Translational Aspects in Metaplastic Breast Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Clinical–Pathologic Features
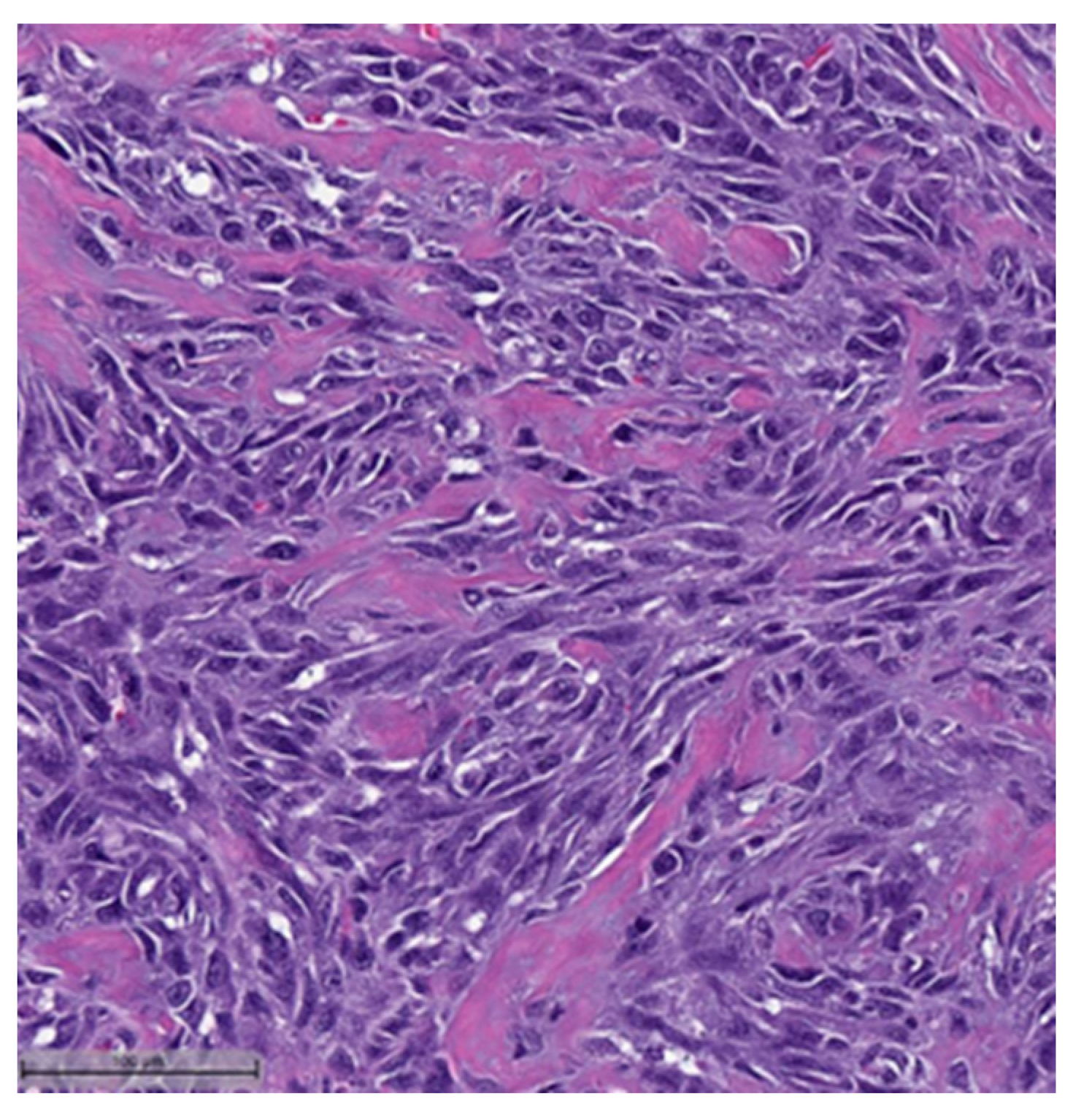
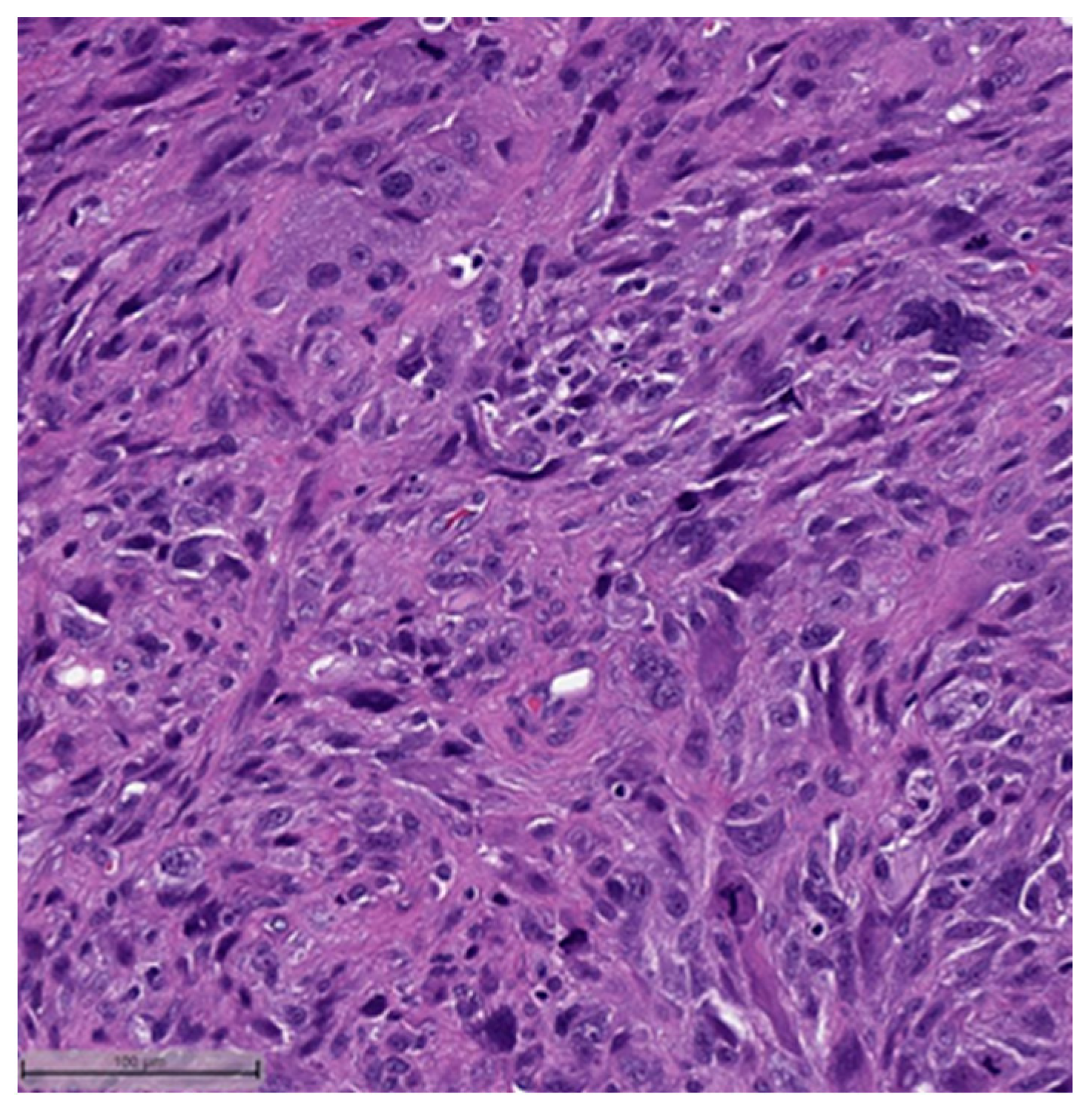
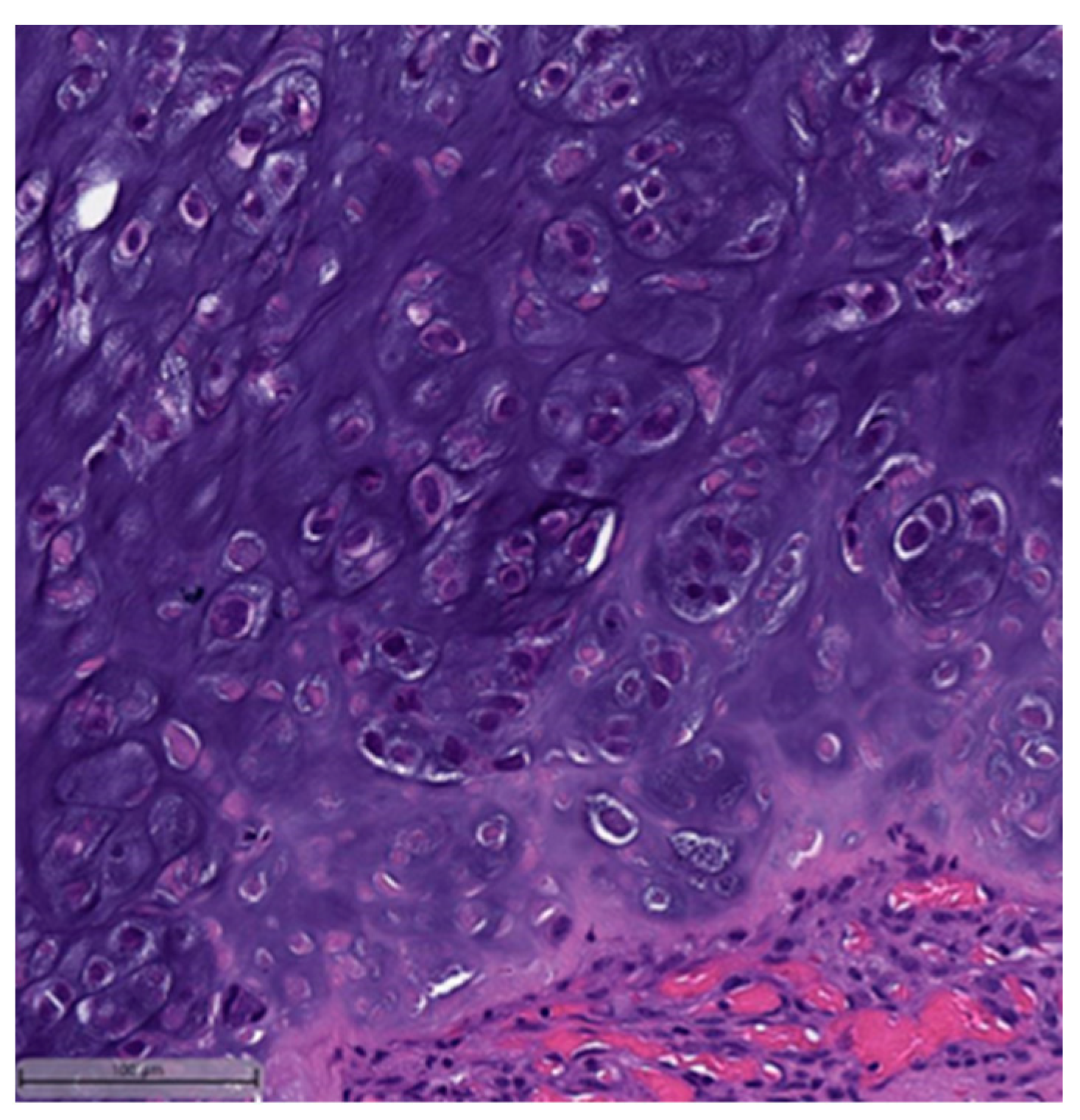
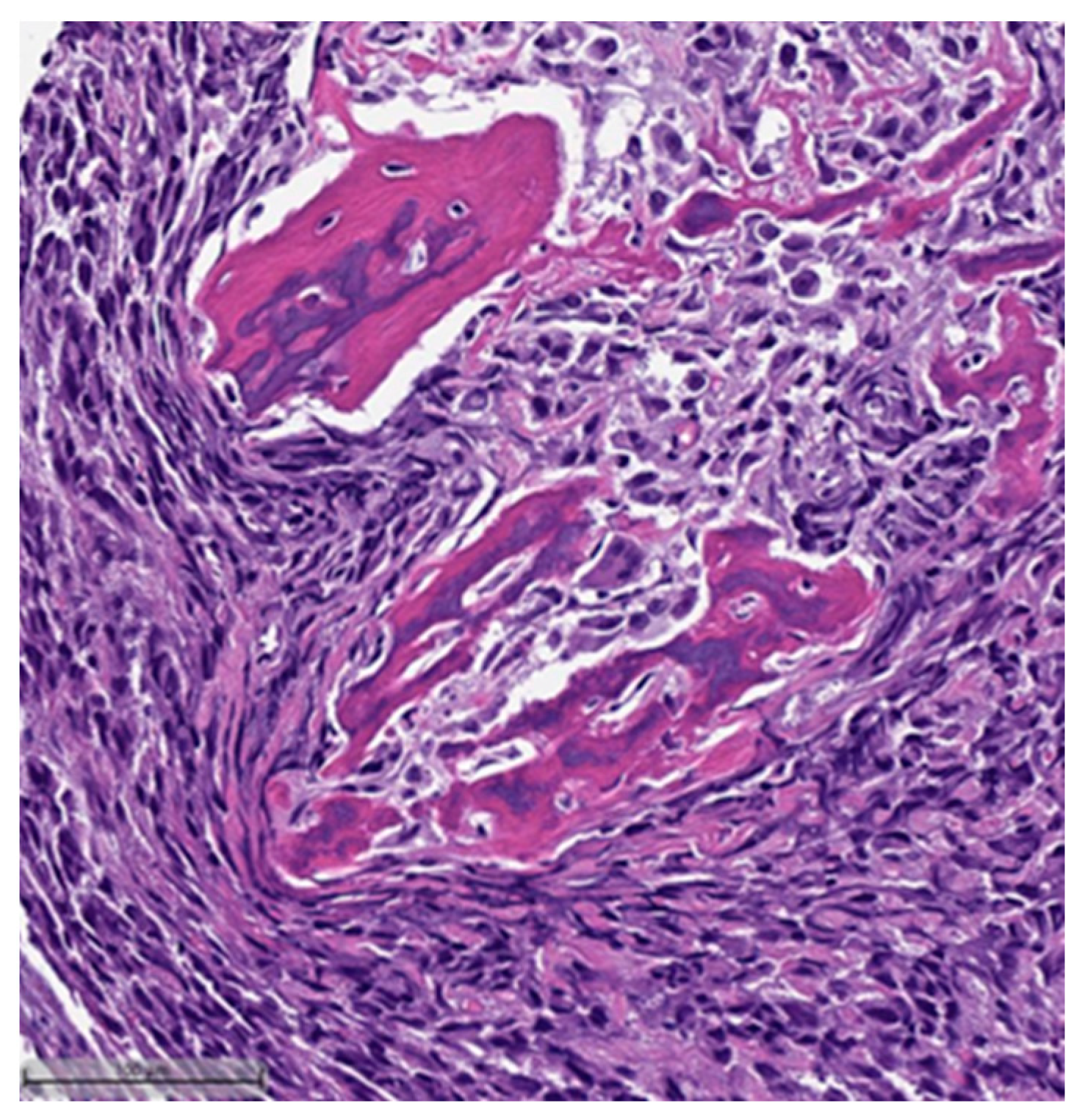
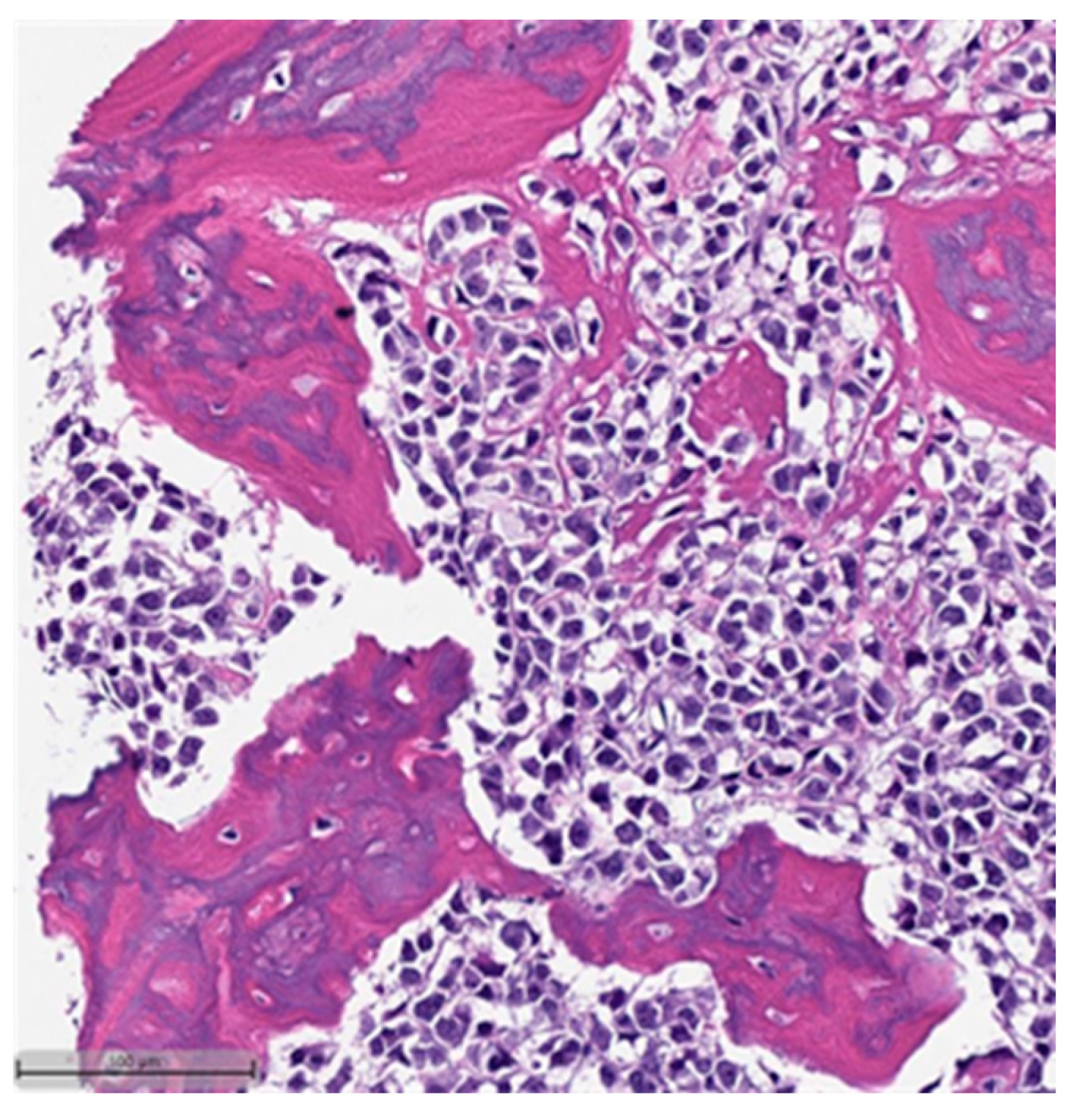
3. Histopathological and Immunohistochemical Features
- Low-grade adenosquamous carcinoma (LGASC);
- Fibromatosis-like metaplastic carcinoma (FLMC);
- Spindle cell carcinoma (SpCC) (Figure 3);
- Metaplastic carcinoma with heterologous mesenchymal differentiation (MCHMD);
- Mixed metaplastic carcinoma (MMC), where the tumor exhibits mixed components.
4. Molecular Aspects in Metaplastic Breast Carcinoma: Associated Mutations, Involved Pathways, and Transcriptomic Features
5. Tumor Immune Microenvironment
6. Epithelial–Mesenchymal Transition
6.1. Cadherins and Cadherin-Switching Mechanism
6.2. Epithelial and Mesenchymal Markers
6.3. Epithelial–Mesenchymal Transition—Transcriptional Factors
6.4. Hypoxia
6.5. miRNAs
6.6. Gene Expression
7. Treatment and Prognosis
8. Clinical Trials
9. Conclusions
10. Limitation of the Study and Current Challenges
11. Future Directions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- IARC. WHO Classification on Tumours Online. Breast Tumours (5th ed.). 2023. Available online: https://tumourclassification.iarc.who.int/ (accessed on 11 August 2023).
- David, J.; Dabbs, E.A.R. Metaplastic Breast Carcinoma. In Breast Pathology, 2nd ed.; Dabbs, D.J., Ed.; Elsevier: Philadelphia, PA, USA, 2017; pp. 532–555. [Google Scholar]
- Nelson, R.A.; Guye, M.L.; Luu, T.; Lai, L.L. Survival Outcomes of Metaplastic Breast Cancer Patients: Results from a US Population-based Analysis. Ann. Surg. Oncol. 2014, 22, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Abouharb, S.; Moulder, S. Metaplastic Breast Cancer: Clinical Overview and Molecular Aberrations for Potential Targeted Therapy. Curr. Oncol. Rep. 2015, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Tzanninis, I.-G.; Kotteas, E.A.; Ntanasis-Stathopoulos, I.; Kontogianni, P.; Fotopoulos, G. Management and Outcomes in Metaplastic Breast Cancer. Clin. Breast Cancer 2016, 16, 437–443. [Google Scholar] [CrossRef]
- Zhai, J.; Giannini, G.; Ewalt, M.D.; Zhang, E.Y.; Invernizzi, M.; Niland, J.; Lai, L.L. Molecular characterization of metaplastic breast carcinoma via next-generation sequencing. Hum. Pathol. 2018, 86, 85–92. [Google Scholar] [CrossRef]
- Han, M.; Salamat, A.; Zhu, L.; Zhang, H.; Clark, B.Z.; Dabbs, D.J.; Carter, G.J.; Brufsky, A.M.; Jankowitz, R.C.; Puhalla, S.L.; et al. Metaplastic breast carcinoma: A clinical-pathologic study of 97 cases with subset analysis of response to neoadjuvant chemotherapy. Mod. Pathol. 2019, 32, 807–816. [Google Scholar] [CrossRef]
- Wargotz, E.S.; Deos, P.H.; Norris, H.J. Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum. Pathol. 1989, 20, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Wargotz, E.S.; Norris, H.J. Metaplastic carcinomas of the breast. III. Carcinosarcoma. Cancer 1989, 64, 1490–1499. [Google Scholar] [CrossRef]
- Wargotz, E.S.; Norris, H.J. Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal origin. Cancer 1990, 65, 272–276. [Google Scholar] [CrossRef]
- Wargotz, E.S.; Norris, H.J. Metaplastic carcinomas of the breast: V. Metaplastic carcinoma with osteoclastic giant cells. Hum. Pathol. 1990, 21, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Wargotz, E.S.; Norris, H.J. Metaplastic carcinomas of the breast. I. Matrix-producing carcinoma. Hum. Pathol. 1989, 20, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Castilla, M.Á.; Díaz-Martín, J.; Sarrió, D.; Romero-Pérez, L.; López-García, M.; Vieites, B.; Biscuola, M.; Ramiro-Fuentes, S.; Isacke, C.M.; Palacios, J. MicroRNA-200 Family Modulation in Distinct Breast Cancer Phenotypes. PLoS ONE 2012, 7, e47709. [Google Scholar] [CrossRef]
- Papatheodoridi, A.; Papamattheou, E.; Marinopoulos, S.; Ntanasis-Stathopoulos, I.; Dimitrakakis, C.; Giannos, A.; Kaparelou, M.; Liontos, M.; Dimopoulos, M.-A.; Zagouri, F. Metaplastic Carcinoma of the Breast: Case Series of a Single Institute and Review of the Literature. Med. Sci. 2023, 11, 35. [Google Scholar] [CrossRef]
- Huang, C.; Tian, H.; Xu, J.; Tong, F.; Fang, D. Metaplastic breast carcinoma with osseous differentiation: A report of a rare case and literature review. Open Life Sci. 2023, 18, 20220640. [Google Scholar] [CrossRef]
- Thomas, H.R.; Hu, B.; Boyraz, B.; Johnson, A.; Bossuyt, V.I.; Spring, L.; Jimenez, R.B. Metaplastic breast cancer: A review. Crit. Rev. Oncol. 2023, 182, 103924. [Google Scholar] [CrossRef]
- González-Martínez, S.; Pérez-Mies, B.; Carretero-Barrio, I.; Palacios-Berraquero, M.L.; Perez-García, J.; Cortés, J.; Palacios, J. Molecular Features of Metaplastic Breast Carcinoma: An Infrequent Subtype of Triple Negative Breast Carcinoma. Cancers 2020, 12, 1832. [Google Scholar] [CrossRef]
- Zawati, I.; Jlassi, A.; Adouni, O.; Nouira, M.; Manai, M.; Rahal, K.; Driss, M.; Manai, M. Association of ZEB1 and Vimentin with poor prognosis in metaplastic breast cancer. Ann. Diagn. Pathol. 2022, 59, 151954. [Google Scholar] [CrossRef] [PubMed]
- A Rakha, E.; Tan, P.H.; Varga, Z.; Tse, G.M.; Shaaban, A.M.; Climent, F.; van Deurzen, C.H.M.; Purnell, D.; Dodwell, D.; Chan, T.; et al. Prognostic factors in metaplastic carcinoma of the breast: A multi-institutional study. Br. J. Cancer 2014, 112, 283–289. [Google Scholar] [CrossRef]
- Beatty, J.D.; Atwood, M.; Tickman, R.; Reiner, M. Metaplastic breast cancer: Clinical significance. Am. J. Surg. 2006, 191, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Wu, Y.; Gilcrease, M.Z. Primary squamous cell carcinoma of the breast: Predictors of locoregional recurrence and overall survival. Am. J. Surg. Pathol. 2013, 37, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Khoury, T. Metaplastic Breast Carcinoma Revisited; Subtypes Determine Outcomes: Comprehensive Pathologic, Clinical, and Molecular Review. Surg. Pathol. Clin. 2022, 15, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Khoury, T. Metaplastic Breast Carcinoma Revisited; Subtypes Determine Outcomes: Comprehensive Pathologic, Clinical, and Molecular Review. Clin. Lab. Med. 2023, 43, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Rungta, S.; Kleer, C.G. Metaplastic Carcinomas of the Breast: Diagnostic Challenges and New Translational Insights. Arch. Pathol. Lab. Med. 2012, 136, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Toy, K.A.; Griffith, K.A.; Awuah, B.; Quayson, S.; Newman, L.A.; Kleer, C.G. Invasive breast carcinomas in Ghana: High frequency of high grade, basal-like histology and high EZH2 expression. Breast Cancer Res. Treat. 2012, 135, 59–66. [Google Scholar] [CrossRef]
- McMullen, E.R.; Zoumberos, N.A.; Kleer, C.G. Metaplastic Breast Carcinoma: Update on Histopathology and Molecular Alterations. Arch. Pathol. Lab. Med. 2019, 143, 1492–1496. [Google Scholar] [CrossRef]
- Adem, C.; Reynolds, C.; Adlakha, H.; Roche, P.C.; Nascimento, A.G. Wide spectrum screening keratin as a marker of metaplastic spindle cell carcinoma of the breast: An immunohistochemical study of 24 patients. Histopathology 2002, 40, 556–562. [Google Scholar] [CrossRef]
- Koker, M.M.; Kleer, C.G. p63 expression in breast cancer: A highly sensitive and specific marker of metaplastic carcinoma. Am. J. Surg. Pathol. 2004, 28, 1506–1512. [Google Scholar] [CrossRef]
- A Rakha, E.; Coimbra, N.D.M.; Hodi, Z.; Juneinah, E.; O Ellis, I.; Lee, A.H.S. Immunoprofile of metaplastic carcinomas of the breast. Histopathology 2016, 70, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Reis-Filho, J.; Pinheiro, C.; Lambros, M.; Milanezi, F.; Carvalho, S.; Savage, K.; Simpson, P.; Jones, C.; Swift, S.; Mackay, A.; et al. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J. Pathol. 2006, 209, 445–453. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.-L.; Sun, H.; Huo, L.; Wu, Y.; Chen, H.; Gan, Q.; Meis, J.M.; Maloney, N.; Lazar, A.J.; et al. Expression of TRPS1 in phyllodes tumor and sarcoma of the breast. Hum. Pathol. 2022, 121, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.C.; Wang, G.; Parkinson, B.; Huo, L.; Peng, Y.; Wang, J.; Salisbury, T.; Wu, Y.; Chen, H.; Albarracin, C.T.; et al. TRPS1, GATA3, and SOX10 expression in triple-negative breast carcinoma. Hum. Pathol. 2022, 125, 97–107. [Google Scholar] [CrossRef]
- A Rakha, E.; Quinn, C.M.; Foschini, M.P.; Martin, M.M.; Dabbs, D.J.; Lakhani, S.; Varga, Z.; E Pinder, S.; Schmitt, F.C.; Reis-Filho, J.S.; et al. Metaplastic carcinomas of the breast without evidence of epithelial differentiation: A diagnostic approach for management. Histopathology 2020, 78, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Gonzalez-Angulo, A.-M.; Stemke-Hale, K.; Gilcrease, M.Z.; Krishnamurthy, S.; Lee, J.-S.; Fridlyand, J.; Sahin, A.; Agarwal, R.; Joy, C.; et al. Characterization of a Naturally Occurring Breast Cancer Subset Enriched in Epithelial-to-Mesenchymal Transition and Stem Cell Characteristics. Cancer Res. 2009, 69, 4116–4124. [Google Scholar] [CrossRef]
- Weigelt, B.; Kreike, B.; Reis-Filho, J.S. Metaplastic breast carcinomas are basal-like breast cancers: A genomic profiling analysis. Breast Cancer Res. Treat. 2008, 117, 273–280. [Google Scholar] [CrossRef]
- Weigelt, B.; Ng, C.K.; Shen, R.; Popova, T.; Schizas, M.; Natrajan, R.; Mariani, O.; Stern, M.H.; Norton, L.; Vincent-Salomon, A.; et al. Metaplastic breast carcinomas display genomic and transcriptomic heterogeneity [corrected]. Mod. Pathol. 2015, 28, 340–351. [Google Scholar] [CrossRef]
- Lien, H.C.; Hsiao, Y.H.; Lin, Y.S.; Yao, Y.T.; Juan, H.F.; Kuo, W.H.; Hung, M.-C.; Chang, K.J.; Hsieh, F.J. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: Identification of genes potentially related to epithelial–mesenchymal transition. Oncogene 2007, 26, 7859–7871. [Google Scholar] [CrossRef]
- Krings, G.; Chen, Y.-Y. Genomic profiling of metaplastic breast carcinomas reveals genetic heterogeneity and relationship to ductal carcinoma. Mod. Pathol. 2018, 31, 1661–1674. [Google Scholar] [CrossRef]
- Lee, K.-H.; Hwang, H.-J.; Noh, H.J.; Shin, T.-J.; Cho, J.-Y. Somatic Mutation of PIK3CA (H1047R) Is a Common Driver Mutation Hotspot in Canine Mammary Tumors as Well as Human Breast Cancers. Cancers 2019, 11, 2006. [Google Scholar] [CrossRef] [PubMed]
- Tray, N.; Taff, J.; Singh, B.; Suh, J.; Ngo, N.; Kwa, M.; Troxel, A.B.; Chae, Y.K.; Kurzrock, R.; Patel, S.P.; et al. Metaplastic breast cancers: Genomic profiling, mutational burden and tumor-infiltrating lymphocytes. Breast 2019, 44, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.; Piscuoglio, S.; Geyer, F.C.; Burke, K.A.; Pareja, F.; Eberle, C.A.; Lim, R.S.; Natrajan, R.; Riaz, N.; Mariani, O.; et al. The Landscape of Somatic Genetic Alterations in Metaplastic Breast Carcinomas. Clin. Cancer Res. 2017, 23, 3859–3870. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Thomas, D.; Emmons, A.; Giordano, T.J.; Kleer, C.G. Genetic Changes of Wnt Pathway Genes Are Common Events in Metaplastic Carcinomas of the Breast. Clin. Cancer Res. 2008, 14, 4038–4044. [Google Scholar] [CrossRef]
- Geyer, F.C.; Li, A.; Papanastasiou, A.D.; Smith, A.; Selenica, P.; Burke, K.A.; Edelweiss, M.; Wen, H.-C.; Piscuoglio, S.; Schultheis, A.M.; et al. Recurrent hotspot mutations in HRAS Q61 and PI3K-AKT pathway genes as drivers of breast adenomyoepitheliomas. Nat. Commun. 2018, 9, 1816. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.E.; Sung, K.-J.; Tran, H.; Song, W.; Ginter, P.S. Mammary Epithelial-Myoepithelial Carcinoma: Report of a Case with HRAS and PIK3CA Mutations by Next-Generation Sequencing. Int. J. Surg. Pathol. 2018, 27, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Breuer, A.; Kandel, M.; Fisseler-Eckhoff, A.; Sutter, C.; Schwaab, E.; Lück, H.-J.; du Bois, A. BRCA1 Germline Mutation in a Woman with Metaplastic Squamous Cell Breast Cancer. Oncol. Res. Treat. 2007, 30, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Noël, J.-C.; Buxant, F.; Engohan-Aloghe, C. Low-grade adenosquamous carcinoma of the breast—A case report with a BRCA1 germline mutation. Pathol. Res. Pract. 2010, 206, 511–513. [Google Scholar] [CrossRef]
- Rashid, M.U.; Shah, M.A.; Azhar, R.; Syed, A.A.; Amin, A.; Hamann, U. A deleterious BRCA1 mutation in a young Pakistani woman with metaplastic breast carcinoma. Pathol. Res. Pract. 2011, 207, 583–586. [Google Scholar] [CrossRef]
- Ghilli, M.; Mariniello, D.M.; Fanelli, G.; Cascione, F.; Fontana, A.; Cristaudo, A.; Cilotti, A.; Caligo, A.M.; Manca, G.; Colizzi, L.; et al. Carcinosarcoma of the Breast: An Aggressive Subtype of Metaplastic Cancer. Report of a Rare Case in a Young BRCA-1 Mutated Woman. Clin. Breast Cancer 2016, 17, e31–e35. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, M.; Schmolze, D.; Yost, S.E.; Frankel, P.H.; Dagis, A.; Amanam, I.U.; Telatar, M.; Nguyen, K.; Yu, K.W.; Luu, T.; et al. Mutation and immune profiling of metaplastic breast cancer: Correlation with survival. PLoS ONE 2019, 14, e0224726. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Abuhadra, N.; Sun, R.; Adrada, B.E.; Ding, Q.-Q.; White, J.B.; Ravenberg, E.E.; Clayborn, A.R.; Valero, V.; Tripathy, D.; et al. Molecular Characterization and Prospective Evaluation of Pathologic Response and Outcomes with Neoadjuvant Therapy in Metaplastic Triple-Negative Breast Cancer. Clin. Cancer Res. 2022, 28, 2878–2889. [Google Scholar] [CrossRef]
- Lien, H.-C.; Hsu, C.-L.; Lu, Y.-S.; Chen, T.W.-W.; Chen, I.-C.; Li, Y.-C.; Huang, C.-S.; Cheng, A.-L.; Lin, C.-H. Transcriptomic alterations underlying metaplasia into specific metaplastic components in metaplastic breast carcinoma. Breast Cancer Res. 2023, 25, 11. [Google Scholar] [CrossRef]
- Luini, A.; Aguilar, M.; Gatti, G.; Fasani, R.; Botteri, E.; Brito, J.A.D.; Maisonneuve, P.; Vento, A.R.; Viale, G. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European Institute of Oncology and review of the literature. Breast Cancer Res. Treat. 2006, 101, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-Y.; Kim, H.Y.; Nam, B.-H.; Min, S.Y.; Lee, S.J.; Park, C.; Kwon, Y.; Kim, E.-A.; Ko, K.L.; Shin, K.H.; et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res. Treat. 2010, 120, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Joneja, U.; Vranic, S.; Swensen, J.; Feldman, R.; Chen, W.; Kimbrough, J.; Xiao, N.; Reddy, S.; Palazzo, J.; Gatalica, Z. Comprehensive profiling of metaplastic breast carcinomas reveals frequent overexpression of programmed death-ligand 1. J. Clin. Pathol. 2016, 70, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Gonda, K.; Akama, T.; Nakamura, T.; Hashimoto, E.; Kyoya, N.; Rokkaku, Y.; Maejima, Y.; Horita, S.; Tachibana, K.; Abe, N.; et al. Cluster of differentiation 8 and programmed cell death ligand 1 expression in triple-negative breast cancer combined with autosomal dominant polycystic kidney disease and tuberous sclerosis complex: A case report. J. Med. Case Rep. 2019, 13, 381. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Dill, E.A.; Gru, A.A.; Atkins, K.A.; Friedman, L.A.; Moore, M.E.; Bullock, T.N.; Cross, J.V.; Dillon, P.M.; Mills, A.M. PD-L1 Expression and Intratumoral Heterogeneity Across Breast Cancer Subtypes and Stages: An Assessment of 245 Primary and 40 Metastatic Tumors. Am. J. Surg. Pathol. 2017, 41, 334–342. [Google Scholar] [CrossRef]
- Vranic, S.; Stafford, P.; Palazzo, J.; Skenderi, F.; Swensen, J.; Xiu, J.; Spetzler, D.; Gatalica, Z. Molecular Profiling of the Metaplastic Spindle Cell Carcinoma of the Breast Reveals Potentially Targetable Biomarkers. Clin. Breast Cancer 2020, 20, 326–331.e1. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Liu, L.; Sun, P.; Yang, X.; Li, M.; Luo, R.; Huang, Y.; He, J.; Yun, J. Immune parameters associated with survival in metaplastic breast cancer. Breast Cancer Res. 2020, 22, 92. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.-C.; Lee, Y.-H.; Chen, I.-C.; Lin, C.-H.; Chen, T.W.-W.; Lu, Y.-T.; Lu, Y.-S. Tumor-infiltrating lymphocyte abundance and programmed death-ligand 1 expression in metaplastic breast carcinoma: Implications for distinct immune microenvironments in different metaplastic components. Virchows Arch. 2020, 478, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Kalaw, E.; Lim, M.; Kutasovic, J.R.; Sokolova, A.; Taege, L.; Johnstone, K.; Bennett, J.; Saunus, J.M.; Niland, C.; Ferguson, K.; et al. Metaplastic breast cancers frequently express immune checkpoint markers FOXP3 and PD-L1. Br. J. Cancer 2020, 123, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.T.; Anderson, K.S.; Lenkiewicz, E.; Andreozzi, M.; Cunliffe, H.E.; Klassen, C.L.; Dueck, A.C.; McCullough, A.E.; Reddy, S.K.; Ramanathan, R.K.; et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget 2015, 6, 26483–26493. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. Tumor-Intrinsic PD-L1 Signaling in Cancer Initiation, Development and Treatment: Beyond Immune Evasion. Front. Oncol. 2018, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Mandapati, A.; Lukong, K.E. Triple negative breast cancer: Approved treatment options and their mechanisms of action. J. Cancer Res. Clin. Oncol. 2022, 149, 3701–3719. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Kruse, M.; Tran, J. Progress in immune checkpoint inhibition in early-stage triple-negative breast cancer. Expert Rev. Anticancer. Ther. 2023, 23, 1071–1084. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Micalizzi, D.S.; Farabaugh, S.M.; Ford, H.L. Epithelial-Mesenchymal Transition in Cancer: Parallels between Normal Development and Tumor Progression. J. Mammary Gland Biol. Neoplasia 2010, 15, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, S.; Pérez-Mies, B.; Pizarro, D.; Caniego-Casas, T.; Cortés, J.; Palacios, J. Epithelial Mesenchymal Transition and Immune Response in Metaplastic Breast Carcinoma. Int. J. Mol. Sci. 2021, 22, 7398. [Google Scholar] [CrossRef] [PubMed]
- Beca, F.; Sebastiao, A.P.M.; Pareja, F.; Dessources, K.; Lozada, J.R.; Geyer, F.; Selenica, P.; Zeizafoun, N.; Wen, H.Y.; Norton, L.; et al. Whole-exome analysis of metaplastic breast carcinomas with extensive osseous differentiation. Histopathology 2020, 77, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs 2021, 211, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; A Mani, S.; Donaher, J.L.; Ramaswamy, S.; A Itzykson, R.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; A Weinberg, R. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Krebs, A.M.; Mitschke, J.; Lasierra Losada, M.; Schmalhofer, O.; Boerries, M.; Busch, H.; Boettcher, M.; Mougiakakos, D.; Reichardt, W.; Bronsert, P.; et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017, 19, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lee, D.K.; Feng, Z.; Xu, Y.; Bu, W.; Li, Y.; Liao, L.; Xu, J. Breast tumor cell-specific knockout of Twist1 inhibits cancer cell plasticity, dissemination, and lung metastasis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 11494–11499. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhang, Q.; Zhang, Q.; Sun, P.; Xiang, R.; Ren, G.; Yang, S. ZEB1 confers chemotherapeutic resistance to breast cancer by activating ATM. Cell Death Dis. 2018, 9, 57. [Google Scholar] [CrossRef]
- Tran, H.D.; Luitel, K.; Kim, M.; Zhang, K.; Longmore, G.D.; Tran, D.D. Transient SNAIL1 Expression Is Necessary for Metastatic Competence in Breast Cancer. Cancer Res. 2014, 74, 6330–6340. [Google Scholar] [CrossRef]
- Khan, A.; Chen, H.-C.; Zhang, D.; Fu, J. Twist: A molecular target in cancer therapeutics. Tumor Biol. 2013, 34, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, P.; Windh, P.; Lauritzen, C.; Emanuelsson, M.; Grönberg, H.; Stenman, G. Women with Saethre-Chotzen syndrome are at increased risk of breast cancer. Genes Chromosom. Cancer 2007, 46, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Goyal, A.; Watkins, G.; Jiang, W.G. Expression of the Transcription Factors Snail, Slug, and Twist and Their Clinical Significance in Human Breast Cancer. Ann. Surg. Oncol. 2005, 12, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.T.; Dobrovic, A.; Yan, M.; Karim, R.Z.; Lee, C.S.; Lakhani, S.R.; Fox, S.B. DNA methylation profiling of phyllodes and fibroadenoma tumours of the breast. Breast Cancer Res. Treat. 2010, 124, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Vesuna, F.; Lisok, A.; Kimble, B.; Domek, J.; Kato, Y.; van der Groep, P.; Artemov, D.; Kowalski, J.; Carraway, H.; van Diest, P.; et al. Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-α. Oncogene 2011, 31, 3223–3234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fang, T.; Lv, Y. Prognostic and clinicopathological value of Slug protein expression in breast cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2022, 20, 361. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kim, H.J.; Kim, E.J.; Chung, Y.R.; Park, S.Y. Expression of epithelial-mesenchymal transition–related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum. Pathol. 2015, 46, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, A.; Bévant, K.; Blanpain, C. Cancer cell plasticity during tumor progression, metastasis and response to therapy. Nat. Cancer 2023, 4, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Nushtaeva, A.; Ermakov, M.; Abdurakhmanova, M.; Troitskaya, O.; Belovezhets, T.; Varlamov, M.; Gayner, T.; Richter, V.; Koval, O. “Pulsed Hypoxia” Gradually Reprograms Breast Cancer Fibroblasts into Pro-Tumorigenic Cells via Mesenchymal-Epithelial Transition. Int. J. Mol. Sci. 2023, 24, 2494. [Google Scholar] [CrossRef]
- Rouschop, K.M.; van den Beucken, T.; Dubois, L.; Niessen, H.; Bussink, J.; Savelkouls, K.; Keulers, T.; Mujcic, H.; Landuyt, W.; Voncken, J.W.; et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investig. 2010, 120, 127–141. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Wu, K.-J. TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle 2008, 7, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.H.; Herschkowitz, J.I.; Komurov, K.; Zhou, A.Y.; Gupta, S.; Yang, J.; Hartwell, K.; Onder, T.T.; Gupta, P.B.; Evans, K.W.; et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc. Natl. Acad. Sci. USA 2010, 107, 15449–15454. [Google Scholar] [CrossRef]
- Oon, M.L.; Thike, A.A.; Tan, S.Y.; Tan, P.H. Cancer stem cell and epithelial–mesenchymal transition markers predict worse outcome in metaplastic carcinoma of the breast. Breast Cancer Res. Treat. 2015, 150, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.P.; Gregory, P.A.; Kolesnikoff, N.; Bert, A.G.; Wang, J.; Shannon, M.F.; Goodall, G.J. A Double-Negative Feedback Loop between ZEB1-SIP1 and the microRNA-200 Family Regulates Epithelial-Mesenchymal Transition. Cancer Res. 2008, 68, 7846–7854. [Google Scholar] [CrossRef] [PubMed]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. Embo Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Howe, E.N.; Cochrane, D.R.; Richer, J.K. The miR-200 and miR-221/222 microRNA families: Opposing effects on epithelial identity. J. Mammary Gland Biol. Neoplasia 2012, 17, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Davalos, V.; Moutinho, C.; Villanueva, A.; Boque, R.; Silva, P.; Carneiro, F.; Esteller, M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene 2011, 31, 2062–2074. [Google Scholar] [CrossRef]
- Russo, J.; Snider, K.; Pereira, J.S.; Russo, I.H. Estrogen-induced breast cancer is the result of disruption of asymmetric cell division of the stem cell. Horm. Mol. Biol. Clin. Investig. 2009, 1, 53–65. [Google Scholar] [CrossRef]
- Cooper, C.L.; Karim, R.Z.; Selinger, C.; Carmalt, H.; Lee, C.S.; A O’Toole, S. Molecular alterations in metaplastic breast carcinoma. J. Clin. Pathol. 2013, 66, 522–528. [Google Scholar] [CrossRef]
- Sarrió, D.; Rodriguez-Pinilla, S.M.; Hardisson, D.; Cano, A.; Moreno-Bueno, G.; Palacios, J. Epithelial-Mesenchymal Transition in Breast Cancer Relates to the Basal-like Phenotype. Cancer Res. 2008, 68, 989–997. [Google Scholar] [CrossRef]
- Bulfoni, M.; Gerratana, L.; Del Ben, F.; Marzinotto, S.; Sorrentino, M.; Turetta, M.; Scoles, G.; Toffoletto, B.; Isola, M.; Beltrami, C.A.; et al. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 2016, 18, 30. [Google Scholar] [CrossRef]
- Buyuk, B.; Jin, S.; Ye, K. Epithelial-to-Mesenchymal Transition Signaling Pathways Responsible for Breast Cancer Metastasis. Cell. Mol. Bioeng. 2021, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef] [PubMed]
- Trapani, D.; Giugliano, F.; Uliano, J.; Zia, V.A.A.; Marra, A.; Viale, G.; Ferraro, E.; Esposito, A.; Criscitiello, C.; D’amico, P.; et al. Benefit of adjuvant chemotherapy in patients with special histology subtypes of triple-negative breast cancer: A systematic review. Breast Cancer Res. Treat. 2021, 187, 323–337. [Google Scholar] [CrossRef]
- Corso, G.; D’ecclesiis, O.; Magnoni, F.; Mazzotta, E.; Conforti, F.; Veronesi, P.; Sajjadi, E.; Venetis, K.; Fusco, N.; Gandini, S. Metaplastic breast cancers and triple-negative breast cancers of no special type: Are they prognostically different? A systematic review and meta-analysis. Eur. J. Cancer Prev. 2021, 31, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta-Caldarola, G.; Nenna, R.; Lanotte, L.; Doronzo, A.; Gadaleta-Caldarola, A.; de Roma, I.; Lombardi, L.; Infusino, S. Metaplastic breast cancer: An old histotype but a current therapeutic problem. Futur. Oncol. 2021, 17, 955–963. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ji, J.; Dong, R.; Liu, H.; Dai, X.; Wang, C.; Esteva, F.J.; Yeung, S.-C.J. Prognosis in different subtypes of metaplastic breast cancer: A population-based analysis. Breast Cancer Res. Treat. 2018, 173, 329–341. [Google Scholar] [CrossRef]
- Barquet-Muñoz, S.A.; Villarreal-Colin, S.P.; Herrera-Montalvo, L.A.; Soto-Reyes, E.; Pérez-Plasencia, C.; Coronel-Martínez, J.; Pérez-Montiel, D.; Vázquez-Romo, R.; de León, D.C. Metaplastic breast cancer: A comparison between the most common histologies with poor immunohistochemistry factors. BMC Cancer 2015, 15, 75. [Google Scholar] [CrossRef]
- Reed, A.E.M.; Kalaw, E.; Nones, K.; Bettington, M.; Lim, M.; Bennett, J.; Johnstone, K.; Kutasovic, J.R.; Saunus, J.M.; Kazakoff, S.; et al. Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications. J. Pathol. 2018, 247, 214–227. [Google Scholar] [CrossRef]
- Downs-Kelly, E.; Nayeemuddin, K.M.; Albarracin, C.; Wu, Y.; Hunt, K.K.; Gilcrease, M.Z. Matrix-producing carcinoma of the breast: An aggressive subtype of metaplastic carcinoma. Am. J. Surg. Pathol. 2009, 33, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.T.; Campbell, B.M.; Thomas, S.M.; Greenup, R.A.; Plichta, J.K.; Rosenberger, L.H.; Force, J.; Hall, A.; Hyslop, T.; Hwang, E.S.; et al. Metaplastic Breast Cancer Treatment and Outcomes in 2500 Patients: A Retrospective Analysis of a National Oncology Database. Ann. Surg. Oncol. 2018, 25, 2249–2260. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, F.; Yang, Y.; Qian, X.; Lang, R.; Fan, Y.; Liu, F.; Li, Y.; Li, S.; Shen, B.; et al. Clinicopathological Features and Prognosis of Metaplastic Breast Carcinoma: Experience of a Major Chinese Cancer Center. PLoS ONE 2015, 10, e0131409. [Google Scholar] [CrossRef]
- Lee, H.; Jung, S.-Y.; Ro, J.Y.; Kwon, Y.; Sohn, J.H.; Park, I.H.; Lee, K.S.; Lee, S.; Kim, S.W.; Kang, H.S.; et al. Metaplastic breast cancer: Clinicopathological features and its prognosis. J. Clin. Pathol. 2012, 65, 441–446. [Google Scholar] [CrossRef]
- Aydiner, A.; Sen, F.; Tambas, M.; Ciftci, R.; Eralp, Y.; Saip, P.; Karanlik, H.; Fayda, M.; Kucucuk, S.; Onder, S.; et al. Metaplastic Breast Carcinoma Versus Triple-Negative Breast Cancer: Survival and Response to Treatment. Medicine 2015, 94, e2341. [Google Scholar] [CrossRef]
- Nagao, T.; Kinoshita, T.; Hojo, T.; Tsuda, H.; Tamura, K.; Fujiwara, Y. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: The relationship between the outcome and the clinicopathological characteristics. Breast 2012, 21, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Lin, C.H.; Huang, C.S.; Lien, H.C.; Hsu, C.; Kuo, W.H.; Lu, Y.S.; Cheng, A.L. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res. Treat. 2011, 130, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Moulder, S.; Moroney, J.; Helgason, T.; Wheler, J.; Booser, D.; Albarracin, C.; Morrow, P.K.; Koenig, K.; Kurzrock, R. Responses to Liposomal Doxorubicin, Bevacizumab, and Temsirolimus in Metaplastic Carcinoma of the Breast: Biologic Rationale and Implications for Stem-Cell Research in Breast Cancer. J. Clin. Oncol. 2011, 29, e572–e575. [Google Scholar] [CrossRef]
- Al-Hilli, Z.; Choong, G.; Keeney, M.G.; Visscher, D.W.; Ingle, J.N.; Goetz, M.P.; Jakub, J.W. Metaplastic breast cancer has a poor response to neoadjuvant systemic therapy. Breast Cancer Res. Treat. 2019, 176, 709–716. [Google Scholar] [CrossRef]
- Cimino-Mathews, A.; Verma, S.; Figueroa-Magalhaes, M.C.; Jeter, S.C.; Zhang, Z.; Argani, P.; Stearns, V.; Connolly, R.M. A Clinicopathologic Analysis of 45 Patients with Metaplastic Breast Carcinoma. Am. J. Clin. Pathol. 2016, 145, 365–372. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Othus, M.; Patel, S.P.; Miller, K.D.; Chugh, R.; Schuetze, S.M.; Chamberlin, M.D.; Haley, B.J.; Storniolo, A.M.V.; Reddy, M.P.; et al. A Multicenter Phase II Trial of Ipilimumab and Nivolumab in Unresectable or Metastatic Metaplastic Breast Cancer: Cohort 36 of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART, SWOG S1609). Clin. Cancer Res. 2021, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Gorshein, E.; Matsuda, K.; Riedlinger, G.; Sokol, L.; Rodriguez-Rodriguez, L.; Eladoumikdachi, F.; Grandhi, M.; Ganesan, S.; Toppmeyer, D.L.; Potdevin, L.; et al. Durable Response to PD1 Inhibitor Pembrolizumab in a Metastatic, Metaplastic Breast Cancer. Case Rep. Oncol. 2021, 14, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Al Sayed, A.D.; Elshenawy, M.A.; Tulbah, A.; Al-Tweigeri, T.; Ghebeh, H. Complete Response of Chemo-Refractory Metastatic Metaplastic Breast Cancer to Paclitaxel-Immunotherapy Combination. Am. J. Case Rep. 2019, 20, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients with Operable Breast Cancer with a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liu, C.; Hou, H.; Zhang, C.; Liu, D.; Wang, G.; Liu, K.; Zhu, J.; Lv, H.; Li, T.; et al. Response to apatinib in chemotherapy-failed advanced spindle cell breast carcinoma. Oncotarget 2016, 7, 72373–72379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, M.-H.; Chen, I.-C.; Lu, Y.-S. PI3K inhibitor provides durable response in metastatic metaplastic carcinoma of the breast: A hidden gem in the BELLE-4 study. J. Formos. Med Assoc. 2018, 118, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.; Noguchi, E.; Yoshida, M.; Mori, T.; Tanioka, M.; Sudo, K.; Shimomura, A.; Yonemori, K.; Fujiwara, Y.; Tamura, K. Response to Dabrafenib and Trametinib of a Patient with Metaplastic Breast Carcinoma Harboring a BRAF V600E Mutation. Case Rep. Oncol. Med. 2020, 2020, 2518383. [Google Scholar] [CrossRef]
- Thomas, A.; Douglas, E.; Reis-Filho, J.S.; Gurcan, M.N.; Wen, H.Y. Metaplastic Breast Cancer: Current Understanding and Future Directions. Clin. Breast Cancer 2023, 23, 775–783. [Google Scholar] [CrossRef]
- Seoul National University Hospital. Neoadjuvant Chemotherapy Response in Metaplastic Carcinoma of Triple Negative Breast Cancer 2020 [Updated 17 December 2020]. Available online: https://classic.clinicaltrials.gov/show/NCT04549584 (accessed on 29 December 2023).
- Casali, P.G.; Bruzzi, P.; Bogaerts, J.; Blay, J.-Y.; Aapro, M.; Adamous, A.; Berruti, A.; Bressington, J.; Bruzzi, B.; Capocaccia, R.; et al. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: A European consensus position paper. Ann. Oncol. 2014, 26, 300–306. [Google Scholar] [CrossRef]
- Gerss, J.W.; Köpcke, W. Clinical trials and rare diseases. Adv. Exp. Med. Biol. 2010, 686, 173–190. [Google Scholar] [PubMed]
- Jing, L.; Yang, L.; Jianbo, C.; Yuqiu, W.; Yehui, Z. CircSETD2 inhibits YAP1 by interaction with HuR during breast cancer progression. Cancer Biol. Ther. 2023, 24, 2246205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, C.; Jia, J.; Wang, Z.; Li, L.; Deng, X.; Cai, Z.; Yang, L.; Wang, D.; Ma, S.; et al. Regulatory network of metformin on adipogenesis determined by combining high-throughput sequencing and GEO database. Adipocyte 2022, 11, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- ’t Hoen, P.A.C.; The GEUVADIS Consortium; Friedländer, M.R.; Almlöf, J.; Sammeth, M.; Pulyakhina, I.; Anvar, S.Y.; Laros, J.F.J.; Buermans, H.P.J.; Karlberg, O.; et al. Reproducibility of high-throughput mRNA and small RNA sequencing across laboratories. Nat. Biotechnol. 2013, 31, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Monkman, J.; Taheri, T.; Warkiani, M.E.; O’leary, C.; Ladwa, R.; Richard, D.; O’byrne, K.; Kulasinghe, A. High-Plex and High-Throughput Digital Spatial Profiling of Non-Small-Cell Lung Cancer (NSCLC). Cancers 2020, 12, 3551. [Google Scholar] [CrossRef]
- Hernandez, S.; Lazcano, R.; Serrano, A.; Powell, S.; Kostousov, L.; Mehta, J.; Khan, K.; Lu, W.; Solis, L.M. Challenges and Opportunities for Immunoprofiling Using a Spatial High-Plex Technology: The NanoString GeoMx® Digital Spatial Profiler. Front. Oncol. 2022, 12, 890410. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrientos-Toro, E.N.; Ding, Q.; Raso, M.G. Translational Aspects in Metaplastic Breast Carcinoma. Cancers 2024, 16, 1433. https://doi.org/10.3390/cancers16071433
Barrientos-Toro EN, Ding Q, Raso MG. Translational Aspects in Metaplastic Breast Carcinoma. Cancers. 2024; 16(7):1433. https://doi.org/10.3390/cancers16071433
Chicago/Turabian StyleBarrientos-Toro, Elizve Nairoby, Qingqing Ding, and Maria Gabriela Raso. 2024. "Translational Aspects in Metaplastic Breast Carcinoma" Cancers 16, no. 7: 1433. https://doi.org/10.3390/cancers16071433
APA StyleBarrientos-Toro, E. N., Ding, Q., & Raso, M. G. (2024). Translational Aspects in Metaplastic Breast Carcinoma. Cancers, 16(7), 1433. https://doi.org/10.3390/cancers16071433






