Modulation of sHLA-G, PD-1, and PD-L1 Expression in Cervical Lesions Following Imiquimod Treatment and Its Association with Treatment Success
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
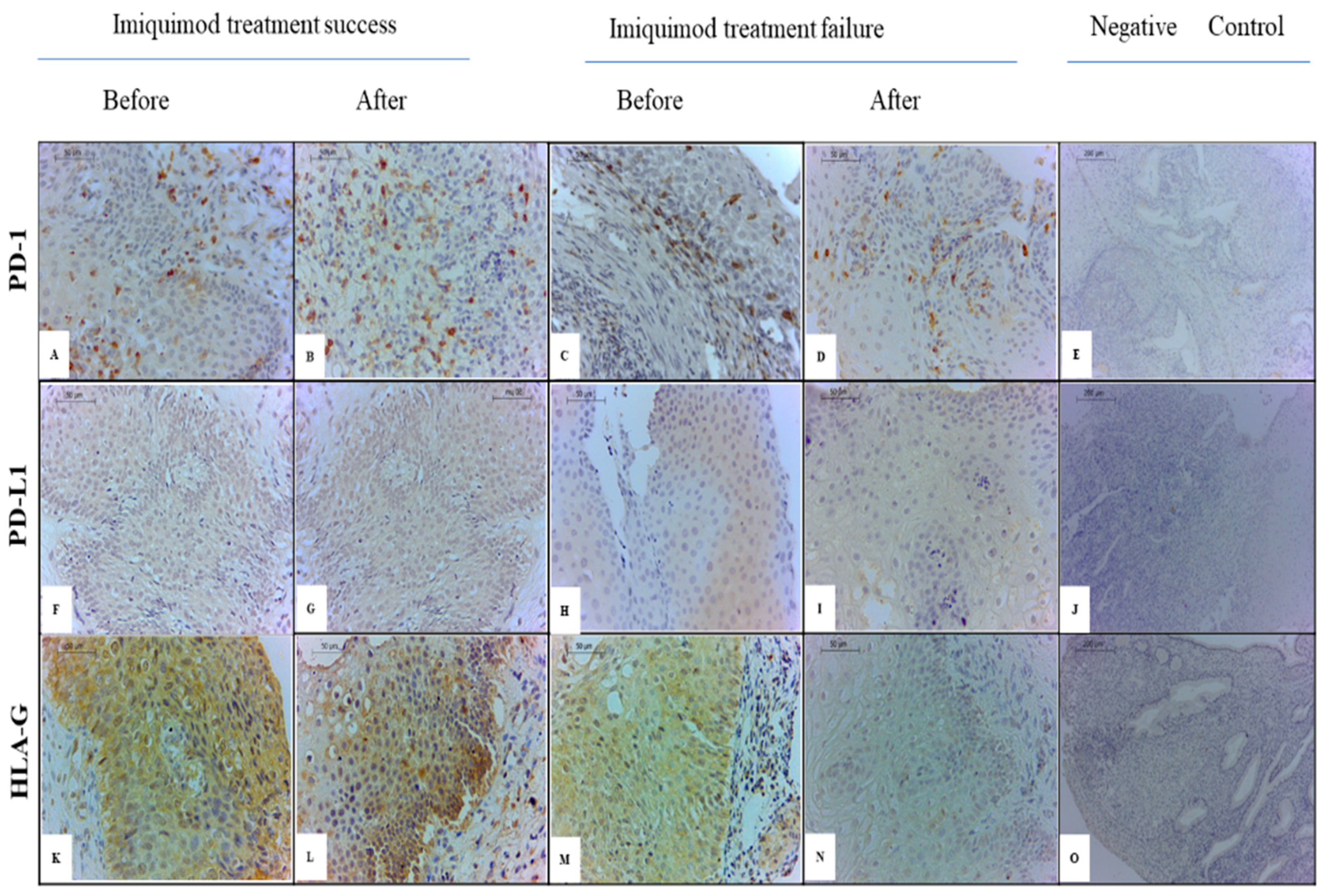
2.2. IHC Evaluation
2.3. Immunofluorescence
2.4. Statistical Analysis
3. Results
3.1. Characterisation of the Cervical Lesion Regression and Vaginal Inflammation Reaction after IMQ Therapy
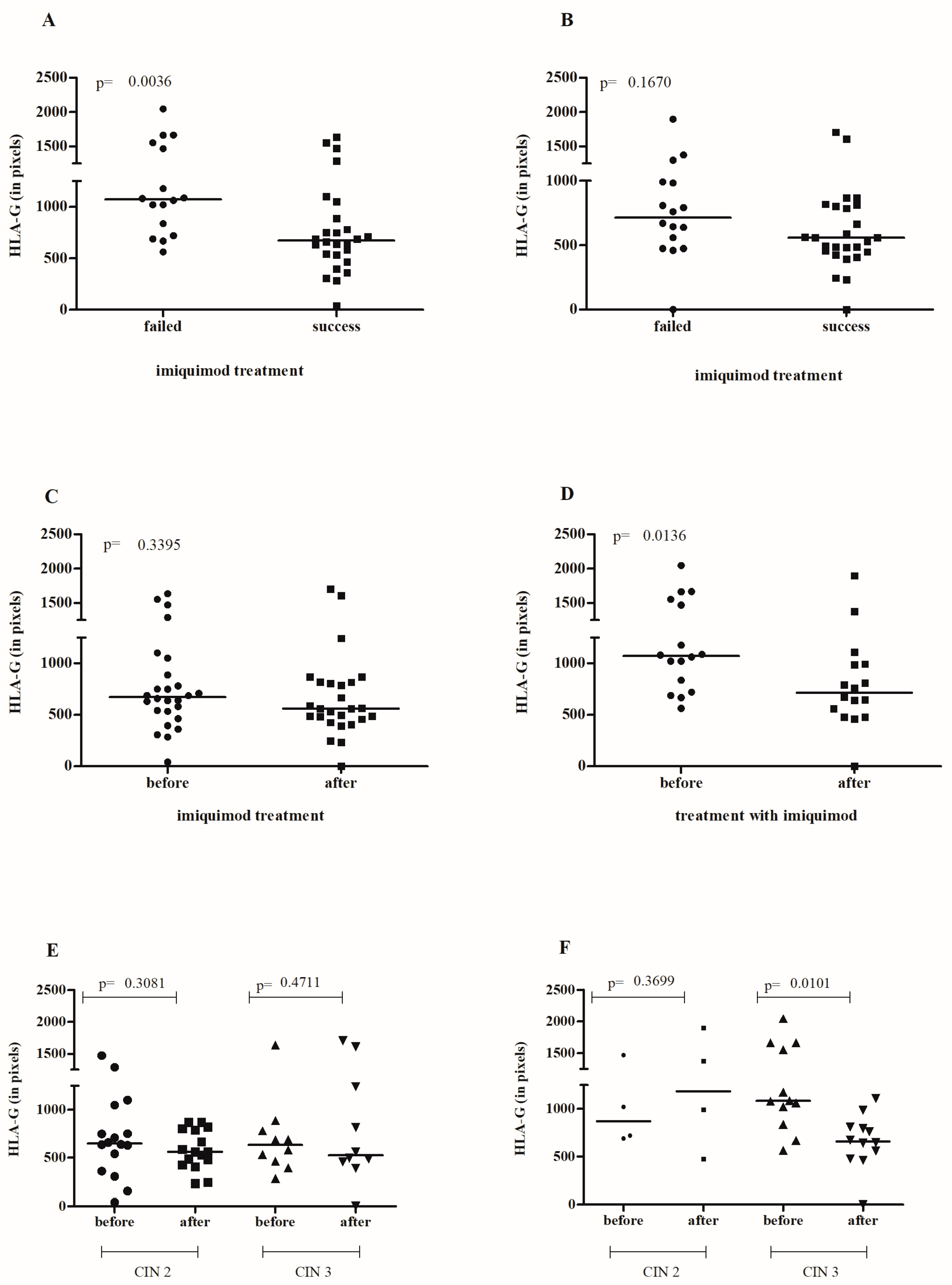
3.2. Effect of IMQ Treatment on Cervical sHLA-G Levels
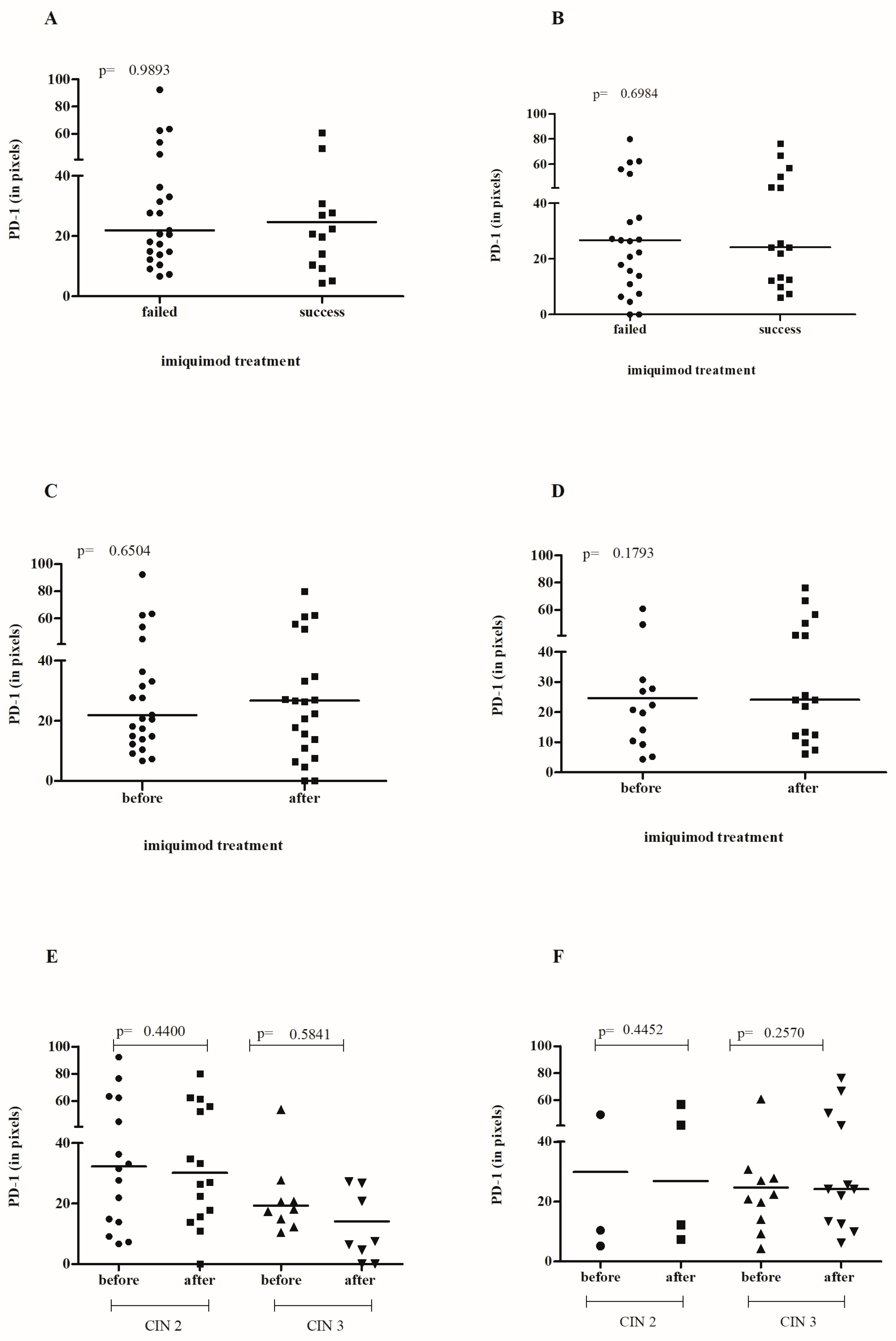
3.3. Effect of IMQ Treatment on Cervical PD-1/PD-L1 Levels
3.4. The Model of Immune Modulation in Cervical Lesions Associated with IMQ Treatment Outcome
3.5. The Effect of IMQ Treatment on HPV E7 Oncoprotein Expression in Cervical Lesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castle, P.E.; Murokora, D.; Perez, C.; Alvarez, M.; Quek, S.C.; Campbell, C. Treatment of cervical intraepithelial lesions. Int. J. Gynaecol. Obstet. 2017, 138 (Suppl. S1), 20–25. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Athanasiou, A.; Kalliala, I.E.J.; Paraskevaidi, M.; Mitra, A.; Martin-Hirsch, P.P.L.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst. Rev. 2017, 11, CD012847. [Google Scholar] [CrossRef]
- Trutnovsky, G.; Reich, O.; Joura, E.A.; Holter, M.; Ciresa-König, A.; Widschwendter, A.; Schauer, C.; Bogner, G.; Jan, Z.; Boandl, A.; et al. Topical imiquimod versus surgery for vulvar intraepithelial neoplasia: A multicentre, randomised, phase 3, non-inferiority trial. Lancet 2022, 399, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, M.; van Hees, C.L.; van Beurden, M.; Bulten, J.; van Dorst, E.B.; Esajas, M.D.; Meeuwis, K.A.; Boll, D.; van Poelgeest, M.I.; de Hullu, J.A. The Paget Trial: Topical 5% imiquimod cream for noninvasive vulvar Paget disease. Am. J. Obstet. Gynecol. 2022, 227, 250.e1–250.e8. [Google Scholar] [CrossRef] [PubMed]
- Inayama, Y.; Takamatsu, S.; Hamanishi, J.; Mizuno, K.; Horinouchi, N.; Yamanoi, K.; Taki, M.; Murakami, R.; Yamaguchi, K.; Kosaka, K.; et al. Imiquimod for Cervical and Vaginal Intraepithelial Neoplasia: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2023, 142, 307–318. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, A.J.M.; Kengsakul, M.; Koeneman, M.M.; Jozwiak, M.; Gerestein, C.G.; Kruse, A.J.; van Esch, E.M.G.; de Vos van Steenwijk, P.J.; Muntinga, C.L.P.; Bramer, W.M.; et al. The efficacy of topical imiquimod in high-grade cervical intraepithelial neoplasia: A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2023, 164, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in dermatology: An overview. Int. J. Dermatol. 2016, 55, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, M.; Valsecchi, A.A.; Tuninetti, V.; Scotto, G.; Borella, F.; Valabrega, G. Immunotherapy for Cervical Cancer: Are We Ready for Prime Time? Int. J. Mol. Sci. 2022, 23, 3559. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Shang, J.; Cheng, Y.; Tian, S.; Yao, Y. Human leukocyte antigen-G in gynaecological tumours. Int. J. Immunogenet. 2023, 50, 163–176. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, X.; Lin, A.; Ruan, Y.Y.; Yan, W.H. Human leukocyte antigen-G (HLA-G) expression in cervical cancer lesions is associated with disease progression. Hum. Immunol. 2012, 73, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Cokan, A.; Pakiž, M.; Serdinšek, T.; Dovnik, A.; Kodrič, T.; Repše Fokter, A.; Kavalar, R.; But, I. Comparison of Conservative Treatment of Cervical Intraepithelial Lesions with Imiquimod with Standard Excisional Technique Using LLETZ: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 5777. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Abdulrahman, Z.; Hendriks, N.; Kruse, A.J.; Somarakis, A.; van de Sande, A.J.M.; van Beekhuizen, H.J.; Piek, J.M.J.; de Miranda, N.F.C.C.; Kooreman, L.F.S.; Slangen, F.M.B.; et al. Immune-based biomarker accurately predicts response to imiquimod immunotherapy in cervical high-grade squamous intraepithelial lesions. J. Immunother. Cancer 2022, 10, e005288. [Google Scholar] [CrossRef]
- Castelli, E.C.; de Almeida, B.S.; Muniz, Y.C.; Silva, N.S.; Passos, M.R.; Souza, A.S.; Page, A.E.; Dyble, M.; Smith, D.; Aguileta, G.; et al. HLA-G genetic diversity and evolutive aspects in worldwide populations. Sci. Rep. 2021, 11, 23070. [Google Scholar] [CrossRef]
- Dong, D.D.; Yang, H.; Li, K.; Xu, G.; Song, L.H.; Fan, X.L.; Jiang, X.-L.; Yie, S.-M. Human leukocyte antigen-G (HLA-G) expression in cervical lesions: Association with cancer progression, HPV 16/18 infection, and host immune response. Reproductive 2010, 17, 718–723. [Google Scholar] [CrossRef]
- da Silva, N.C.H.; Sonon, P.; Medeiros, F.S.; da Silva, M.C.; Dos Santos Gomes, F.O.; Peixoto, C.A.; Crispim, J.C.; Paiva, L.A.; Rygaard, M.C.V.; Menezes, M.L.B.; et al. Contribution of HLA-G and FOXP3 genes and proteins in the severity of cervical intraepithelial neoplasia during HPV infection. Hum. Immunol. 2023, 84, 408–417. [Google Scholar] [CrossRef]
- Crispim-Freitas, J.C.O.; Morais, R.S.P.; Oliveira, V.S.; Sadissou, I.; Palomino, G.M.; Cobucci, R.N.O.; Lira, G.A.; Carvalho, K.T.C.; Silva, E.B.O.; Silva, N.L.; et al. Influence of immune-checkpoint inhibitor and HLA-G in patients with Cervical Cancer. J. Immunol. 2019, 202 (Suppl. S1), 136.10. [Google Scholar] [CrossRef]
- Xu, H.H.; Yan, W.H.; Lin, A. The Role of HLA-G in Human Papillomavirus Infections and Cervical Carcinogenesis. Front. Immunol. 2020, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, K.; Patta, J.; Pergialiotis, V.; Stefanidi, D.; Loutradis, D. Imiquimod treatment effectively reduces the percentage of viable cells in a cervical carcinoma cell line but does not affect the expression of HLA-G or OCT-4. J. Stem Cells 2015, 10, 217–223. [Google Scholar] [PubMed]
- Hemmat, N.; Bannazadeh Baghi, H. Association of human papillomavirus infection and inflammation in cervical cancer. Pathog. Dis. 2019, 77, ftz048. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.C.; Medeiros, F.S.; da Silva, N.C.H.; Paiva, L.A.; Gomes, F.; Costa, E.S.M.; Gomes, T.T.; Peixoto, C.A.; Rygaard, M.C.V.; Menezes, M.L.B.; et al. Increased PD-1 Level in Severe Cervical Injury Is Associated with the Rare Programmed Cell Death 1 (PDCD1) rs36084323 A Allele in a Dominant Model. Front. Cell. Infect. Microbiol. 2021, 11, 587932. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Ayithan, N.; Wu, X.; Yuan, Y.; Wang, L.; Hwang, S.T. Cutting Edge: PD-1 Regulates Imiquimod-Induced Psoriasiform Dermatitis through Inhibition of IL-17A Expression by Innate γδ-Low T Cells. J. Immunol. 2015, 195, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Oya, K.; Nakamura, Y.; Zhenjie, Z.; Tanaka, R.; Okiyama, N.; Ichimura, Y.; Ishitsuka, Y.; Saito, A.; Kubota, N.; Watanabe, R.; et al. Combination Treatment of Topical Imiquimod Plus Anti-PD-1 Antibody Exerts Significantly Potent Antitumor Effect. Cancers 2021, 13, 3948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G. PD-1/PD-L1 blockade in cervical cancer: Current studies and perspectives. Front. Med. 2019, 13, 438–450. [Google Scholar] [CrossRef]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Konishi, R.; Nakamura, Y.; Mizuno, S.; Takahashi, S.; Fujimoto, M.; Nomura, T.; Okiyama, N. The Role of PD-L1 on Langerhans Cells in the Regulation of Psoriasis. J. Investig. Dermatol. 2022, 142, 3167–3174.e9. [Google Scholar] [CrossRef]
- Mezache, L.; Paniccia, B.; Nyinawabera, A.; Nuovo, G.J. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod. Pathol. 2015, 28, 1594–1602. [Google Scholar] [CrossRef]



| Univariate Analysis | Success of Treatment | OR | CI (95%) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| N | % | N | % | Low | Up | |||
| HLAG | ||||||||
| <898,216 | 5 | 31.25 | 20 | 76.92 | 1.00 | |||
| ≥898,216 | 11 | 68.75 | 6 | 23.08 | 0.14 | 0.03 | 0.52 | 0.0052 |
| PDL1 | ||||||||
| <189,626 | 8 | 50.00 | 3 | 11.54 | 1.00 | |||
| ≥189,626 | 8 | 50.00 | 23 | 88.46 | 7.67 | 1.76 | 42.23 | 0.0101 |
| Age | ||||||||
| <28.49 | 11 | 68.75 | 13 | 48.15 | 1.00 | |||
| ≥28.49 | 5 | 31.25 | 14 | 51.85 | 2.37 | 0.67 | 9.29 | 0.1931 |
| Multivariate Model | ||||||||
| HLAG | ||||||||
| ≥898,216 | 0.10 | 0.01 | 0.48 | 0.0079 | ||||
| PDL1 | ||||||||
| ≥189,626 | 10.11 | 1.76 | 84.04 | 0.0160 | ||||
| Age | ||||||||
| ≥28.49 | 4.90 | 0.95 | 35.03 | 0.0770 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cokan, A.; da Silva, N.C.H.; Kavalar, R.; But, I.; Pakiž, M.; Andrade de Oliveira, S.; dos Santos Gomes, F.O.; da Silva, R.S.; Peixoto, C.A.; Lucena-Silva, N. Modulation of sHLA-G, PD-1, and PD-L1 Expression in Cervical Lesions Following Imiquimod Treatment and Its Association with Treatment Success. Cancers 2024, 16, 1272. https://doi.org/10.3390/cancers16071272
Cokan A, da Silva NCH, Kavalar R, But I, Pakiž M, Andrade de Oliveira S, dos Santos Gomes FO, da Silva RS, Peixoto CA, Lucena-Silva N. Modulation of sHLA-G, PD-1, and PD-L1 Expression in Cervical Lesions Following Imiquimod Treatment and Its Association with Treatment Success. Cancers. 2024; 16(7):1272. https://doi.org/10.3390/cancers16071272
Chicago/Turabian StyleCokan, Andrej, Neila Caroline Henrique da Silva, Rajko Kavalar, Igor But, Maja Pakiž, Sheilla Andrade de Oliveira, Fabiana Oliveira dos Santos Gomes, Rodrigo Soares da Silva, Christina Alves Peixoto, and Norma Lucena-Silva. 2024. "Modulation of sHLA-G, PD-1, and PD-L1 Expression in Cervical Lesions Following Imiquimod Treatment and Its Association with Treatment Success" Cancers 16, no. 7: 1272. https://doi.org/10.3390/cancers16071272
APA StyleCokan, A., da Silva, N. C. H., Kavalar, R., But, I., Pakiž, M., Andrade de Oliveira, S., dos Santos Gomes, F. O., da Silva, R. S., Peixoto, C. A., & Lucena-Silva, N. (2024). Modulation of sHLA-G, PD-1, and PD-L1 Expression in Cervical Lesions Following Imiquimod Treatment and Its Association with Treatment Success. Cancers, 16(7), 1272. https://doi.org/10.3390/cancers16071272






