Long-Term Survival Rates and Treatment Trends of Burkitt Lymphoma in Patients with HIV—A National Cancer Database (NCDB) Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
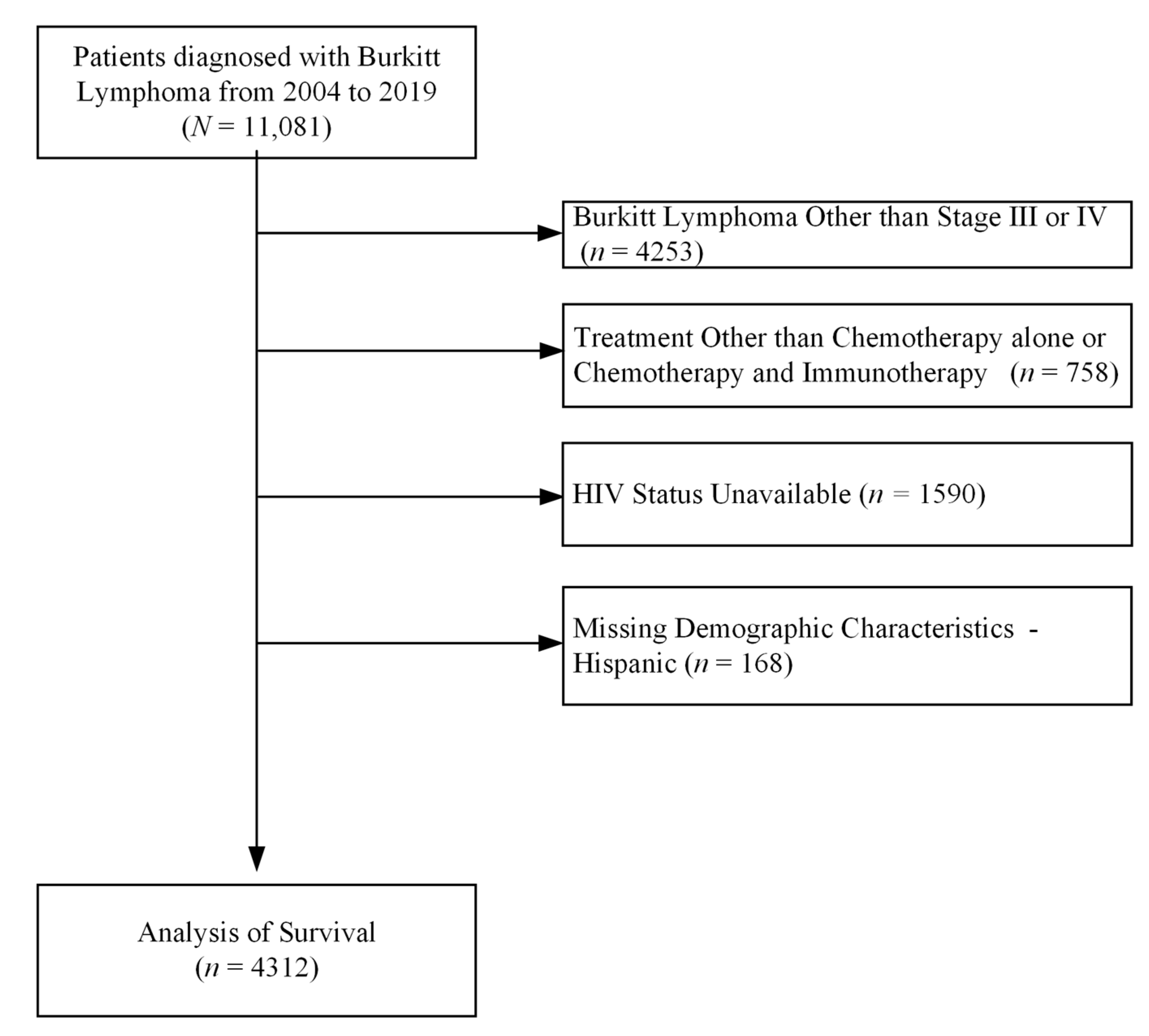
2.2. Study Cohort
2.3. Statistical Analysis
3. Results
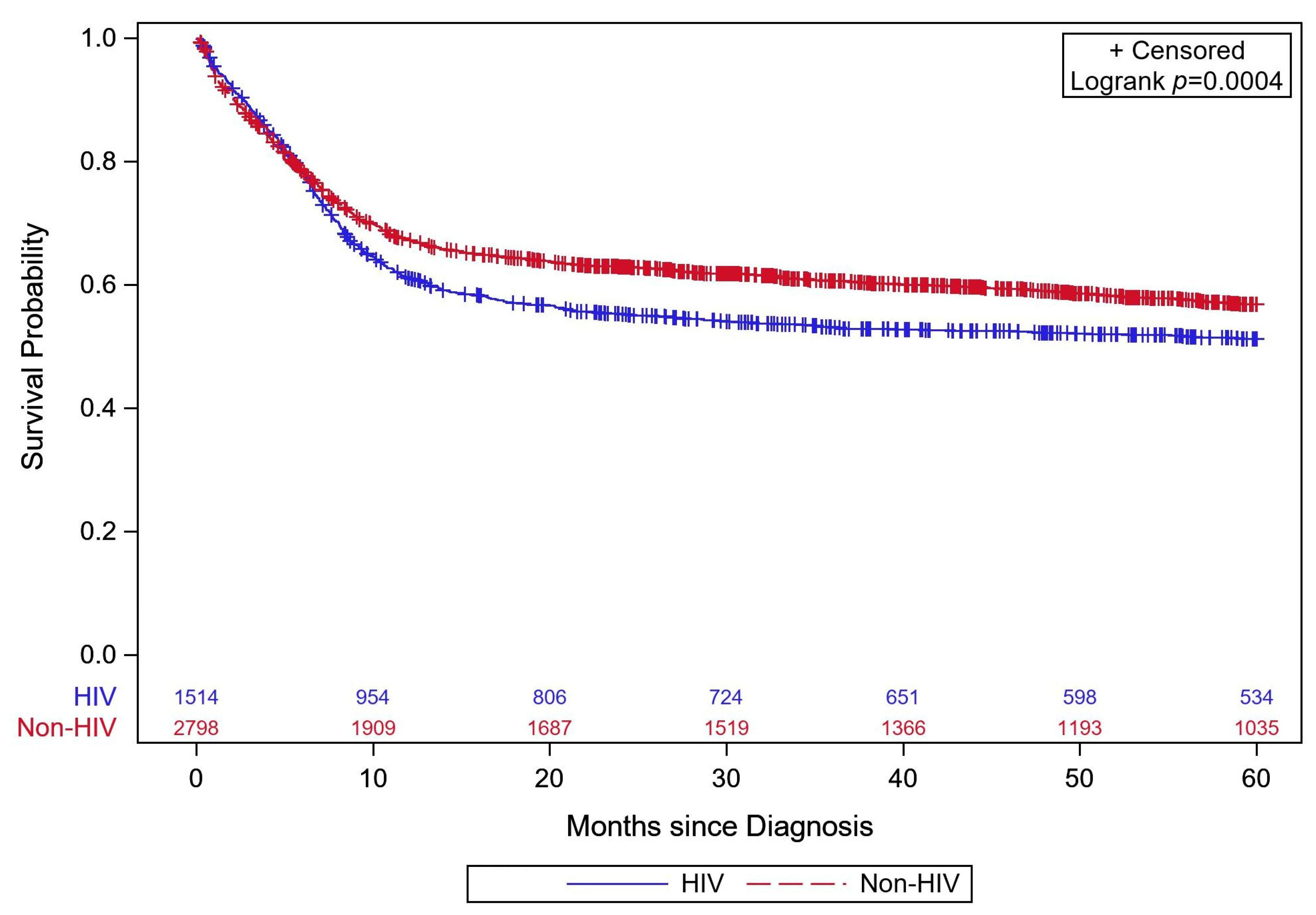
Mortality and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- HIV Data and Statistics—Global HIV Programme. World Health Organization Web Site. Updated 2023. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 27 August 2023).
- HIV Basics—Data and Trends: U.S. Statistics. HIV.Gov Web Site. Updated 2022. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics/ (accessed on 27 August 2023).
- HIV Surveillance Report, 2021. Centers for Disease Control and Prevention. 2023. (Volume 34). Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed on 27 August 2023).
- HIV Treatment and Care. Centers for Disease Control and Prevention. 26 September 2023. Available online: https://www.cdc.gov/hiv/clinicians/treatment-care/index.html#:~:text=Current%20treatment%20guidelines%20recommend%20antiretroviral,importance%20of%20adhering%20to%20ART (accessed on 18 March 2024).
- HIV.Gov. The Global HIV and AIDS Epidemic. HIV.Gov Web Site. Updated 2023. Available online: https://www.hiv.gov/federal-response/pepfar-global-aids/global-hiv-aids-overview/ (accessed on 18 December 2023).
- Dolcetti, R.; Gloghini, A.; Caruso, A.; Carbone, A. A lymphomagenic role for HIV beyond immune suppression? Am. J. Hematol. 2016, 127, 1403–1409. [Google Scholar] [CrossRef]
- Brunnberg, U.; Hentrich, M.; Hoffmann, C.; Wolf, T.; Huebel, K. HIV-associated malignant lymphoma. Oncol. Res. Treat. 2017, 40, 82–87. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Abramson, J.S. HIV-associated Hodgkin’s lymphoma: Prognosis and therapy in the era of cART. Adv. Hematol. 2012, 2012, 507257. [Google Scholar] [CrossRef]
- Riedel, D.; Rositch, A.; Redfield, R.; Blattner, W. HIV-associated lymphoma sub-type distribution, immunophenotypes and survival in an urban clinic population. Leuk. Lymphoma 2016, 57, 306–312. [Google Scholar] [CrossRef]
- Gloghini, A.; Dolcetti, R.; Carbone, A. Lymphomas occurring specifically in HIV-infected patients: From pathogenesis to pathology. Semin. Cancer Biol. 2013, 23, 457–467. [Google Scholar] [CrossRef]
- Frisch, M.; Biggar, R.J.; Engels, E.A.; Goedert, J.J.; the AIDS-Cancer Match Registry Study Group. Association of cancer with AIDS-related immunosuppression in adults. JAMA J. Am. Med. Assoc. 2001, 285, 1736–1745. [Google Scholar] [CrossRef]
- Brauninger, A.; Hansmann, M.; Strickler, J.G.; Dummer, R.; Burg, G.; Rajewsky, K.; Küppers, R. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin’s disease and non-Hodgkin’s lymphoma. N. Engl. J. Med. 1999, 340, 1239–1247. [Google Scholar] [CrossRef]
- Coghill, A.E.; Han, X.; Suneja, G.; Lin, C.C.; Jemal, A.; Shiels, M.S. Advanced stage at diagnosis and elevated mortality among HIV-infected US cancer patients in the national cancer database. Cancer 2019, 125, 2868–2876. [Google Scholar] [CrossRef]
- Berhan, A.; Bayleyegn, B.; Getaneh, Z. HIV/AIDS associated lymphoma: Review. Blood Lymphat. Cancer 2022, 12, 31–45. [Google Scholar] [CrossRef]
- Birlutiu, V.; Birlutiu, R.M.; Zaharie, I.S.; Sandu, M. Burkitt lymphoma associated with human immunodeficiency virus infection and pulmonary tuberculosis: A case report. Medicine 2020, 99, e23853. [Google Scholar] [CrossRef] [PubMed]
- Atallah-Yunes, S.A.; Murphy, D.J.; Noy, A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020, 7, e594–e600. [Google Scholar] [CrossRef]
- Chao, C.; Xu, L.; Abrams, D.; Leyden, W.; Horberg, M.; Towner, W.; Klein, D.; Tang, B.; Silverberg, M. Survival of non-Hodgkin lymphoma patients with and without HIV infection in the era of combined antiretroviral therapy. AIDS 2010, 24, 1765–1770. [Google Scholar] [CrossRef]
- Han, X.; Jemal, A.; Hulland, E.; Simard, E.P.; Nastoupil, L.; Ward, E.; Flowers, C.R. HIV infection and survival of lymphoma patients in the era of highly active antiretroviral therapy. Cancer Epidemiol. Biomark. Prev. 2017, 26, 303–311. [Google Scholar] [CrossRef]
- Howlader, N.; Shiels, M.S.; Mariotto, A.B.; Engels, E.A. Contributions of HIV to non-Hodgkin lymphoma mortality trends in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.C.; Marques, M.d.O.; Pereira, J.; Braga, W.M.T.; Hamerschlak, N.; Tabacof, J.; Ferreira, P.R.A.; Colleoni, G.W.B.; Baiocchi, O.C. Factors associated with survival in patients with lymphoma and HIV. AIDS 2023, 37, 1217–1226. [Google Scholar] [CrossRef]
- Alderuccio, J.P.; Olszewski, A.J.; Evens, A.M.; Collins, G.P.; Danilov, A.V.; Bower, M.; Jagadeesh, D.; Zhu, C.; Sperling, A.; Kim, S.-H.; et al. HIV-associated Burkitt lymphoma: Outcomes from a US-UK collaborative analysis. Blood Adv. 2021, 5, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Qiu, T.Y.; Chiang, J.; Tan, Y.H.; Yang, V.S.; Chang, E.W.Y.; Poon, E.; Somasundaram, N.; Farid, M.; Tao, M.; et al. Burkitt lymphoma—No impact of HIV status on outcomes with rituximab-based chemoimmunotherapy. Leuk. Lymphoma 2023, 64, 586–596. [Google Scholar] [CrossRef]
- Barnes, J.A.; LaCasce, A.S.; Feng, Y.; Toomey, C.E.; Neuberg, D.; Michaelson, J.S.; Hochberg, E.P.; Abramson, J.S. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: A retrospective analysis. Ann. Oncol. 2011, 22, 1859–1864. [Google Scholar] [CrossRef]
- Ribrag, V.; Koscielny, S.; Bosq, J.; Leguay, T.; Casasnovas, O.; Fornecker, L.-M.; Recher, C.; Ghesquieres, H.; Morschhauser, F.; Girault, S.; et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.R.C.; Barta, S.K.; Lee, J.; Rudek, M.A.; Sparano, J.A.; Noy, A. Combination antiretroviral therapy accelerates immune recovery in patients with HIV-related lymphoma treated with EPOCH: A comparison within one prospective trial AMC034. Leuk. Lymphoma 2018, 59, 1851–1860. [Google Scholar] [CrossRef]
- About The National Cancer Database. American College of Surgeons. Available online: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/about/ (accessed on 21 July 2023).
- North American Association of Central Cancer Registries. Site Specific Data Items (SSDI)/Grade—Lymphoma. Available online: https://apps.naaccr.org/ssdi/schema/lymphoma/?breadcrumbs=(~schema_list~),(~view_schema~,~lymphoma~),(~view_naaccr_item~,~3859~) (accessed on 21 July 2023).
- National Cancer Institute—SEER Registrar Staging Assistant. EOD Data—NAACCR Item #3859: HIV Status. National Cancer Institute—SEER Registrar Staging Assistant Web Site. Updated 2023. Available online: https://staging.seer.cancer.gov/naaccr/item/eod_public/3.0/3859/?breadcrumbs=(~view_schema~,~lymphoma_cll_sll~) (accessed on 12 February 2024).
- Kanbar, A.H. Burkitt Lymphoma and Burkitt-Like Lymphoma Treatment & Management. Medscape Web Site. Updated 2022. Available online: https://emedicine.medscape.com/article/1447602-treatment#d9 (accessed on 31 August 2023).
- Wang, Z.; Zhang, R.; Liu, L.; Shen, Y.; Chen, J.; Qi, T.; Song, W.; Tang, Y.; Sun, J.; Lin, Y.; et al. Incidence and spectrum of infections among HIV/AIDS patients with lymphoma during chemotherapy. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2021, 27, 1459–1464. [Google Scholar] [CrossRef]
- Noy, A. HIV Lymphoma and Burkitts Lymphoma. Cancer J. 2020, 26, 260–268. [Google Scholar] [CrossRef]
- Jayakrishnan, T.T.; Bakalov, V.; Samhouri, Y.; Wegner, R.E.; Sadashiv, S. Outcomes of treatment for HIV-Infected Lymphoma patients: A National Cancer Database (NCDB) analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e864–e870. [Google Scholar] [CrossRef]
- Torres, H.A.; Mulanovich, V. Management of HIV infection in patients with cancer receiving chemotherapy. Clin. Infect. Dis. 2014, 59, 106–114. [Google Scholar] [CrossRef]
- Wildes, T.M.; Farrington, L.; Yeung, C.; Harrington, A.M.; Foyil, K.V.; Liu, J.; Kreisel, F.; Bartlett, N.L.; Fenske, T.S. Rituximab is associated with improved survival in Burkitt lymphoma: A retrospective analysis from two US academic medical centers. Ther. Adv. Hematol. 2014, 5, 3–12. [Google Scholar] [CrossRef]
- Oriol, A.; Ribera, J.; Bergua, J.; Mesa, E.G.; Grande, C.; Esteve, J.; Brunet, S.; Moreno, M.; Escoda, L.; Hernandez-Rivas, J.; et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: Comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer 2008, 113, 117–125. [Google Scholar] [CrossRef]
- Thomas, D.A.; Faderl, S.; O’Brien, S.; Bueso-Ramos, C.; Cortes, J.; Garcia-Manero, G.; Giles, F.J.; Verstovsek, S.; Wierda, W.G.; Pierce, S.A.; et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 2006, 106, 1569–1580. [Google Scholar] [CrossRef]
- Dunleavy, K.; Pittaluga, S.; Janik, J.; Grant, N.; Shovlin, M.; Little, R.; Yarchoan, R.; Steinberg, S.; Jaffe, E.S.; Wilson, W.H. Novel treatment of Burkitt lymphoma with dose-adjusted EPOCH-rituximab: Preliminary results showing excellent outcome. Blood 2006, 108, 2736. [Google Scholar] [CrossRef]
- Nie, M.; Wang, Y.; Bi, X.; Xia, Y.; Sun, P.; Liu, P.-P.; Li, Z.-M.; Jiang, W.-Q. Effect of rituximab on adult Burkitt’s lymphoma: A systematic review and meta-analysis. Ann. Hematol. 2016, 95, 19–26. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Song, K.W.; Connors, J.M.; Leitch, H.; Barnett, M.J.; Ramadan, K.; Slack, G.W.; Mourad, Y.A.; Forrest, D.L.; Hogge, D.E.; et al. Excellent real-world outcomes of adults with Burkitt lymphoma treated with CODOX-M/IVAC plus or minus rituximab. Br. J. Haematol. 2018, 181, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Barta, S.K.; Lee, J.Y.; Kaplan, L.D.; Noy, A.; Sparano, J.A. Pooled analysis of AIDS malignancy consortium trials evaluating rituximab plus CHOP or infusional EPOCH chemotherapy in HIV-associated non-Hodgkin lymphoma. Cancer 2012, 118, 3977–3983. [Google Scholar] [CrossRef]
- Kaplan, L.D. Rituximab does not improve clinical outcome in a randomized phase 3 trial of chop with or without rituximab in patients with HIV-associated non-Hodgkin Lymphoma: AIDS-Malignancies Consortium Trial 010. Blood 2005, 106, 1538–1543. [Google Scholar] [CrossRef]
- Pellowski, J.A.; Kalichman, S.C.; Matthews, K.A.; Adler, N. A pandemic of the poor: Social disadvantage and the U.S. HIV epidemic. Am. Psychol. 2013, 68, 197–209. [Google Scholar] [CrossRef]
- Dray-Spira, R.; Gueguen, A.; Lert, F. Disease severity, self-reported experience of workplace discrimination and employment loss during the course of chronic HIV disease: Differences according to gender and education. Occup. Environ. Med. 2008, 65, 112–119. [Google Scholar] [CrossRef]
| N = 4312 | Overall | HIV Negative | HIV Positive | p |
|---|---|---|---|---|
| Age 1 | 48 (36, 60) | 54 (37, 66) | 43 (35, 50) | <0.0001 |
| Female | 997 (23.1%) | 799 (28.6%) | 198 (13.1%) | <0.0001 |
| Race | <0.0001 | |||
| White | 3448 (80.0%) | 2444 (87.4%) | 1004 (66.3%) | |
| Black | 613 (14.2%) | 176 (6.3%) | 437 (28.9%) | |
| Other | 251 (5.8%) | 178 (6.4%) | 73 (4.8%) | |
| Hispanic | 567 (13.2%) | 292 (10.4%) | 275 (18.2%) | <0.001 |
| Primary Payer | <0.001 | |||
| Not Insured | 320 (7.4%) | 151 (5.4%) | 169 (11.2%) | |
| Private | 2221 (51.5%) | 1481 (52.9%) | 740 (48.9%) | |
| Medicaid | 734 (17.0%) | 317 (11.3%) | 417 (27.5%) | |
| Medicare | 902 (20.9%) | 758 (27.1%) | 144 (9.5%) | |
| Other Gov. | 57 (1.3%) | 42 (1.5%) | 15 (1.0%) | |
| Unknown | 78 (1.8%) | 49 (1.8%) | 29 (1.9%) | |
| Stage of Disease | ||||
| 3 | 697 (16.2%) | 457 (16.3) | 240 (15.9) | 0.682 |
| 4 | 3615 (83.8%) | 2341 (83.7) | 1274 (84.2) | |
| Extranodal Disease | 914 (21.2%) | 652 (23.3%) | 262 (17.3%) | <0.001 |
| Treatment | <0.001 | |||
| Chemo Alone | 2848 (66.0%) | 1745 (62.4%) | 1103 (72.9%) | |
| Chemo and Immuno | 1464 (34.0%) | 1053 (37.6%) | 411 (27.2%) | |
| Metastatic Brain Involvement 2 | 32 (3.0%) | 22 (2.9%) | 10 (3.3%) | 0.7011 |
| N = 4312 | Overall | HIV Negative | HIV Positive | p |
|---|---|---|---|---|
| Overall | 54.9 (53.3–56.4) | 56.8 (54.9–58.7) | 51.2 (48.6–53.8) | <0.001 |
| Age | ||||
| <50 | 62.7 (60.6–64.7) | 72.1 (69.4–74.7) | 52.9 (49.9–55.8) | <0.001 |
| 50+ | 45.9 (43.6–48.2) | 45.8 (43.3–48.3) | 46.3 (41.1–51.3) | 0.958 |
| Sex | ||||
| Male | 54.1 (52.3–55.8) | 55.6 (53.3–57.9) | 51.6 (48.8–54.4) | 0.019 |
| Female | 57.6 (54.3–60.7) | 59.8 (56.2–63.2) | 48.5 (41.0–55.5) | 0.007 |
| Race | ||||
| White | 55.1 (53.4–56.8) | 56.3 (54.3–58.3) | 52.1 (48.9–55.2) | 0.027 |
| Black | 51.5 (47.3–55.5) | 58.6 (50.8–65.7) | 48.5 (43.6–53.3) | 0.025 |
| Other | 60.0 (53.2–66.2) | 62.2 (54.1–69.4) | 54.7 (41.9–65.8) | 0.174 |
| Ethnicity | ||||
| Not Hispanic | 54.0 (52.3–55.7) | 55.9 (53.9–57.9) | 50.1 (47.3–53.0) | 0.001 |
| Hispanic | 60.7 (56.4–64.8) | 64.9 (58.8–70.3) | 56.3 (50.1–62.1) | 0.031 |
| Primary Payer | ||||
| Not Insured | 51.8 (46.0–57.3) | 53.9 (45.3–61.6) | 50.2 (42.2–57.6) | 0.370 |
| Private | 62.0 (59.8–64.0) | 65.0 (62.4–67.4) | 55.9 (52.1–59.4) | <0.001 |
| Medicaid | 54.2 (50.4–57.9) | 62.5 (56.6–67.8) | 48.1 (43.1–52.9) | <0.001 |
| Medicare | 40.1 (36.7–43.4) | 40.0 (36.4–43.6) | 40.3 (32.0–48.3) | 0.697 |
| Other Gov. | 53.6 (38.6–66.4) | 57.1 (39.0–71.7) | 44.4 (18.5–67.7) | 0.342 |
| Unknown | 45.4 (33.6–56.7) | 46.8 (31.8–60.4) | 42.8 (23.0–61.2) | 0.833 |
| Stage of Disease | ||||
| 3 | 67.8 (64.1–71.2) | 69.4 (64.8–73.6) | 64.6 (58.0–70.5) | 0.245 |
| 4 | 52.4 (50.7–54.1) | 54.4 (52.2–56.4) | 48.7 (45.9–51.5) | 0.001 |
| Involvement | ||||
| Extranodal | 54.6 (52.8–56.3) | 56.0 (53.8–58.2) | 52.0 (49.1–54.8) | 0.025 |
| Nodal | 56.2 (52.8–59.4) | 59.6 (55.6–63.4) | 47.5 (41.2–53.5) | <0.001 |
| Treatment | ||||
| Chemo Alone | 49.9 (48.0–51.7) | 51.8 (49.4–54.2) | 46.9 (43.8–49.8) | 0.020 |
| Chemo and Immuno | 65.1 (62.4–67.6) | 65.8 (62.6–68.8) | 63.3 (58.1–68.0) | 0.379 |
| Metastatic Brain Involvement | ||||
| No | 54.9 (53.3–56.4) | 56.9 (54.9–58.8) | 51.1 (48.5–53.8) | <0.001 |
| Yes | 58.2 (38.8–73.3) | 52.9 (29.8–71.5) | 70.0 (32.9–89.2) | 0.395 |
| Parameter | Hazard Ratio | 95% CI | p | |
|---|---|---|---|---|
| HIV vs. NO HIV 0–3 Months | 1.04 | 0.86 | 1.26 | 0.6668 |
| HIV vs. NO HIV 3–60 Months | 1.55 | 1.38 | 1.75 | <0.0001 |
| Chemo and Immuno vs. Chemo Only | 0.59 | 0.53 | 0.65 | <0.0001 |
| Extranodal vs. Nodal | 0.93 | 0.83 | 1.04 | 0.2083 |
| Age (1 year change) | 1.03 | 1.02 | 1.03 | <0.0001 |
| Female vs. Male | 0.86 | 0.77 | 0.96 | 0.0090 |
| Black vs. White | 1.11 | 0.97 | 1.27 | 0.1300 |
| Other vs. White | 1.00 | 0.81 | 1.23 | 0.9922 |
| Hispanic vs. Not Hispanic | 0.84 | 0.73 | 0.98 | 0.0231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieland, C.M.; Tuin, A.M.; Dort, E.J.; Hall, A.G.; Krishnan, M.; Velagapudi, M. Long-Term Survival Rates and Treatment Trends of Burkitt Lymphoma in Patients with HIV—A National Cancer Database (NCDB) Study. Cancers 2024, 16, 1397. https://doi.org/10.3390/cancers16071397
Wieland CM, Tuin AM, Dort EJ, Hall AG, Krishnan M, Velagapudi M. Long-Term Survival Rates and Treatment Trends of Burkitt Lymphoma in Patients with HIV—A National Cancer Database (NCDB) Study. Cancers. 2024; 16(7):1397. https://doi.org/10.3390/cancers16071397
Chicago/Turabian StyleWieland, Clare M., Ashley M. Tuin, Elizabeth J. Dort, Alexander G. Hall, Mridula Krishnan, and Manasa Velagapudi. 2024. "Long-Term Survival Rates and Treatment Trends of Burkitt Lymphoma in Patients with HIV—A National Cancer Database (NCDB) Study" Cancers 16, no. 7: 1397. https://doi.org/10.3390/cancers16071397
APA StyleWieland, C. M., Tuin, A. M., Dort, E. J., Hall, A. G., Krishnan, M., & Velagapudi, M. (2024). Long-Term Survival Rates and Treatment Trends of Burkitt Lymphoma in Patients with HIV—A National Cancer Database (NCDB) Study. Cancers, 16(7), 1397. https://doi.org/10.3390/cancers16071397






