Evaluating Leukocyte Telomere Length and Myeloid-Derived Suppressor Cells as Biomarkers for Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample and Data Collection
2.2. LTL Measurement
2.3. MDSC Measurement
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Patients’ Characteristics
3.2. Prostate Cancer Patients Have Shorter Leukocyte Telomere Lengths
3.3. Prostate Cancer Patients Have a Higher Percentage of M-MDSC
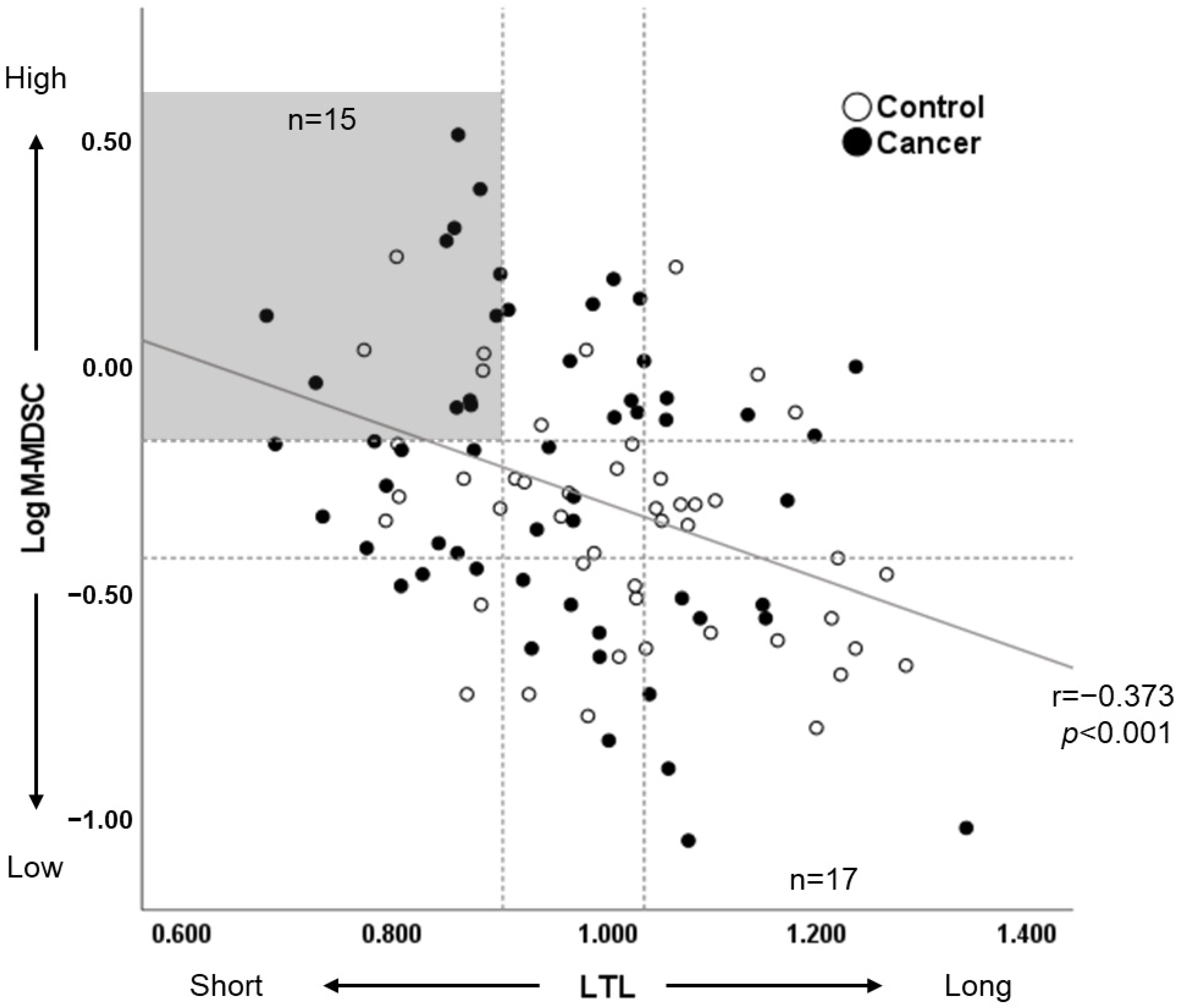
3.4. Leukocyte Telomere Length Correlates with the Percentage of M-MDSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Verdun, R.E.; Karlseder, J. Replication and protection of telomeres. Nature 2007, 447, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.Z.; Allsopp, R.C.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere end-replication problem and cell aging. J. Mol. Biol. 1992, 225, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. eBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; De Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sonugür, F.G.; Akbulut, H. The Role of Tumor Microenvironment in Genomic Instability of Malignant Tumors. Front. Genet. 2019, 10, 1063. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Myeloid derived-suppressor cells: Their role in cancer and obesity. Curr. Opin. Immunol. 2018, 51, 68–75. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Johnstone, J.; Millar, J.; Dorrington, M.G.; Habibagahi, M.; Lelic, A.; Loeb, M.; Bramson, J.L.; Bowdish, D.M.E. Blood CD33(+)HLA-DR(−) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 2013, 93, 633–637. [Google Scholar] [CrossRef]
- Salminen, A. Activation of immunosuppressive network in the aging process. Ageing Res. Rev. 2020, 57, 100998. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Immunosenescence: The potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell. Mol. Life Sci. 2019, 76, 1901–1918. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten public health impacts of cancer—An overview. Arch. Ind. Hyg. Toxicol. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hu, R.; Hua, X.G.; Jiang, Q.C. Associations of telomere length in risk and recurrence of prostate cancer: A meta-analysis. Andrologia 2019, 51, e13304. [Google Scholar] [CrossRef]
- Xu, J.; Chang, W.-S.; Tsai, C.-W.; Bau, D.-T.; Xu, Y.; Davis, J.W.; Thompson, T.C.; Logothetis, C.J.; Gu, J. Leukocyte telomere length is associated with aggressive prostate cancer in localized prostate cancer patients. eBioMedicine 2020, 52, 102616. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-W.; Chang, W.-S.; Xu, J.; Xu, Y.; Huang, M.; Pettaway, C.; Bau, D.-T.; Gu, J. Leukocyte telomere length is associated with aggressive prostate cancer in localized African American prostate cancer patients. Carcinogenesis 2020, 41, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Ercolano, G.; Ianaro, A. The New Era of Cancer Immunotherapy: Targeting Myeloid-Derived Suppressor Cells to Overcome Immune Evasion. Front. Immunol. 2020, 11, 1680. [Google Scholar] [CrossRef]
- Idorn, M.; Køllgaard, T.; Kongsted, P.; Sengeløv, L.; Straten, P.T. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol. Immunother. 2014, 63, 1177–1187. [Google Scholar] [CrossRef]
- Chi, N.; Tan, Z.; Ma, K.; Bao, L.; Yun, Z. Increased circulating myeloid-derived suppressor cells correlate with cancer stages, interleukin-8 and -6 in prostate cancer. Int. J. Clin. Exp. Med. 2014, 7, 3181–3192. [Google Scholar]
- Lopez-Bujanda, Z.; Drake, C.G. Myeloid-derived cells in prostate cancer progression: Phenotype and prospective therapies. J. Leukoc. Biol. 2017, 102, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Bruger, A.M.; Dorhoi, A.; Esendagli, G.; Barczyk-Kahlert, K.; van der Bruggen, P.; Lipoldova, M.; Perecko, T.; Santibanez, J.; Saraiva, M.; Van Ginderachter, J.A.; et al. How to measure the immunosuppressive activity of MDSC: Assays, problems and potential solutions. Cancer Immunol. Immunother. 2019, 68, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Apodaca, M.C.; Wright, A.E.; Riggins, A.M.; Harris, W.P.; Yeung, R.S.; Yu, L.; Morishima, C. Characterization of a whole blood assay for quantifying myeloid-derived suppressor cells. J. Immunother. Cancer 2019, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. A systematic review of leukocyte telomere length and age in adults. Ageing Res. Rev. 2013, 12, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Müezzinler, A.; Zaineddin, A.K.; Brenner, H. Body mass index and leukocyte telomere length in adults: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Müezzinler, A.; Mons, U.; Dieffenbach, A.K.; Butterbach, K.; Saum, K.-U.; Schick, M.; Stammer, H.; Boukamp, P.; Holleczek, B.; Stegmaier, C.; et al. Smoking habits and leukocyte telomere length dynamics among older adults: Results from the ESTHER cohort. Exp. Gerontol. 2015, 70, 18–25. [Google Scholar] [CrossRef]
- Vuk-Pavlović, S.; Bulur, P.A.; Lin, Y.I.; Qin, R.; Szumlanski, C.L.; Zhao, X.; Dietz, A.B. Immunosuppressive CD14+ HLA-DRlow/− monocytes in prostate cancer. Prostate 2010, 70, 443–455. [Google Scholar] [CrossRef]
- Brusa, D.; Simone, M.; Gontero, P.; Spadi, R.; Racca, P.; Micari, J.; Degiuli, M.; Carletto, S.; Tizzani, A.; Matera, L. Circulating immunosuppressive cells of prostate cancer patients before and after radical prostatectomy: Profile comparison. Int. J. Urol. 2013, 20, 971–978. [Google Scholar] [CrossRef]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Okuma, A.; Hanyu, A.; Watanabe, S.; Hara, E. p16Ink4a and p21Cip1/Waf1 promote tumour growth by enhancing myeloid-derived suppressor cells chemotaxis. Nat. Commun. 2017, 8, 2050. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Myeloid-derived suppressor cells (MDSC): An important partner in cellular/tissue senescence. Biogerontology 2018, 19, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.; Sant’Anna, O.A.; Lord, J.M. Ageing and myeloid-derived suppressor cells: Possible involvement in immunosenescence and age-related disease. Age 2014, 36, 9729. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Cesare, A.J.; Hayashi, M.T.; Crabbe, L.; Karlseder, J. The Telomere Deprotection Response Is Functionally Distinct from the Genomic DNA Damage Response. Mol. Cell 2013, 51, 141–155. [Google Scholar] [CrossRef]
- Blanco, R.; Munoz, P.; Flores, J.M.; Klatt, P.; Blasco, M.A. Telomerase abroates TRF2-induced epithelial carcinogenesisgation dramatically acceler. Genes Dev. 2007, 21, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Cherfils-Vicini, J.; Iltis, C.; Cervera, L.; Pisano, S.; Croce, O.; Sadouni, N.; Győrffy, B.; Collet, R.; Renault, V.M.; Rey-Millet, M.; et al. Cancer cells induce immune escape via glycocalyx changes controlled by the telomeric protein TRF 2. EMBO J. 2019, 38, e100012. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Castillo, G.; González, J.; Valls, N.; Rodrigo, R. Oxidative Stress in Hypertension: Mechanisms and Therapeutic Opportunities. Exp. Clin. Endocrinol. Diabetes 2015, 123, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free. Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Babcock, M.C.; DuBose, L.E.; Witten, T.L.; Stauffer, B.L.; Hildreth, K.L.; Schwartz, R.S.; Kohrt, W.M.; Moreau, K.L. Oxidative Stress and Inflammation Are Associated with Age-Related Endothelial Dysfunction in Men With Low Testosterone. J. Clin. Endocrinol. Metab. 2022, 107, e500–e514. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.R.; Clauson, C.L.; Cho, J.; Lee, B.C.; McGowan, S.J.; Baker, D.J.; Niedernhofer, L.J.; Robbins, P.D. Expansion of myeloid-derived suppressor cells with aging in the bone marrow of mice through a NF-κB-dependent mechanism. Aging Cell 2017, 16, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Godtman, R.A.; Kollberg, K.S.; Pihl, C.G.; Månsson, M.; Hugosson, J. The Association Between Age, Prostate Cancer Risk, and Higher Gleason Score in a Long-term Screening Program: Results from the Göteborg-1 Prostate Cancer Screening Trial. Eur. Urol. 2022, 82, 311–317. [Google Scholar] [CrossRef]
- Schröder, F.H.; Hugosson, J.; Roobol-Bouts, M.J.; Tammela, T.L.J.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and Prostate-Cancer Mortality in a Randomized European Study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef]
- Julin, B.; Shui, I.; Heaphy, C.M.; Joshu, C.E.; Meeker, A.K.; Giovannucci, E.; De Vivo, I.; Platz, E.A. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br. J. Cancer 2015, 112, 769–776. [Google Scholar] [CrossRef]
- Svenson, U.; Roos, G.; Wikstrom, P. Long leukocyte telomere length in prostate cancer patients at diagnosis is associated with poor metastasis-free and cancer-specific survival. Tumour. Biol. 2017, 39, 1010428317692236. [Google Scholar] [CrossRef] [PubMed]
- Renner, W.; Krenn-Pilko, S.; Gruber, H.-J.; Herrmann, M.; Langsenlehner, T. Relative telomere length and prostate cancer mortality. Prostate Cancer Prostatic Dis. 2018, 21, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Lindrose, A.R.; McLester-Davis, L.W.Y.; Tristano, R.I.; Kataria, L.; Gadalla, S.M.; Eisenberg, D.T.A.; Verhulst, S.; Drury, S. Method comparison studies of telomere length measurement using qPCR approaches: A critical appraisal of the literature. PLoS ONE 2021, 16, e0245582. [Google Scholar] [CrossRef]
- Roux, A.V.D.; Ranjit, N.; Jenny, N.S.; Shea, S.; Cushman, M.; Fitzpatrick, A.; Seeman, T. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell 2009, 8, 251–257. [Google Scholar] [CrossRef]
| Characteristics | Total (N = 102) | Cancer (N = 56) | Control (N = 46) | Mean Difference (t-Test/M-W) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean/n (%) | SD | Mean/n (%) | Min | Max | SD | Mean/n (%) | Min | Max | SD | t/Z | df | p-Value | |
| Age (years) | 67.9 | 8.4 | 70.0 | 56.0 | 87.0 | 7.9 | 65.3 | 47.0 | 81.0 | 8.4 | −2.869 | 100 | 0.005 |
| BMI (kg/m2) | 24.2 | 2.8 | 24.1 | 18.5 | 31.7 | 3.0 | 24.3 | 18.7 | 30.0 | 2.5 | 0.346 | 100 | 0.730 |
| Smoking status | |||||||||||||
| Smoking, n (%) | 66 (64.7) | - | 40 (71.4) | - | - | - | 26 (56.5) | - | - | - | - | - | - |
| Non-smoking, n (%) | 36 (35.3) | - | 16 (28.6) | - | - | - | 20 (43.5) | - | - | - | - | - | - |
| PSA (ng/mL) | 10.6 | 13.3 | 13.1 | 3.4 | 116.4 | 17.3 | 7.5 | 2.3 | 22.2 | 3.7 | 2.206 | - | 0.027 * |
| Testosterone (ng/mL) | 4.90 | 1.66 | 4.96 | 1.24 | 9.58 | 1.78 | 4.83 | 2.54 | 7.93 | 1.51 | −0.398 | 100 | 0.692 |
| CRP (mg/dL) | 0.13 | 0.21 | 0.18 | 0.01 | 1.29 | 0.26 | 0.08 | 0.01 | 0.33 | 0.73 | 1.959 | - | 0.050 * |
| HbA1c (%) | 5.9 | 0.6 | 5.9 | 5.2 | 8.2 | 0.6 | 5.8 | 5.3 | 7.5 | 0.5 | 1.342 | - | 0.180 * |
| LTL | 0.980 | 0.142 | 0.951 | 0.675 | 1.342 | 0.141 | 1.015 | 0.768 | 1.265 | 0.138 | 2.290 | 100 | 0.024 |
| Total MDSC (%/PBMC) | 7.59 | 3.55 | 7.29 | 2.69 | 18.40 | 3.57 | 7.95 | 1.83 | 15.60 | 3.53 | −1.288 | - | 0.198 * |
| PMN-MDSC (%/PBMC) | 0.89 | 0.86 | 0.80 | 0.10 | 4.73 | 0.89 | 1.01 | 0.04 | 3.23 | 0.83 | −1.641 | - | 0.101 * |
| M-MDSC (%/PBMC) | 0.67 | 0.53 | 0.78 | 0.09 | 3.30 | 0.62 | 0.54 | 0.16 | 1.77 | 0.36 | 1.910 | - | 0.056 * |
| LTL * | β | Adjusted OR ** | 95% CI | p-Value |
|---|---|---|---|---|
| Short | 1.092 | 2.979 | 1.001, 8.869 | 0.050 |
| Middle | 0.660 | 1.934 | 0.661, 5.658 | 0.228 |
| Long | Reference |
| M-MDSC * | β | OR ** | 95% CI | p-Value |
|---|---|---|---|---|
| High | 1.109 | 3.031 | 1.152, 7.977 | 0.025 |
| Low | Reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakita, H.; Lu, Y.; Li, X.; Kobayashi, T.; Hachiya, T.; Ide, H.; Horie, S. Evaluating Leukocyte Telomere Length and Myeloid-Derived Suppressor Cells as Biomarkers for Prostate Cancer. Cancers 2024, 16, 1386. https://doi.org/10.3390/cancers16071386
Wakita H, Lu Y, Li X, Kobayashi T, Hachiya T, Ide H, Horie S. Evaluating Leukocyte Telomere Length and Myeloid-Derived Suppressor Cells as Biomarkers for Prostate Cancer. Cancers. 2024; 16(7):1386. https://doi.org/10.3390/cancers16071386
Chicago/Turabian StyleWakita, Haruhiko, Yan Lu, Xiaoxu Li, Takuro Kobayashi, Tsuyoshi Hachiya, Hisamitsu Ide, and Shigeo Horie. 2024. "Evaluating Leukocyte Telomere Length and Myeloid-Derived Suppressor Cells as Biomarkers for Prostate Cancer" Cancers 16, no. 7: 1386. https://doi.org/10.3390/cancers16071386
APA StyleWakita, H., Lu, Y., Li, X., Kobayashi, T., Hachiya, T., Ide, H., & Horie, S. (2024). Evaluating Leukocyte Telomere Length and Myeloid-Derived Suppressor Cells as Biomarkers for Prostate Cancer. Cancers, 16(7), 1386. https://doi.org/10.3390/cancers16071386





