Drainage
3. Why Do We Need Procedures Other Than ERCP?
3.1. Arguments in Favor of EUS-BD
3.1.1. EUS-BD of the Bile Duct
3.1.2. EUS-GBD
3.2. Arguments against EUS-Guided Drainage
4. Should EUS-BD and ERCP Be Performed by the Same Operator?
4.1. Arguments in Favor
4.2. Arguments Against
5. Rendezvous Techniques
5.1. Should Rendezvous Be Used First?
5.2. Which Rendezvous Route Should Be Used?
6. Percutaneous Transhepatic Cholangiography and Biliary Drainage (PTBD)
6.1. Arguments in Favor of PTBD
6.2. Arguments Against
6.3. Should PTBD and EUS-BD Be Performed by the Same Physician?
7. Do We Need Cystotomes?
8. Do We Need Bougies?
9. Are All EUS Needles the Same for EUS-BD?
10. Plastic or Metal Stents?
11. Adverse Events
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDS | choledochoduodenostomy |
| EFSUMB | European Federation of Societies for Ultrasound in Medicine and Biology |
| EUS | EUS |
| EUS-BD | EUS-guided bile duct drainage |
| EUS-GBD | EUS-guided gallbladder drainage |
| ERCP | endoscopic retrograde cholangiopancreatography |
| ESGE | European Society of Gastrointestinal Endoscopy |
| HGS | hepaticogastrostomy |
| HJS | hepaticojejunostomy |
| LAMS | lumen-apposing metal stents |
| PPFC | peripancreatic fluid collection |
| PTBD | percutaneous transhepatic biliary drainage |
| SEMS | self-expandable metal stents |
References
- Wiersema, M.J.; Sandusky, D.; Carr, R.; Wiersema, L.M.; Erdel, W.C.; Frederick, P.K. Endosonography-guided cholangiopancreatography. Gastrointest. Endosc. 1996, 43, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J.R. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Burmester, E.; Niehaus, J.; Leineweber, T.; Huetteroth, T. EUS-cholangio-drainage of the bile duct: Report of 4 cases. Gastrointest. Endosc. 2003, 57, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Mallery, S.; Matlock, J.; Freeman, M.L. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest. Endosc. 2004, 59, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Luigiano, C.; Lisotti, A.; Cennamo, V.; Virgilio, C.; Caletti, G.; Fusaroli, P. Endoscopic ultrasound-guided treatments: Are we getting evidence based—A systematic review. World J. Gastroenterol. 2014, 20, 8424–8448. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Bhandari, S.; Bapat, M.; Maydeo, A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest. Endosc. 2012, 75, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Will, U.; Fueldner, F.; Kern, C.; Meyer, F. EUS-Guided Bile Duct Drainage (EUBD) in 95 Patients. Ultraschall Med.-Eur. J. Ultrasound 2015, 36, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Sofuni, A.; Tsuchiya, T.; Ijima, M.; Iwashita, T. Endoscopic ultrasonography-guided transhepatic antegrade stone removal in patients with surgically altered anatomy: Case series and technical review (with videos). J. Hepatobiliary Pancreat. Sci. 2014, 21, E86–E93. [Google Scholar] [CrossRef]
- Gupta, K.; Perez-Miranda, M.; Kahaleh, M.; Artifon, E.L.; Itoi, T.; Freeman, M.L.; de-Serna, C.; Sauer, B.; Giovannini, M.; In, E.S.G. Endoscopic ultrasound-assisted bile duct access and drainage: Multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J. Clin. Gastroenterol. 2014, 48, 80–87. [Google Scholar] [CrossRef]
- Kahaleh, M.; Artifon, E.L.; Perez-Miranda, M.; Gupta, K.; Itoi, T.; Binmoeller, K.F.; Giovannini, M. Endoscopic ultrasonography guided biliary drainage: Summary of consortium meeting, May 7th, 2011, Chicago. World J. Gastroenterol. 2013, 19, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, P.; Jenssen, C.; Hocke, M.; Burmester, E.; Buscarini, E.; Havre, R.F.; Ignee, A.; Saftoiu, A.; Vilmann, P.; Nolsoe, C.P.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V—EUS-Guided Therapeutic Interventions (short version). Ultraschall Med. 2016, 37, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Artifon, E.L.; Aparicio, D.; Paione, J.B.; Lo, S.K.; Bordini, A.; Rabello, C.; Otoch, J.P.; Gupta, K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: Endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J. Clin. Gastroenterol. 2012, 46, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Bapaye, A.; Dubale, N.; Aher, A. Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP. United Eur. Gastroenterol. J. 2013, 1, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.A.; Valeshabad, A.K.; Afghani, E.; Singh, V.K.; Kumbhari, V.; Messallam, A.; Saxena, P.; El Zein, M.; Lennon, A.M.; Canto, M.I.; et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig. Dis. Sci. 2015, 60, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Hindryckx, P.; Degroote, H.; Tate, D.J.; Deprez, P.H. Endoscopic ultrasound-guided drainage of the biliary system: Techniques, indications and future perspectives. World J. Gastrointest. Endosc. 2019, 11, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Salerno, R.; Davies, S.E.C.; Mezzina, N.; Ardizzone, S. Comprehensive review on EUS-guided biliary drainage. World J. Gastrointest. Endosc. 2019, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Giovannini, M.; Sahai, A.V.; Saftoiu, A.; Dietrich, C.F.; Santo, E.; Fusaroli, P.; Siddiqui, A.A.; Bhutani, M.S.; Bun Teoh, A.Y.; et al. A multi-institution consensus on how to perform EUS-guided biliary drainage for malignant biliary obstruction. Endosc. Ultrasound 2018, 7, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Jeong, S.U.; Lee, B.U.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest. Endosc. 2013, 78, 91–101. [Google Scholar] [CrossRef]
- Tyberg, A.; Desai, A.P.; Kumta, N.A.; Brown, E.; Gaidhane, M.; Sharaiha, R.Z.; Kahaleh, M. EUS-guided biliary drainage after failed ERCP: A novel algorithm individualized based on patient anatomy. Gastrointest. Endosc. 2016, 84, 941–946. [Google Scholar] [CrossRef]
- Artifon, E.L.; Marson, F.P.; Gaidhane, M.; Kahaleh, M.; Otoch, J.P. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest. Endosc. 2015, 81, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Dhir, V. EUS-guided biliary rendezvous: Slow, hesitant, baby steps forward. Gastrointest. Endosc. 2016, 83, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Khashab, M.A.; Messallam, A.A.; Penas, I.; Nakai, Y.; Modayil, R.J.; De la Serna, C.; Hara, K.; El Zein, M.; Stavropoulos, S.N.; Perez-Miranda, M.; et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open 2016, 4, E175–E181. [Google Scholar] [CrossRef] [PubMed]
- Hedjoudje, A.; Sportes, A.; Grabar, S.; Zhang, A.; Koch, S.; Vuitton, L.; Prat, F. Outcomes of endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest. Endosc. 2018, 88, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Woo, Y.S.; Noh, D.H.; Yang, J.I.; Bae, S.Y.; Yun, H.S.; Lee, J.K.; Lee, K.T.; Lee, K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018, 88, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Napoleon, B.; Kunda, R.; Arcidiacono, P.G.; Kongkam, P.; Larghi, A.; Van der Merwe, S.; Jacques, J.; Legros, R.; Thawee, R.E.; et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent versus ERCP with Covered Metallic Stents in Patients with Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology 2023, 165, 473–482. [Google Scholar] [CrossRef] [PubMed]
- van Wanrooij, R.L.J.; Vanella, G.; Bronswijk, M.; de Gooyer, P.; Laleman, W.; van Malenstein, H.; Mandarino, F.V.; Dell’Anna, G.; Fockens, P.; Arcidiacono, P.G.; et al. Endoscopic ultrasound-guided gastroenterostomy versus duodenal stenting for malignant gastric outlet obstruction: An international, multicenter, propensity score-matched comparison. Endoscopy 2022, 54, 1023–1031. [Google Scholar] [CrossRef]
- McCune, W.S.; Shorb, P.E.; Moscovitz, H. Endoscopic cannulation of the ampulla of vater: A preliminary report. Ann. Surg. 1968, 167, 752–756. [Google Scholar] [CrossRef]
- Enochsson, L.; Swahn, F.; Arnelo, U.; Nilsson, M.; Lohr, M.; Persson, G. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest. Endosc. 2010, 72, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Tse, F.; Yuan, Y.; Moayyedi, P.; Leontiadis, G.I. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis. Endoscopy 2013, 45, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, M.; Barnabas, A.; Fernandopulle, N.; Bailey, A.A.; Collier, J.; Phillips-Hughes, J.; Ellis, A.; Chapman, R.; Braden, B. Repeat endoscopic retrograde cholangiopancreaticography after failed initial precut sphincterotomy for biliary cannulation. World J. Gastroenterol. 2014, 20, 13153–13158. [Google Scholar] [CrossRef]
- Domagk, D.; Oppong, K.W.; Aabakken, L.; Czako, L.; Gyokeres, T.; Manes, G.; Meier, P.; Poley, J.W.; Ponchon, T.; Tringali, A.; et al. Performance measures for endoscopic retrograde cholangiopancreatography and endoscopic ultrasound: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. United Eur. Gastroenterol. J. 2018, 6, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.C.; Lee, S.K.; Lee, T.Y.; Kwon, S.; Lee, S.S.; Seo, D.W.; Kim, M.H. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy 2007, 39, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Winick, A.B.; Waybill, P.N.; Venbrux, A.C. Complications of percutaneous transhepatic biliary interventions. Tech. Vasc. Interv. Radiol. 2001, 4, 200–206. [Google Scholar] [CrossRef] [PubMed]
- van Wanrooij, R.L.J.; Bronswijk, M.; Kunda, R.; Everett, S.M.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Badaoui, A.; Law, R.; Arcidiacono, P.G.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2022, 54, 310–332. [Google Scholar] [CrossRef]
- Khashab, M.A.; El Zein, M.H.; Sharzehi, K.; Marson, F.P.; Haluszka, O.; Small, A.J.; Nakai, Y.; Park, D.H.; Kunda, R.; Teoh, A.Y.; et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: An international comparative study. Endosc. Int. Open 2016, 4, E1322–E1327. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; Fugazza, A.; Binda, C.; Zerbi, A.; Jovine, E.; Cennamo, V.; Repici, A.; Anderloni, A. Beyond palliation: Using EUS-guided choledochoduodenostomy with a lumen-apposing metal stent as a bridge to surgery. a case series. J. Gastrointest. Liver Dis. 2019, 28, 125–128. [Google Scholar] [CrossRef]
- Gaujoux, S.; Jacques, J.; Bourdariat, R.; Sulpice, L.; Lesurtel, M.; Truant, S.; Robin, F.; Prat, F.; Palazzo, M.; Schwarz, L.; et al. Pancreaticoduodenectomy following endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents an ACHBT–SFED study. HPB 2020, 23, 154–160. [Google Scholar] [CrossRef]
- Winbladh, A.; Gullstrand, P.; Svanvik, J.; Sandstrom, P. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB 2009, 11, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Leung, C.H.; Tam, P.T.H.; Au Yeung, K.K.Y.; Mok, R.C.Y.; Chan, D.L.; Chan, S.M.; Yip, H.C.; Chiu, P.W.Y.; Ng, E.K.W. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: A propensity score analysis with 1-year follow-up data. Gastrointest. Endosc. 2020, 93, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Kitano, M.; Itoi, T.; Perez-Miranda, M.; Ogura, T.; Chan, S.M.; Serna-Higuera, C.; Omoto, S.; Torres-Yuste, R.; Tsuichiya, T.; et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut 2020, 69, 1085–1091. [Google Scholar] [CrossRef]
- Hayat, U.; Al Shabeeb, R.; Perez, P.; Hensien, J.; Dwivedi, A.; Sakhawat, U.; Ahmad, O.; Haseeb, M.; Siddiqui, A.A.; Adler, D.G. Safety and adverse events of endoscopic ultrasound-guided gallbladder drainage using lumen-apposing metal stents and percutaneous cholecystostomy tubes: A systematic review and meta-analysis. Gastrointest. Endosc. 2023, 99, 444–448. [Google Scholar] [CrossRef]
- Veitch, A.M.; Radaelli, F.; Alikhan, R.; Dumonceau, J.M.; Eaton, D.; Jerrome, J.; Lester, W.; Nylander, D.; Thoufeeq, M.; Vanbiervliet, G.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Endoscopy 2021, 53, 947–969. [Google Scholar] [CrossRef]
- Baron, T.H.; DeSimio, T.M. New ex-vivo porcine model for endoscopic ultrasound-guided training in transmural puncture and drainage of pancreatic cysts and fluid collections (with videos). Endosc. Ultrasound 2015, 4, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Isayama, H.; Itoi, T.; Almadi, M.; Siripun, A.; Teoh, A.Y.B.; Ho, K.Y. Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig. Endosc. 2017, 29, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bekkali, N.L.; Burmeister, S.; Dong, Y.; Everett, S.M.; Hocke, M.; Ignee, A.; On, W.; Hebbar, S.; Oppong, K.; et al. Controversies in ERCP: Technical aspects. Endosc. Ultrasound. 2022, 11, 27–37. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bekkali, N.L.; Burmeister, S.; Dong, Y.; Everett, S.M.; Hocke, M.; Ignee, A.; On, W.; Hebbar, S.; Oppong, K.; et al. Controversies in ERCP: Indications and preparation. Endosc. Ultrasound. 2022, 11, 186–200. [Google Scholar] [CrossRef]
- Tyberg, A.; Mishra, A.; Cheung, M.; Kedia, P.; Gaidhane, M.; Craig, C.; Tarnasky, P.R.; Ardengh, J.C.; Kahaleh, M. Learning curve for EUS-guided biliary drainage: What have we learned? Endosc. Ultrasound 2020, 9, 392–396. [Google Scholar] [CrossRef]
- Tarantino, I.; Barresi, L.; Repici, A.; Traina, M. EUS-guided biliary drainage: A case series. Endoscopy 2008, 40, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Maranki, J.; Hernandez, A.J.; Arslan, B.; Jaffan, A.A.; Angle, J.F.; Shami, V.M.; Kahaleh, M. Interventional endoscopic ultrasound-guided cholangiography: Long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy 2009, 41, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gupta, K.; Mallery, S.; Li, R.; Kinney, T.; Freeman, M.L. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: Cumulative experience at a single center. A case series. Endoscopy 2010, 42, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, T.; Lee, J.G.; Shinoura, S.; Nakai, Y.; Park, D.H.; Muthusamy, V.R.; Chang, K.J. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy 2012, 44, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sanchez, M.V.; Jenssen, C.; Faiss, S.; Napoleon, B. Interventional endoscopic ultrasonography: An overview of safety and complications. Surg. Endosc. 2014, 28, 712–734. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, T.; Doi, S.; Yasuda, I. Endoscopic ultrasound-guided biliary drainage: A review. Clin. J. Gastroenterol. 2014, 7, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.J.; Perez-Miranda, M.; Vazquez-Sequeiros, E.; Abadia, M.A.; Perez-Millan, A.; Gonzalez-Huix, F.; Gornals, J.; Iglesias-Garcia, J.; De la Serna, C.; Aparicio, J.R.; et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012, 76, 1133–1141. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, J.; Xing, L.; Wang, Y.; Jin, Z.; Li, Z. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest. Endosc. 2016, 83, 1218–1227. [Google Scholar] [CrossRef]
- Isayama, H.; Nakai, Y.; Kawakubo, K.; Kawakami, H.; Itoi, T.; Yamamoto, N.; Kogure, H.; Koike, K. The endoscopic ultrasonography-guided rendezvous technique for biliary cannulation: A technical review. J. Hepatobiliary Pancreat. Sci. 2013, 20, 413–420. [Google Scholar] [CrossRef]
- Iwashita, T.; Yasuda, I.; Mukai, T.; Iwata, K.; Ando, N.; Doi, S.; Nakashima, M.; Uemura, S.; Mabuchi, M.; Shimizu, M. EUS-guided rendezvous for difficult biliary cannulation using a standardized algorithm: A multicenter prospective pilot study (with videos). Gastrointest. Endosc. 2016, 83, 394–400. [Google Scholar] [CrossRef]
- Perez-Miranda, M.; De la Serna Higuera, C.; Gil-Simon, P.; Hernandez, V.; Diez-Redondo, P.; Fernandez-Salazar, L. EUS-guided choledochoduodenostomy with lumen-apposing metal stent after failed rendezvous in synchronous malignant biliary and gastric outlet obstruction (with video). Gastrointest. Endosc. 2014, 80, 342; discussion 343–344. [Google Scholar] [CrossRef]
- Okuno, N.; Hara, K.; Mizuno, N.; Hijioka, S.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; Niwa, Y.; et al. Endoscopic Ultrasound-guided Rendezvous Technique after Failed Endoscopic Retrograde Cholangiopancreatography: Which Approach Route Is the Best? Intern. Med. 2017, 56, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.; Wallace, M.J.; Wojak, J.C.; Kundu, S.; Cardella, J.F. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J. Vasc. Interv. Radiol. 2010, 21, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Ba, Y.; Yue, P.; Leung, J.W.; Wang, H.; Lin, Y.; Bai, B.; Zhu, X.; Zhang, L.; Zhu, K.; Wang, W.; et al. Percutaneous transhepatic biliary drainage may be the preferred preoperative drainage method in hilar cholangiocarcinoma. Endosc. Int. Open 2020, 8, E203–E210. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Barkun, A.N.; Martel, M.; Chen, Y.I. Endoscopic ultrasound-guided biliary drainage for distal malignant obstruction: A systematic review and meta-analysis of randomized trials. Endosc. Int. Open 2019, 7, E1563–E1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, N.; Xin, F.; Ke, Q.; Zeng, Y.; Liu, J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J. Surg. Oncol. 2019, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Mohan, B.P.; Jearth, V.; Kale, A.; Angadi, S.; Afzalpurkar, S.; Harindranath, S.; Sundaram, S. Adverse events with EUS-guided biliary drainage: A systematic review and meta-analysis. Gastrointest. Endosc. 2023, 98, 515–523 e518. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, M.; Kitano, M.; Hatamaru, K.; Tamura, T.; Nuta, J.; Kawaji, Y.; Takenaka, M.; Minaga, K.; Kudo, M.; Ogura, T.; et al. Endoscopic ultrasound-guided choledochoduodenostomy using a thin stent delivery system in patients with unresectable malignant distal biliary obstruction: A prospective multicenter study. Dig. Endosc. 2019, 31, 291–298. [Google Scholar] [CrossRef]
- Giovannini, M.; Dotti, M.; Bories, E.; Moutardier, V.; Pesenti, C.; Danisi, C.; Delpero, J.R. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy 2003, 35, 1076–1078. [Google Scholar] [CrossRef]
- Umeda, J.; Itoi, T.; Tsuchiya, T.; Sofuni, A.; Itokawa, F.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Kamada, K.; Tanaka, R.; et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos). Gastrointest. Endosc. 2015, 82, 390–396.e392. [Google Scholar] [CrossRef]
- Khashab, M.A.; Valeshabad, A.K.; Modayil, R.; Widmer, J.; Saxena, P.; Idrees, M.; Iqbal, S.; Kalloo, A.N.; Stavropoulos, S.N. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: Rendezvous versus direct transluminal techniques (with videos). Gastrointest. Endosc. 2013, 78, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.G.; Waxman, I.; Siddiqui, U.D. Endoscopic Ultrasound (EUS)-Guided Pancreatic Duct Drainage: The Basics of When and How to Perform EUS-Guided Pancreatic Duct Interventions. Clin. Endosc. 2016, 49, 161–167. [Google Scholar] [CrossRef]
- Itoi, T.; Yasuda, I.; Kurihara, T.; Itokawa, F.; Kasuya, K. Technique of endoscopic ultrasonography-guided pancreatic duct intervention (with videos). J. Hepatobiliary Pancreat. Sci. 2014, 21, E4–E9. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Akbar, A.; Baron, T.H.; Khan, S.; Kocak, M.; Alastal, Y.; Hammad, T.; Lee, W.M.; Sofi, A.; Artifon, E.L.; et al. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2016, 61, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Varadarajulu, S. EUS followed by endoscopic pancreatic pseudocyst drainage or all-in-one procedure: A review of basic techniques (with video). Gastrointest. Endosc. 2009, 69, S176–S181. [Google Scholar] [CrossRef]
- Song, T.J.; Park, D.H.; Eum, J.B.; Moon, S.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided cholecystoenterostomy with single-step placement of a 7F double-pigtail plastic stent in patients who are unsuitable for cholecystectomy: A pilot study (with video). Gastrointest. Endosc. 2010, 71, 634–640. [Google Scholar] [CrossRef]
- Cremer, M.; Deviere, J.; Baize, M.; Matos, C. New device for endoscopic cystoenterostomy. Endoscopy 1990, 22, 76–77. [Google Scholar] [CrossRef]
- Honjo, M.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Mukai, S.; Sofuni, A.; Nagakawa, Y.; Iwasaki, H.; Kanai, T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc. Ultrasound 2018, 7, 376–382. [Google Scholar] [CrossRef]
- Prachayakul, V.; Aswakul, P. Feasibility and safety of using Soehendra stent retriever as a new technique for biliary access in endoscopic ultrasound-guided biliary drainage. World J. Gastroenterol. 2015, 21, 2725–2730. [Google Scholar] [CrossRef]
- Ryou, M.; Benias, P.C.; Kumbhari, V. Initial clinical experience of a steerable access device for EUS-guided biliary drainage. Gastrointest. Endosc. 2020, 91, 178–184. [Google Scholar] [CrossRef]
- Schmidt, A.; Riecken, B.; Rische, S.; Klinger, C.; Jakobs, R.; Bechtler, M.; Kahler, G.; Dormann, A.; Caca, K. Wing-shaped plastic stents vs. self-expandable metal stents for palliative drainage of malignant distal biliary obstruction: A randomized multicenter study. Endoscopy 2015, 47, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kunda, R.; Perez-Miranda, M.; Will, U.; Ullrich, S.; Brenke, D.; Dollhopf, M.; Meier, M.; Larghi, A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg. Endosc. 2016, 30, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Dollhopf, M.; Larghi, A.; Will, U.; Rimbas, M.; Anderloni, A.; Sanchez-Yague, A.; Teoh, A.Y.B.; Kunda, R. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest. Endosc. 2017, 86, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Ngamruengphong, S.; Teoh, A.; Will, U.; Nieto, J.; Abu Dayyeh, B.K.; Gan, S.I.; Larsen, M.; Yip, H.C.; Topazian, M.D.; et al. Similar Efficacies of Endoscopic Ultrasound Gallbladder Drainage with a Lumen-Apposing Metal Stent Versus Percutaneous Transhepatic Gallbladder Drainage for Acute Cholecystitis. Clin. Gastroenterol. Hepatol. 2017, 15, 738–745. [Google Scholar] [CrossRef] [PubMed]
- El Chafic, A.H.; Shah, J.N.; Hamerski, C.; Binmoeller, K.F.; Irani, S.; James, T.W.; Baron, T.H.; Nieto, J.; Romero, R.V.; Evans, J.A.; et al. EUS-Guided Choledochoduodenostomy for Distal Malignant Biliary Obstruction Using Electrocautery-Enhanced Lumen-Apposing Metal Stents: First US, Multicenter Experience. Dig. Dis. Sci. 2019, 64, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
- Fuldner, F.; Meyer, F.; Will, U. EUS-guided biliary interventions for benign diseases and unsuccessful ERCP—A prospective unicenter feasibility study on a large consecutive patient cohort. Z. Gastroenterol. 2021, 59, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Puspok, A.; Lomoschitz, F.; Dejaco, C.; Hejna, M.; Sautner, T.; Gangl, A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: A case series. Am. J. Gastroenterol. 2005, 100, 1743–1747. [Google Scholar] [CrossRef]
- Kahaleh, M.; Hernandez, A.J.; Tokar, J.; Adams, R.B.; Shami, V.M.; Yeaton, P. Interventional EUS-guided cholangiography: Evaluation of a technique in evolution. Gastrointest. Endosc. 2006, 64, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bories, E.; Pesenti, C.; Caillol, F.; Lopes, C.; Giovannini, M. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy 2007, 39, 287–291. [Google Scholar] [CrossRef]
- Yamao, K.; Bhatia, V.; Mizuno, N.; Sawaki, A.; Ishikawa, H.; Tajika, M.; Hoki, N.; Shimizu, Y.; Ashida, R.; Fukami, N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: Results of long-term follow-up. Endoscopy 2008, 40, 340–342. [Google Scholar] [CrossRef]
- Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Obana, T.; Takasawa, O.; Koshita, S.; Kanno, Y. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig. Endosc. 2009, 21, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Brauer, B.C.; Chen, Y.K.; Fukami, N.; Shah, R.J. Single-operator EUS-guided cholangiopancreatography for difficult pancreaticobiliary access (with video). Gastrointest. Endosc. 2009, 70, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Yamao, K.; Niwa, Y.; Sawaki, A.; Mizuno, N.; Hijioka, S.; Tajika, M.; Kawai, H.; Kondo, S.; Kobayashi, Y.; et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am. J. Gastroenterol. 2011, 106, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, S.H.; Oh, H.J.; Sohn, Y.W.; Lee, S.O. Endoscopic ultrasound-guided biliary drainage with placement of a fully covered metal stent for malignant biliary obstruction. World J. Gastroenterol. 2012, 18, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Vanella, G.; Bronswijk, M.; Maleux, G.; van Malenstein, H.; Laleman, W.; Van der Merwe, S. EUS-guided intrahepatic biliary drainage: A large retrospective series and subgroup comparison between percutaneous drainage in hilar stenoses or postsurgical anatomy. Endosc. Int. Open 2020, 8, E1782–E1794. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalapathy, S.V.; James, M.W.; Huggett, M.T.; Paranandi, B.; Pereira, S.P.; Johnson, G.; Aravinthan, A.D.; Aithal, G.P. Utility of palliative EUS-guided biliary drainage using lumen-apposing metal stents: A prospective multicenter feasibility study (with video). Gastrointest. Endosc. 2021, 94, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.; Caillol, F.; Autret, A.; Ratone, J.P.; Zemmour, C.; Boher, J.M.; Pesenti, C.; Bories, E.; Barthet, M.; Napoleon, B.; et al. EUS-guided hepaticogastrostomy in patients with obstructive jaundice after failed or impossible endoscopic retrograde drainage: A multicenter, randomized phase II Study. Endosc. Ultrasound 2022, 11, 495–502. [Google Scholar] [CrossRef]
- Ragab, K.M.; Abdel-Hameed, M.; Gouda, M.; Katamish, H.; Madkour, A.; Atalla, H.; Hamed, H.; Shiha, G.E.; Abdallah, O.; Agwa, R.H.; et al. Endoscopic ultrasound-guided biliary drainage for distal malignant biliary obstruction: A prospective 3-year multicenter Egyptian study. Acta Gastroenterol. Belg. 2023, 86, 26–35. [Google Scholar] [CrossRef]
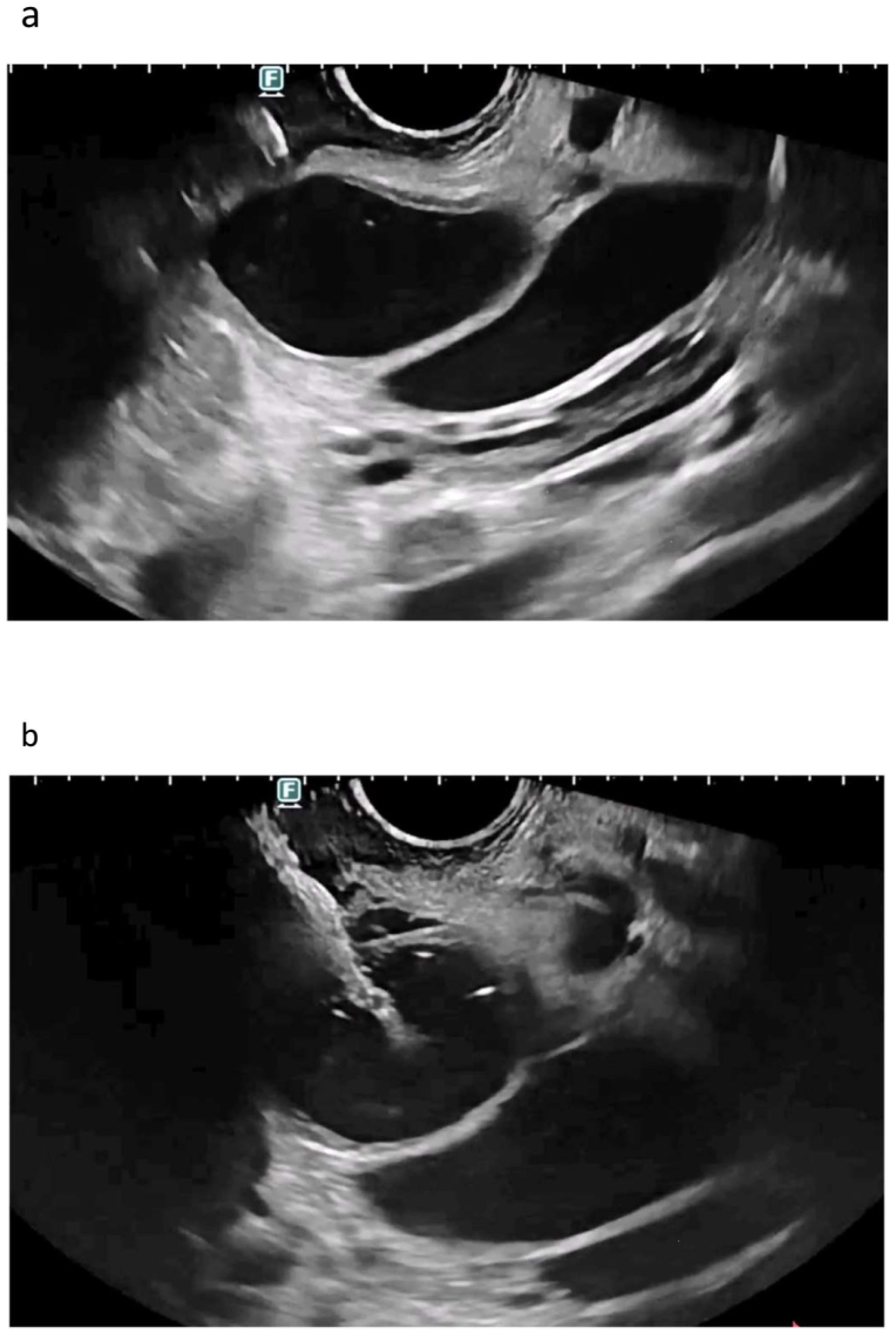
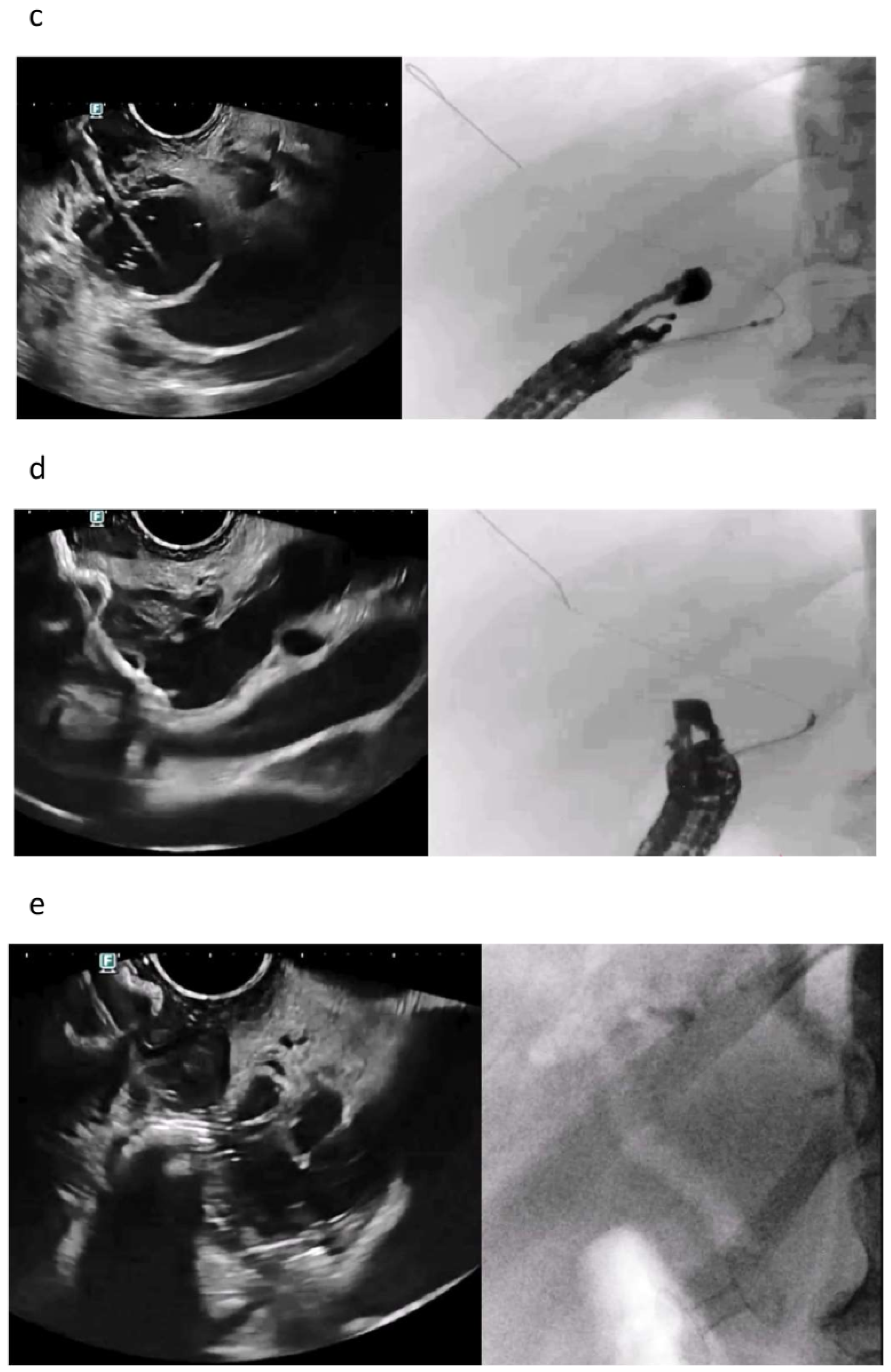
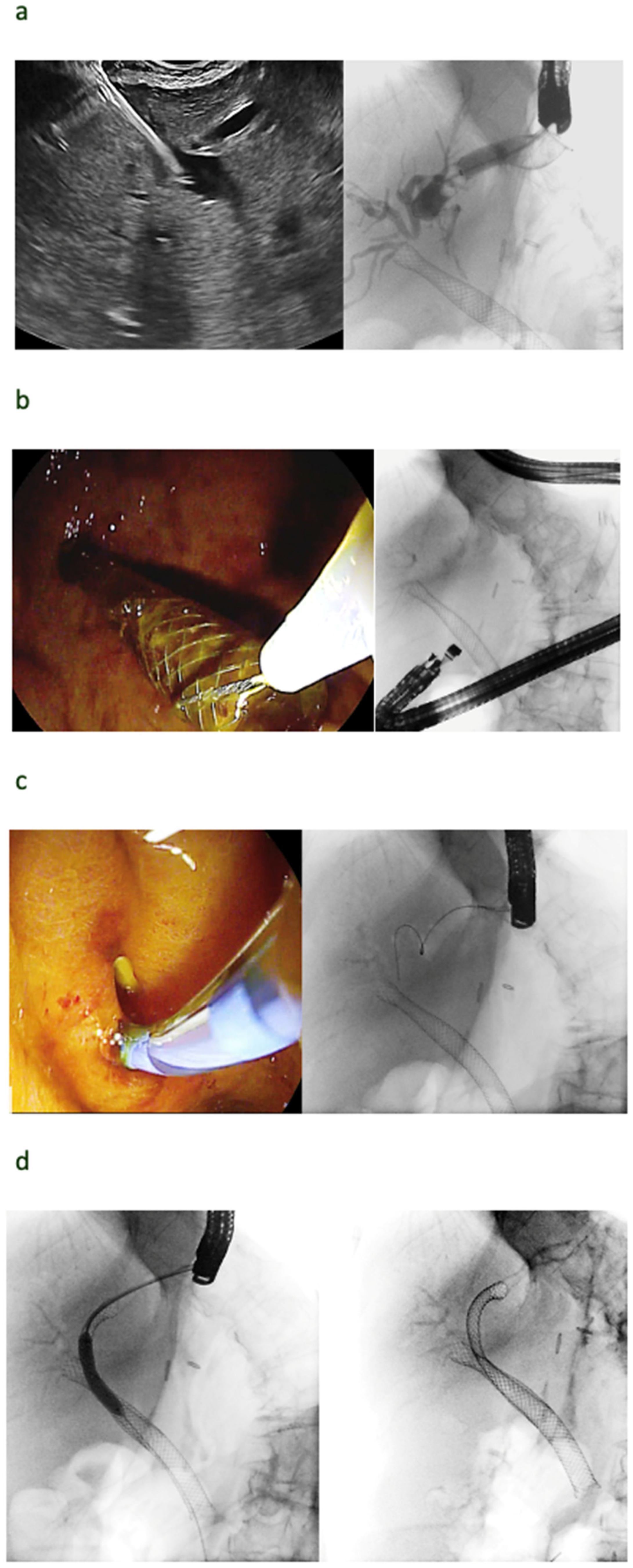
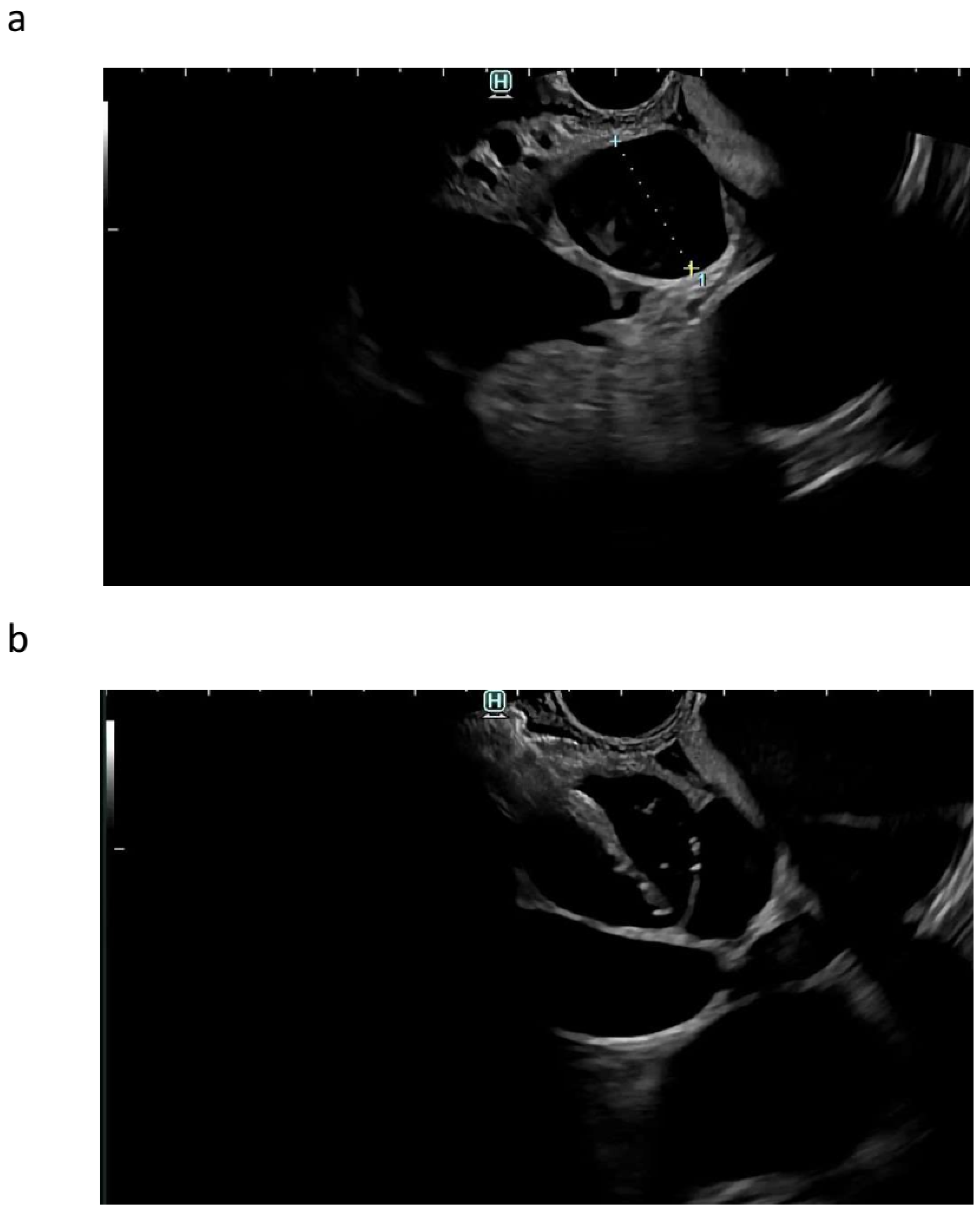
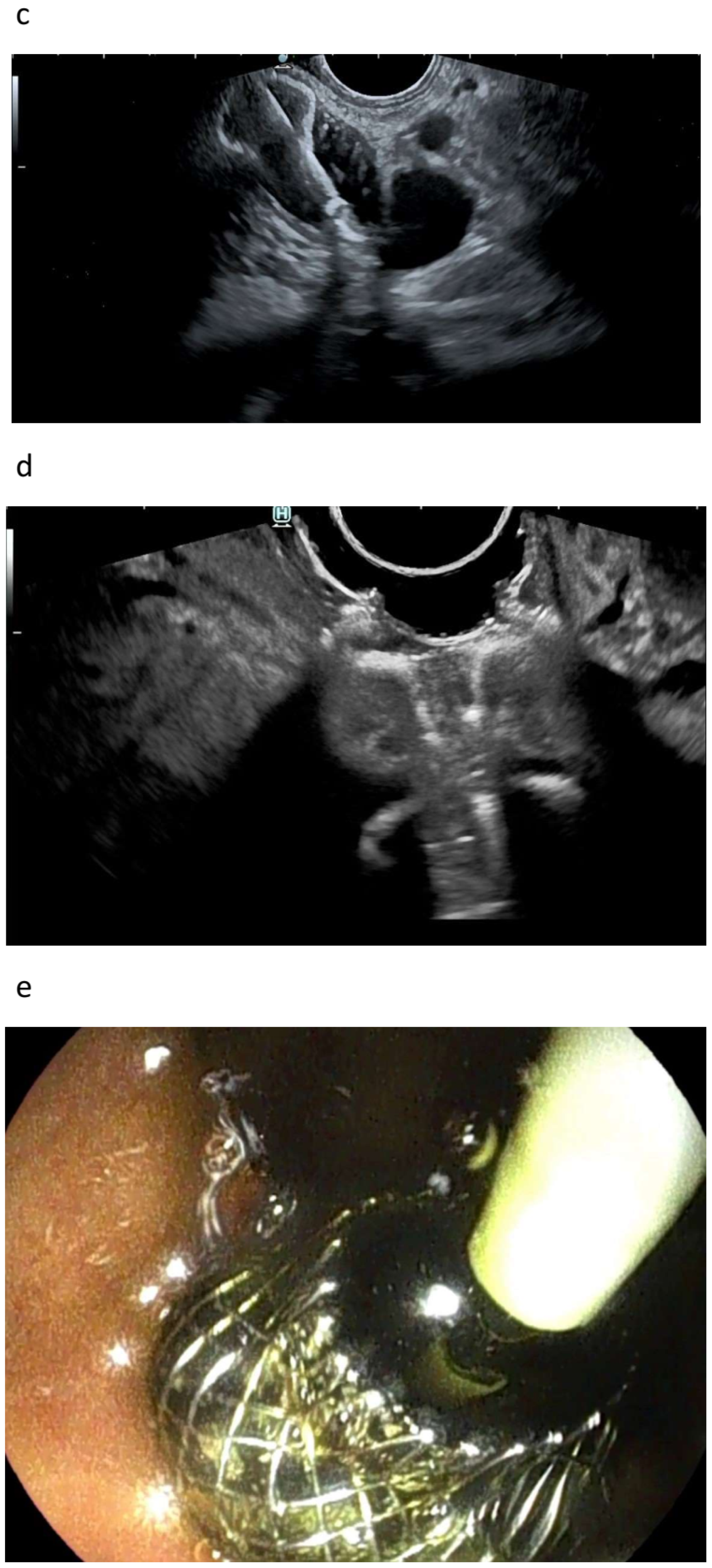
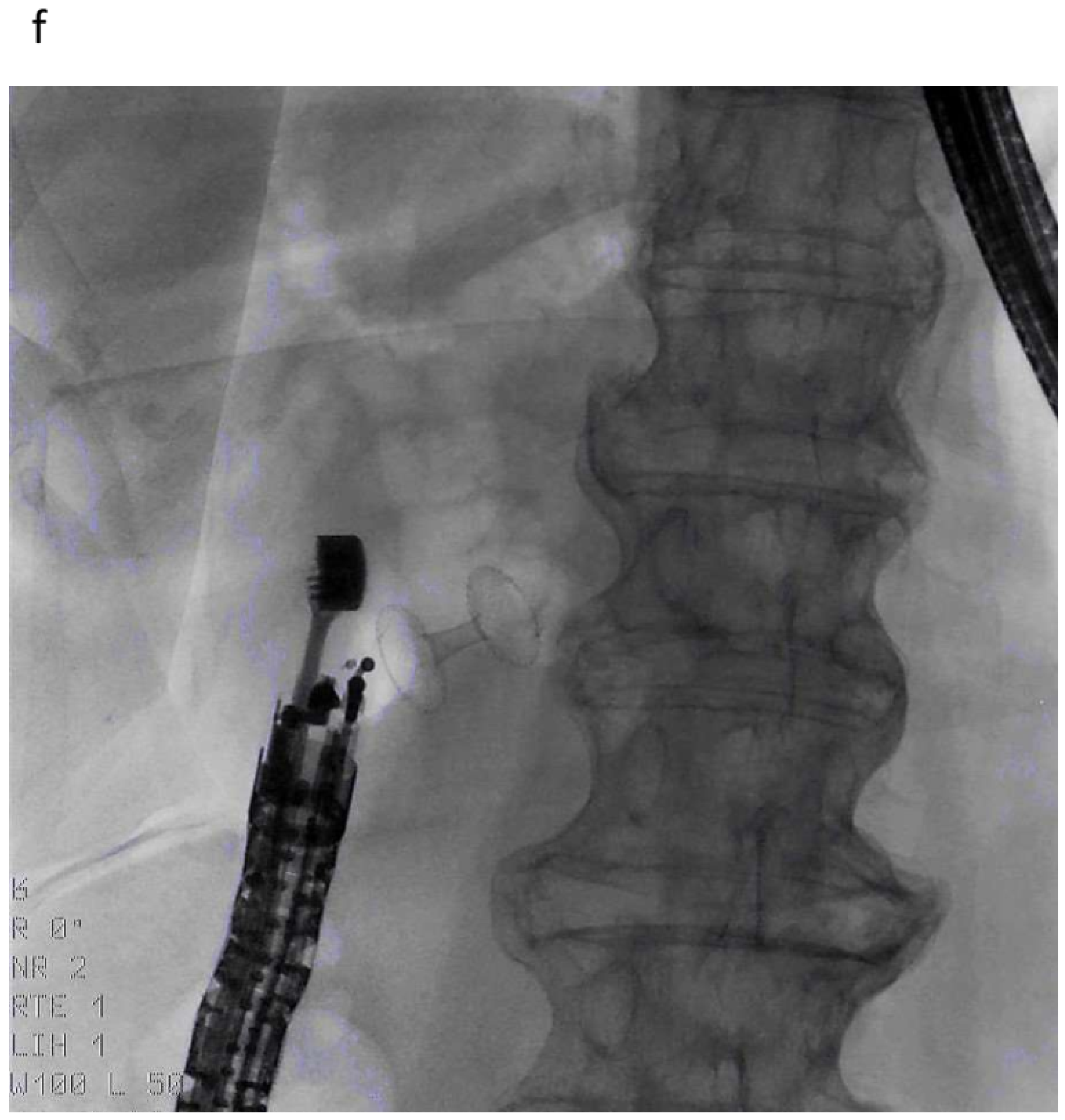
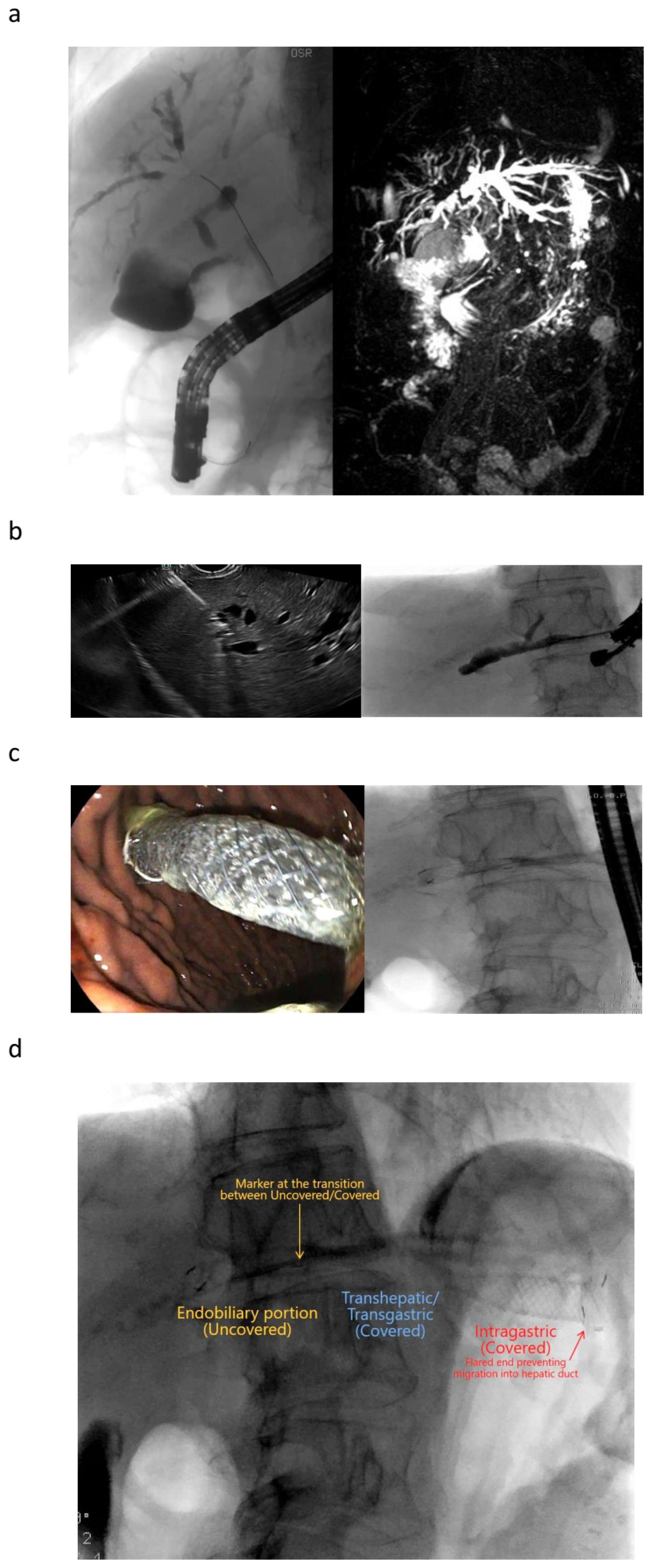
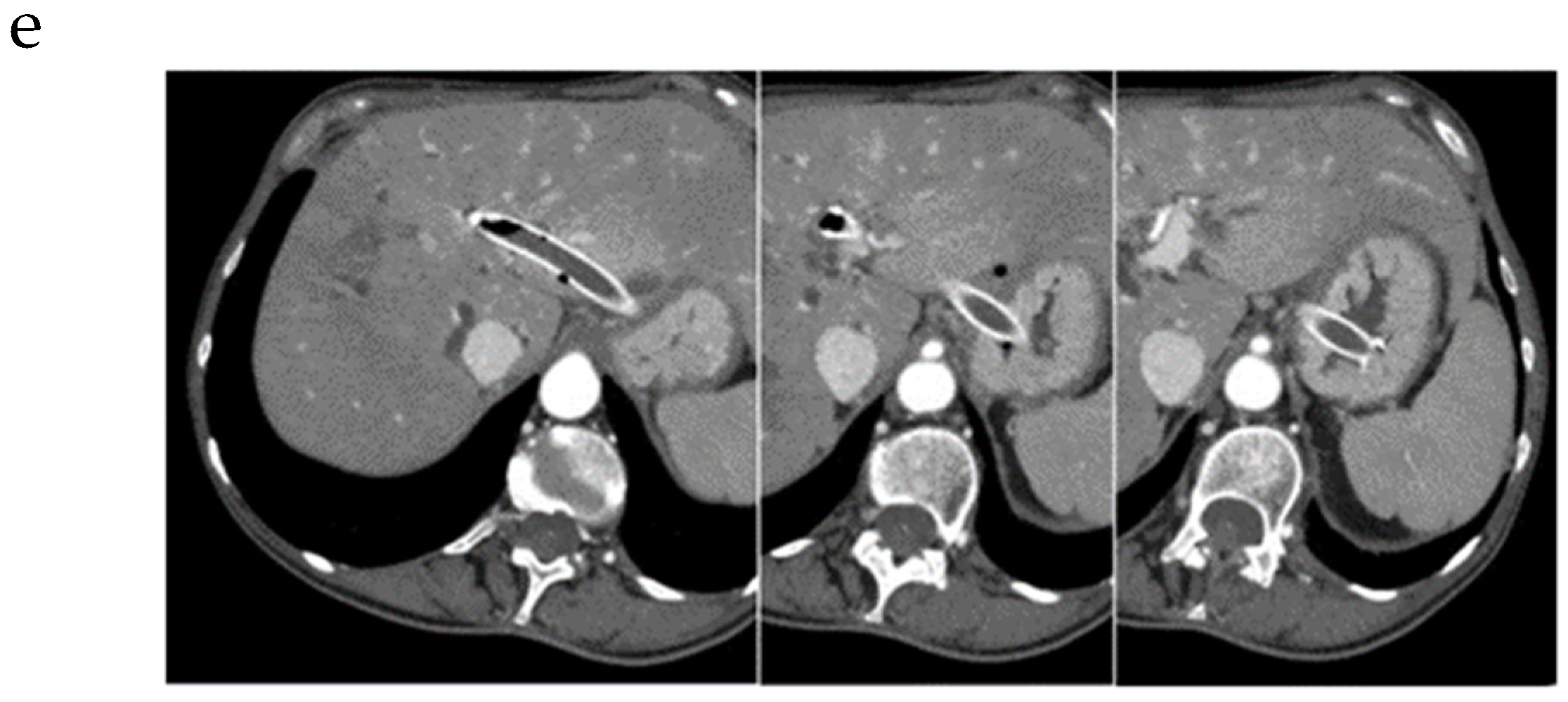
| Autor | Study Type | Patients | Adverse Events | Comments |
|---|---|---|---|---|
| Burmester et al. Gastrointest Endosc 2003 [3] | Single-center case reports | 4 | Bile leak (n = 1) | EUS-CDS, retrograde HJS, EUS-HGS. |
| Puspok et al. Am J Gastroenterol 2005 [87] | Case reports | 6 | Cholecystitis (n = 1) | EUS-guided transduodenal puncture of the common bile duct with stent placement. Transhepatic metal stent |
| Kahaleh et al. Gastrointest Endosc 2006 [88] | Retrospective study | 28 | Minor bleeding (n = 1), self-limited Pneumoperitoneum (n = 2) Bile leak (n = 1) | Rendezvous |
| Bories et al. Endoscopy 2007 [89] | Pilot study, single-center | 11 | Cholangitis (n = 1) | EUS-HGS |
| Yamao et al. Endoscopy 2008 [90] | Case reports | 5 | Pneumoperitoneum (n = 1) | EUS-CDS |
| Tarantino et al. Endoscopy 2008 [51] | Single-center | 9 | Death from liver cirrhosis complication 15 days after intervention (n = 1) | Transduodenal approach |
| Maranki et al. Endoscopy 2009 [52] | Single-center, retrospective | 49 | Pneumoperitoneum (n = 3), Bleeding (n = 1), Aspiration pneumonia (n = 1) | Transgastric-transhepatic (intrahepatic) or transenteric-transcholedochal (extrahepatic) |
| Horaguchi et al. Dig Endosc 2009 [91] | Single-center | 16 | Peritonitis (n = 1) | EUS-BD via duodenum, stomach, esophagus |
| Brauer et al. Gastrointest Endosc 2009 [92] | Comparative study, single-center nonrandomized observational study | 20 | Pneumoperitoneum (n = 1) Respiratory failure (n = 1) | Transenteric-transcholedochal extrahepatic approach for biliary cases |
| Park et al. Gastrointest Endosc 2011 [7] | Comparative study, prospective follow-up study. | 57 | Postoperative adverse effects after EUS-EUS-BD: 20%: bile peritonitis (n = 2), mild bleeding (n = 2), and self-limited pneumoperitoneum (n = 7) Late adverse effecta: distal stent migration—7%. | In multivariate analysis, needle-knife use was the single risk factor for post-procedure adverse events after EUS-BD |
| Hara et al. Am J Gastroenterol 2011 [93] | Prospective study | 18 | Peritonitis (n = 2) Bleeding (1) | EUS-CDS |
| Artifon et al. J Clin Gastroenterol 2012 [13] | Randomized controlled trial | 25 | Bile leak (n = 1) Mild bleeding (n = 1) | Percutaneous transhepatic biliary drainage and EUS-BD |
| Kim et al. World J Gastroenterol 2012 [94] | Two-center study | 13 | Peritonitis (n = 1) | EUS-CDS and EUS- HGS, fully nitinol-covered self-expandable metal stent |
| Gupta et al. J Clin Gastroenterol 2014 [10] | Multicenter, nonrandomized retrospective study. | 240 | Pneumoperitoneum 5%, bleeding 11%, bile leak/peritonitis 10% and cholangitis 5%. | Extra- and intrahepatic BD access. No significant difference between IH and EH approaches; benign and malignant indications |
| Will, et al. Ultraschall Med 2015 [8] | Single-center database over a 10-year period | 95 | Elevated cholestasis parameters (n = 3) | EUS-BD |
| Vanella et al. EIO 2020 [95] | Retrospective | 104 | Perforation (n = 2), Bleeding (n = 3), Bile leak (n = 1), Cholangitis (n = 9), Bacteriemia (n = 3), Acute Pancreatitis (n = 4), Severe abdominal pain (n = 2) | EUS-guided intrahepatic access, including rendezvous, antegrade stenting, and EUS-HGS |
| Füldner et al. Z Gastroenterol 2021 [86] | Prospective EUS-BD registry (2004–2020) | 103 | Complication rate: (n = 26/25%): stent dislocation (n = 11), perforation (n = 1), pain (n = 2), hemorrhage (n = 6), biliary ascites/leakage (n = 3) and bilioma/liver abscess (n = 3); major complication rate (n = 12/68—17.6%). | Different approaches of EUS-BD |
| Venkatachalapathy et al. Gastrointest Endosc 2021 [96] | Prospective multicenter study | 20 | Cholangitis (n = 1/5%), Stent migration (n = 1/5%) | EUS-CDS, lumen-apposing metal stents |
| Marx et al. Endosc Ultrasound 2022 [97] | RCT | 35 | Bleeding (n = 3/8.6%) Cholangitis (n = 1/2.9%) Peritonitis (n = 1/2.9%) Sepsis (n = 7/20%) | EUS-HGS |
| Ragab et al. Acta Gastroenterol Belg 2023 [98] | Prospective multicenter study | 91 | AE rate 18.7% for EUS-HGS, AE rate 8.9% for EUS-CDS. AE not specified. | EUS-HGS (n = 35) EUS-CDS (n = 48) |
| Bun Theo et al. Gastroenterology 2023 [28] | RCT | 83 | Cholangitis (n = 5) Stent misdeployment (n = 2) Stent migration (n = 1) Multi-organ failure (n = 2) Fatal (n = 4) | EUS-CDS with Hot Axios |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, C.F.; Arcidiacono, P.G.; Bhutani, M.S.; Braden, B.; Burmester, E.; Fusaroli, P.; Hocke, M.; Ignee, A.; Jenssen, C.; Al-Lehibi, A.; et al. Controversies in Endoscopic Ultrasound-Guided Biliary Drainage. Cancers 2024, 16, 1616. https://doi.org/10.3390/cancers16091616
Dietrich CF, Arcidiacono PG, Bhutani MS, Braden B, Burmester E, Fusaroli P, Hocke M, Ignee A, Jenssen C, Al-Lehibi A, et al. Controversies in Endoscopic Ultrasound-Guided Biliary Drainage. Cancers. 2024; 16(9):1616. https://doi.org/10.3390/cancers16091616
Chicago/Turabian StyleDietrich, Christoph Frank, Paolo Giorgio Arcidiacono, Manoop S. Bhutani, Barbara Braden, Eike Burmester, Pietro Fusaroli, Michael Hocke, Andrè Ignee, Christian Jenssen, Abed Al-Lehibi, and et al. 2024. "Controversies in Endoscopic Ultrasound-Guided Biliary Drainage" Cancers 16, no. 9: 1616. https://doi.org/10.3390/cancers16091616
APA StyleDietrich, C. F., Arcidiacono, P. G., Bhutani, M. S., Braden, B., Burmester, E., Fusaroli, P., Hocke, M., Ignee, A., Jenssen, C., Al-Lehibi, A., Aljahdli, E., Napoléon, B., Rimbas, M., & Vanella, G. (2024). Controversies in Endoscopic Ultrasound-Guided Biliary Drainage. Cancers, 16(9), 1616. https://doi.org/10.3390/cancers16091616








