Could Periodontitis Increase the Risk of Suffering from Pancreatic Cancer?—A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
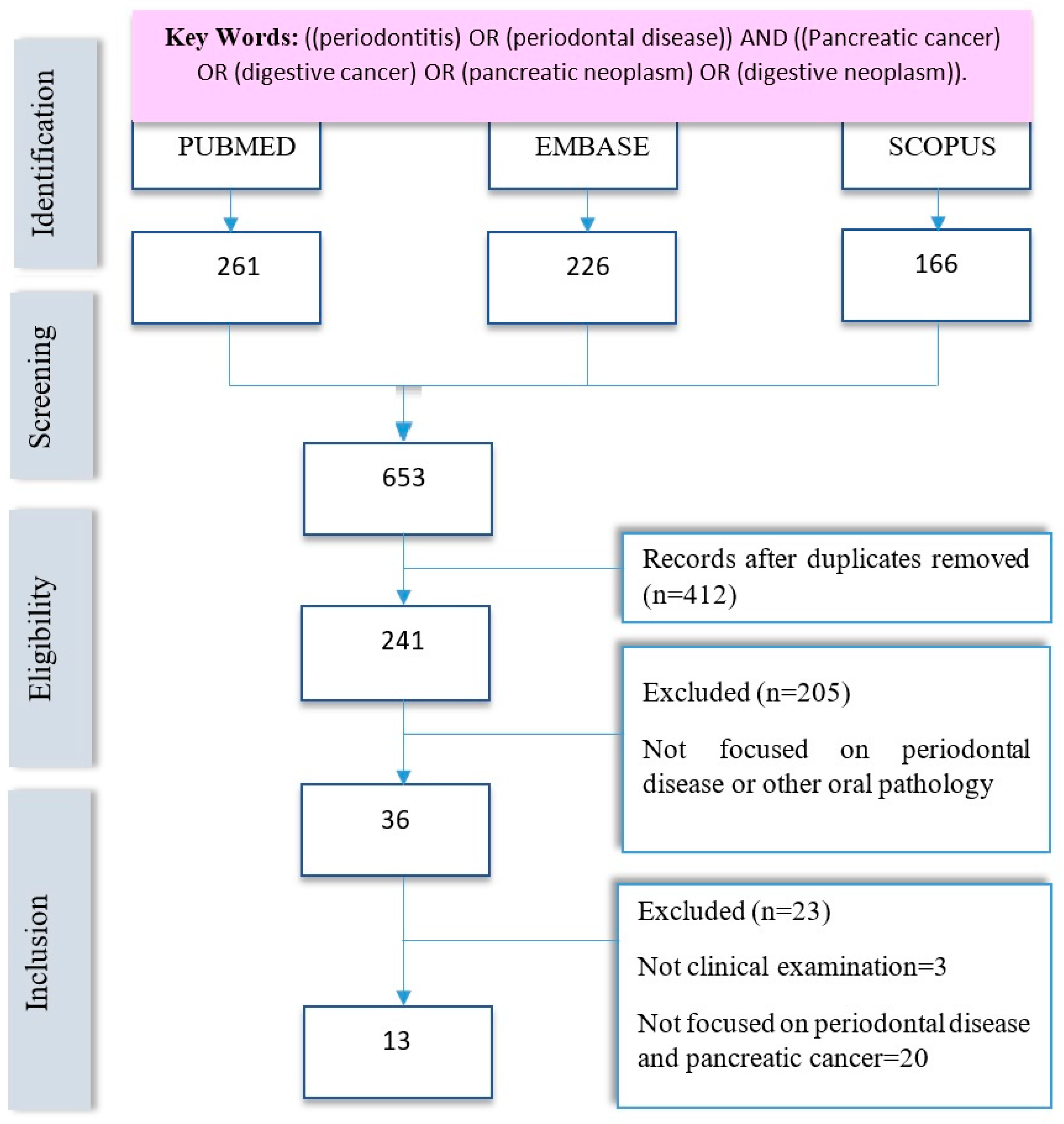
2. Materials and Methods
2.1. Protocol and Literature Search
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Synthesis
2.5. Quality Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Linden, G.J.; Lyons, A.; Scannapieco, F.A. Periodontal Systemic Associations: Review of the Evidence. J. Clin. Periodontol. 2013, 40 (Suppl. S14), 8–19. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Tang, C.Y.; Tan, T.-S.; Chen, K.-H.; Liao, K.-H.; Liou, M.-L. Subgingival Microbiota in Individuals with Severe Chronic Periodontitis. J. Microbiol. Immunol. Infect. 2016, 51, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Periodoncia y Osteointegración. Las enfermedades periodontales en España. SEPA 2010, 56, 1–20. [Google Scholar]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Becerra Ruiz, J.S.; Guzman Flores, J.M. Enfermedad Periodontal y Sistema Inmune. Elementos 2021, 123, 51–54. Available online: https://elementos.buap.mx/directus/storage/uploads/123-A9-p51-Enfermedad_periodontal_y_sistema_inmune_M.pdf (accessed on 29 February 2024).
- Tonetti, M.S. Periodontitis and Risk for Atherosclerosis: An Update on Intervention Trials. J. Clin. Periodontol. 2009, 36, 15–19. [Google Scholar] [CrossRef]
- Pulcini, R.; D’Agostino, S.; Dolci, M.; Bissioli, A.; Caporaso, L.; Iarussi, F. The Impact of COVID-19 on Oral Cancer Diagnosis: A Systematic Review. J. Multidiscip. Appl. Nat. Sci. 2022, 2, 65–69. [Google Scholar] [CrossRef]
- Southerland, J.H.; Moss, K.; Taylor, G.W.; Beck, J.D.; Pankow, J.; Gangula, P.R.; Offenbacher, S. Periodontitis and Diabetes Associations with Measures of Atherosclerosis and CHD. Atherosclerosis 2012, 222, 196–201. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis Induced by Porphyromonas gingivalis Drives Periodontal Microbiota Dysbiosis and Insulin Resistance via an Impaired Adaptive Immune Response. Gut 2017, 66, 872–885. [Google Scholar] [CrossRef]
- Sugiyama, N.; Uehara, O.; Morikawa, T.; Paudel, D.; Ebata, K.; Hiraki, D.; Harada, F.; Yoshida, K.; Kato, S.; Nagasawa, T.; et al. Gut Flora Alterations Due to Lipopolysac Charide Derived from Porphyromonas gingivalis. Odontology 2022, 110, 673–681. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, M.; Kashyap, P.C.; Chia, N.; Tran, N.H.; McWilliams, R.R.; Bekaii-Saab, T.S.; Ma, W.W. The Role of Microbiome in Pancreatic Cancer. Cancer Metastasis Rev. 2021, 40, 777–789. [Google Scholar] [CrossRef]
- Heikkilä, P.; But, A.; Sorsa, T.; Haukka, J. Periodontitis and Cancer Mortality: Register-based Cohort Study of 68,273 adults in 10-year Follow-up. Int. J. Cancer 2018, 142, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. The Inflammophilic Character of the Periodontitis-associated Microbiota. Mol. Oral Microbiol. 2014, 29, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.T.; Listyarifah, D.; Hagström, J.; Haglund, C.; Grenier, D.; Nordström, D.; Uitto, V.-J.; Hernandez, M.; Yucel-Lindberg, T.; Tervahartiala, T.; et al. Treponema Denticola Chymotrypsin-like Proteinase may Contribute to Orodigestive Carcinogenesis through Immunomodulation. Br. J. Cancer 2018, 118, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Tung, Y.C.; Wu, L.S.; Chang, C.J.; Kung, S.; Chu, P.H. Severity of Chronic Periodontitis and Risk of Gastrointestinal Cancers. Medicine 2018, 97, e11386. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, M.; Silvestre, F.J.; Silvestre-Rangil, J.; Bañuls, C.; Rocha, M.; Hernández-Mijares, A. Involvement of Insulin Resistance in Normoglycaemic Obese Patients with Periodontitis: A Cross-sectional Study. J. Clin. Periodontol. 2017, 44, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Arrico, C.F.; Silvestre-Rangil, J.; Gutiérrez-Castillo, L.; Martinez-Herrera, M.; Silvestre, F.J.; Rocha, M. Association between Periodontal Diseases and Polycystic Ovary Syndrome: A Systematic Review. J. Clin. Med. 2020, 9, 1586. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rocha, M.; Bañuls, C.; Alvarez, A.; de Pablo, C.; Sanchez-Serrano, M.; Gomez, M.; Hernandez-Mijares, A. Induction of Oxidative Stress and Human Leukocyte/Endothelial Cell Interactions in Polycystic Ovary Syndrome Patients with Insulin Resistance. J. Clin. Endocrinol. Metab. 2011, 96, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, R.V.; Weidlich, P.; Musskopf, M.L. Periodontal Disease and Systemic Complications. Braz. Oral Res. 2012, 26 (Suppl. S1), 39–47. [Google Scholar] [CrossRef]
- Tan, Q.; Ma, X.; Yang, B.; Liu, Y.; Xie, Y.; Wang, X.; Yuan, W.; Ma, J. Periodontitis Pathogen Porphyromonas gingivalis Promotes Pancreatic Tumorigenesis via Neutrophil Elastase from Tumor-associated Neutrophils. Gut Microbes 2022, 14, 2073785. [Google Scholar] [CrossRef]
- Nwizu, N.N.; Marshall, J.R.; Moysich, K.; Genco, R.J.; Hovey, K.M.; Mai, X.; LaMonte, M.J.; Freudenheim, J.L.; Wactawski-Wende, J. Periodontal Disease and Incident Cancer Risk among Postmenopausal Women: Results from the Women’s Health Initiative Observational Cohort. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Gerlovin, H. Oral Health in Relation to Pancreatic Cancer Risk in African American Women. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Miskiewicz, A.; Szparecki, G.; Durlik, M.; Rydzewska, G.; Ziobrowski, I.; Górska, R. The Correlation between Pancreatic Dysfunction Markers and Selected Indices of Periodontitis. Adv. Clin. Exp. Med. 2018, 27, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, Aging, and Periodontal Disease: Basic Biologic Considerations. Periodontology 2000 2021, 87, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic Stress Accelerates Pancreatic Cancer Growth and Invasion: A Critical Role for Beta-adrenergic Signaling in the Pancreatic Microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, J.; Gallimidi, A.B.; Saba, E.; Pandi, K.; Berchoer, L.E.; Hermano, E.; Angabo, S.; Makkawi, H.; Khashan, A.; Daoud, A.; et al. Intracellular Porphyromonas gingivalis Promotes the Tumorigenic Behavior of Pancreatic Carcinoma Cells. Cancers 2020, 12, 2331. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.; Carneiro, F.; Hruban, R.; Theise, N. WHO Classification of Tumours: Digestive System Tumours, 5th ed.; World Health Organization: Geneva, Switzerland, 2019; Volume 1, ISBN 978-92-832-4499-8.
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The Methodological Quality Assessment Tools for Preclinical and Clinical Studies, Systematic Review and Meta-analysis, and Clinical Practice Guideline: A Systematic Review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and Validation of a Risk-of-bias Tool for Assessing in Vitro Studies Conducted in Dentistry: The QUIN. J. Prosthet. Dent. 2022, 22, 00345–00346. [Google Scholar] [CrossRef]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human Oral Microbiome and Prospective Risk for Pancreatic Cancer: A Population-based Nested Case-control Study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Miskiewicz, A.; Szparecki, G.; Durlik, M.; Rydzewska, G.; Ziobrowski, I.; Górska, R. The Q705K and F359L Single-Nucleotide Polymorphisms of NOD-Like Receptor Signaling Pathway: Association with Chronic Pancreatitis, Pancreatic Cancer, and Periodontitis. Arch. Immunol. Ther. Exp. 2015, 63, 485–494. [Google Scholar] [CrossRef]
- Vogtmann, E.; Han, Y.; Caporaso, J.G.; Bokulich, N.; Mohamadkhani, A.; Moayyedkazemi, A.; Hua, X.; Kamangar, F.; Wan, Y.; Suman, S.; et al. Oral Microbial Community Composition is Associated with Pancreatic Cancer: A Case-control Study in Iran. Cancer Med. 2020, 9, 797–806. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma Antibodies to Oral Bacteria and Risk of Pancreatic Cancer in a Large European Prospective Cohort Study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium Species in Pancreatic Cancer Tissues with Molecular Features and Prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef] [PubMed]
- Kriauciunas, A.; Zekonis, G.; Liutkeviciene, R. Periodontitis association with IL-8 gene polymorphisms. Biomed. Pap. 2022, 166, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Xu, J.N.; Huang, Y.Q.; Wang, J.; Wang, H.L.; Sun, C.; Shi, W.; Jiang, X. Association between healthy lifestyle combinations and periodontitis in NHANES. BMC Oral Health 2024, 24, 182. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, R.; Yuan, S.; Fan, R.; Wang, L.; Larsson, S.C.; Theodoratou, E.; Zhu, Y.; Wu, S.; Ding, Y.; et al. Healthy lifestyle and cancer survival: A multinational cohort study. Int. J. Cancer 2024. ahead of print. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, C.; Gao, R.; Shu, S.; Shu, C. Association between DEFB1 polymorphisms and periodontitis: A meta-analysis. Pharmazie 2019, 74, 390–396. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, M.; Wu, L.; Jia, X.W.; Jin, Y.H.; Zeng, X.T. DEFB1rs11362 polymorphism and risk of chronic periodontitis: A meta-analysis of unadjusted and adjusted data. Front Genet. 2019, 12, 179. [Google Scholar] [CrossRef]
- Terashima, T.; Chubachi, S.; Matsuzaki, T.; Nakajima, T.; Satoh, M.; Iwami, E.; Yoshida, K.; Katakura, A.; Betsuyaku, T. The association between dental health and nutritional status in chronic obstructive pulmonary disease. Chronic Respir. Dis. 2017, 14, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, M.; Abad-Jiménez, Z.; Silvestre, F.J.; López-Domènech, S.; Silvestre-Rangil, J.; Márquez-Arrico, C.F.; Víctor, V.M.; Rocha, M. Effect of Non-Surgical Periodontal Treatment on Oxidative Stress Markers in Leukocytes and Their Interaction with the Endothelium in Obese Subjects with Periodontitis: A Pilot Study. J. Clin. Med. 2020, 9, 2117. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.F.; Navarro, T.P.; Silva, T.A.; Cota, L.O.M.; Soares Dutra Oliveira, A.M.; Costa, F.O. Periodontitis and Endothelial Dysfunction: Periodontal Clinical Parameters and Levels of Salivary Markers Interleukin-1β, Tumor Necrosis Factor-α, Matrix Metalloproteinase-2, Tissue Inhibitor of Metalloproteinases-2 Complex, and Nitric Oxide. J. Periodontol. 2017, 88, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, B.S.; Gheorghe, D.N.; Nicolae, F.M.; Râmboiu, S.; Radu, P.A.; Șurlin, V.M.; Strâmbu, V.D.E.; Gheonea, D.I.; Roman, A.; Șurlin, P. Could there be an interplay between periodontal changes and pancreatic malignancies? World J. Clin. Cases 2023, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Minoli, M.; Michaud, D.S.; Aimetti, M.; Sanz, M.; Loos, B.G.; Romandini, M. Periodontitis and risk of cancer: Mechanistic evidence. Periodontology 2000 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Ribaldone, D.G.; Romano, F.; Aimetti, M.; Romandini, M. The Gum-Gut Axis: Periodontitis and the Risk of Gastrointestinal Cancers. Cancers 2023, 15, 4594. [Google Scholar] [CrossRef] [PubMed]
- Kebschull, M.; Papapanou, P.N. Mini but Mighty—microRNAs in the Pathobiology of Periodontal Disease. Periodontology 2000 2015, 69, 201. [Google Scholar] [CrossRef]
- Vezza, T.; de Marañón, A.M.; Canet, F.; Díaz-Pozo, P.; Marti, M.; D’ocon, P.; Apostolova, N.; Rocha, M.; Víctor, V.M. MicroRNAs and Oxidative Stress: An Intriguing Crosstalk to Be Exploited in the Management of Type 2 Diabetes. Antioxidants 2021, 10, 802. [Google Scholar] [CrossRef]
- Luan, X.; Zhou, X.; Fallah, P.; Pandya, M.; Lyu, H.; Foyle, D.; Burch, D.; Diekwisch, T.G. MicroRNAs: Harbingers and shapers of periodontal inflammation. Semin. Cell Dev. Biol. 2022, 124, 85–98. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, S.M.; Edmisson, J.S.; Jimenez-Flores, E. Human neutrophils and oral microbiota: A constant tug-of-war between a harmonious and a discordant coexistence. Immunol Rev. 2016, 273, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Authors | Selection | Comparability | Exposure | Total Score/9 |
|---|---|---|---|---|
| Fan et al., 2017 [31] | * | * | ** | 4/9 |
| Miskiewicz et al., 2018 [23] | *** | ** | ** | 8/9 |
| Miskiewicz et al., 2015 [32] | *** | ** | ** | 7/9 |
| Tan et al., 2022 [20] | ** | * | * | 6/9 |
| Vogtmann et al., 2019 [33] | *** | ** | ** | 7/9 |
| Authors | Selection Items | Comparability Items | Exposure Items | Total Score/9 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Chou S-H et al., 2018 [15] | * | * | * | * | * | * | * | 7/9 | |
| Heikkilä P et al., 2018 [12] | * | * | * | ** | * | * | 7/9 | ||
| Michaud et al., 2013 [34] | * | * | * | * | * | * | 7/9 | ||
| Authors | Selection Items | Comparability Items | Outcomes Items | Total Score/9 | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Gerlovin et al., 2019 [22] | * | * | * | * | ** | 6/9 | ||
| Kei et al., 2015 [35] | * | * | * | * | ** | 6/9 | ||
| The QUIN Scale | Authors | ||
|---|---|---|---|
| Criteria: | Gnanasekaran et al., 2020 [26] | Nieminen et al., 2018 [14] | Sugiyama et al., 2022 [10] |
| Clearly stated aims/objectives | 2 | 2 | 2 |
| Detailed explanation of sample size calculation | 0 | 0 | 1 |
| Detailed explanation of sampling technique | 2 | 2 | 1 |
| Details of comparison group | N/A | N/A | N/A |
| Detailed explanation of methodology | 2 | 2 | 1 |
| Operator details | 1 | 2 | 1 |
| Randomization | 0 | 0 | 0 |
| Method of measurement of outcome | 2 | 2 | 2 |
| Outcome assessor details | 2 | 2 | 2 |
| Blinding | 0 | 0 | 0 |
| Statistical analysis | 2 | 2 | 2 |
| Presentation of results | 2 | 2 | 2 |
| Author, Year | Study Design | Sample Size | Principal Findings |
|---|---|---|---|
| Miskiewicz et al., 2015 [32] | Case control study | Pancreatic cancer n = 18, chronic pancreatitis n = 39, controls n = 119 | The activation of the NLRP3 inflammasome present in subjects with periodontitis and pancreatic cancer is analyzed. This activation is linked to both pathologies. A genomic study of this receptor is carried out. All periodontal parameters (BOP and CAL) were significantly worse (p = 0.001 and p = 0.001, respectively) in patients with chronic pancreatitis than in the two other groups. The NLRP2 polymorphism was associated with chronic pancreatitis, whereas the NLRP3 polymorphism was comorbid with pancreatic cancer and the increase of CAL. |
| Chou et al., 2018 [15] | Retrospective Cohorts | n = 25,485 individuals with periodontitis. Gastrointestinal cancers: 275 mild periodontitis; 324 severe periodontitis | Severe periodontitis not associated with an increased risk of total individual gastrointestinal cancers compared con mild periodontitis |
| Fan et al., 2017 [31] | Case control study | Pancreatic cancer cases= 361 Control = 371 (were drawn from 2 cohorts the American Cancer Society and National Cancer Institute Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial) | P. gingivalis and Aa > pancreatic cancer risk (OR 1.60). Phylum Fusobacteria and genus Leptotrichia < risk. Oral microbiota may be a role pancreatic cancer aetiology. |
| Gerlovin et al., 2019 [22] | Cross sectional study (with a Biennial follow-up questionnaire by mail). | n = 59,000 African American women | A total of 78 incidents of pancreatic cancer occurred during follow-ups from 2007 through 2016, with participants contributing an average of 9.85 years of follow-up. Relative to the reference category of women who never reported either tooth loss or periodontal disease, multivariable HRs were 1.77 for periodontal disease with no tooth loss, 1.58, for periodontal disease with tooth loss, and 2.05 for tooth loss without periodontal disease. Results from this study suggest that poor oral health may play a role in racial disparities in pancreatic cancer incidence. |
| Gnanasekaran et al., 2020 [26] | Experimental study | 3 cellular lines of Pancreatic ductal adenocarcinoma (2 humans and 1 mouse cells line). Cells with absence of P. gingivalis vs. cells with P. gingivalis. | This study focuses on the relationship between the mechanism of cell cycle impairment in the event of superinfection by periodontopathogens. Specifically, this study is the first to test the mechanistic involvement of P. gingivalis in pancreatic tumorigenesis, applying in vitro tools and a xenograft pancreatic carcinoma model in vivo. Our results reveal a previously unknown direct effect of P. gingivalis on PDAC progression, highlighting the importance of the interplay between hypoxia and P. gingivalis intracellular survival. P. gingivalis infection enhances PDAC cell proliferation. |
| Heikkila et al., 2018 [12] | Cohort Longitudinal study | n = 68,273 Periodontal status was defined based on periodontal treatment procedure codes. | This research analyzes the reported history of dental status (number of teeth, health indices, initial caries, decayed/missing/filled teeth) and CPITN. Furthermore, it defines periodontitis as a binary variable (no/yes) based on the above codes. Data support the association between periodontitis and mortality in all types of cancer, especially pancreatic cancer. |
| Michaud et al., 2013 [34] | Cohort study to select cases and control from a data base | Blood samples from 405 pancreatic cancer cases and 416 matched controls. | This study performed blood tests to detect antibodies to periodontal bacteria. Individuals with high levels of antibodies against P. gingivalis P. gingivalis ATTC 53,978, a pathogenic periodontal bacterium, had a 2-fold higher risk of pancreatic cancer than individuals with lower levels of these antibodies (odds ratio [OR], 2.14; 95% confidence interval [CI], 1.05–4.36; >200 ng/mL vs. ≤200 ng/mL). People with high levels of antibodies against common oral bacteria had a 45% lower risk of pancreatic cancer compared to those with a profile of lower antibody levels. Periodontal disease might increase the risk of pancreatic cancer. |
| Miskiewiez et al., 2018 [23] | Case control study | Evaluated oral health level and periodontal status among 3 groups: n = 29 cancer pancreas; n = 41 chronic pancreatitis; n = 50 controls | Both pathologies are linked based on the systemic inflammatory mediators present in the blood and the correlation with local inflammation measured with the BOP. Periodontitis in pancreatic cancer is independent of the state of oral hygiene. BOP 62.5%, enzyme activity (lipase and amilasa) and chronic pancreatitis were interrelated. |
| Kei et al., 2015 [35] | Transversal study | 283 pancreatic cancer tissue specimens | The presence of periodontopathogens is detected as biomarkers of malignancy in pancreatic tumors. This study associates bacterial tissue infection with cell cycle changes and carcinogenic potential. Periodontal bacteria were found in those samples from patients with the highest risk. The presence of FN was in 25 samples (8.8%) and largely coincided with high mortality rates. |
| Nieminen et al., 2018 [14] | Experimental study | 149 orodigestive tumor tissue samples Tissue samples comprised squamous cell carcinomas (SCCs) of tongue (n = 29), tonsil (n = 25), esophagus (n = 3), and adenocarcinoma of stomach (n = 32), pancreas (n = 6), and colon (n = 54). | This study associates bacterial tissue infection with cell cycle changes and the inhibition of protective factors. Td-CTLP was present in the majority of orodigestive tumor samples. Td-CTLP was found to convert pro MMP-8 and -9 into their active forms. In addition, Td-CTLP was able to degrade the proteinase inhibitors TIMP-1, TIMP-2, and a-1-antichymotrypsin, as well as complement C1q. |
| Sugiyama et al., 2022 [10] | Experimental study | 2 groups of 5 mice: Received P. gingivalis P. gingivalis -LPS and controls. Effect on P. gingivalis -LPS on the gut flora | Infection with periodontopathogens increases TFNα and other inflammatory mediators that are also increased in cancer patients. This route of infection increases the risk of developing cancerous pathologies. The administration of periodontal pathogens can cause changes in the intestinal flora, affecting its physiological functions increasing the risk of cancer. |
| Tan et al., 2022 [20] | Case control study | Study intrapancreatic microbiome composition on resected cancer tissue and matched normal adjacent tissues | In this research, a path of association is indicated due to the increase in low-grade systemic inflammation that occurs due to periodontitis because of the invasion of P. gingivalis. Neutrophils (main cells of the innate response to bacterial aggression) are secreters of elastase and are involved in cellular changes that occur in cancer. P. gingivalis modifies the inflammatory tumor microenvironment and recruits neutrophils and enhances the secretion of neutrophils elastase to promote cancer pancreatic |
| Vogtmann et al., 2019 [33] | Case control study | A total of 273 pancreatic adenocarcinoma cases and 285 controls | The abundance of some specific microbial taxa was also associated with pancreatic cancer, including Haemophilus, Enterobacteriaceae, Lachnospiraceae G7, Bacteroidaceae, and Staphylococcaceae. The microbial community and taxa level differences could be related to the presence of pancreatic cancer or the risk of developing pancreatic cancer. P. gingivalis was not associated with pancreatic cancer and was detected in 76.92% of cases and 76.49% of controls. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Arrico, C.F.; Silvestre, F.J.; Marquez-Arrico, J.E.; Silvestre-Rangil, J. Could Periodontitis Increase the Risk of Suffering from Pancreatic Cancer?—A Systematic Review. Cancers 2024, 16, 1257. https://doi.org/10.3390/cancers16071257
Márquez-Arrico CF, Silvestre FJ, Marquez-Arrico JE, Silvestre-Rangil J. Could Periodontitis Increase the Risk of Suffering from Pancreatic Cancer?—A Systematic Review. Cancers. 2024; 16(7):1257. https://doi.org/10.3390/cancers16071257
Chicago/Turabian StyleMárquez-Arrico, Cecilia Fabiana, Francisco Javier Silvestre, Julia Elena Marquez-Arrico, and Javier Silvestre-Rangil. 2024. "Could Periodontitis Increase the Risk of Suffering from Pancreatic Cancer?—A Systematic Review" Cancers 16, no. 7: 1257. https://doi.org/10.3390/cancers16071257
APA StyleMárquez-Arrico, C. F., Silvestre, F. J., Marquez-Arrico, J. E., & Silvestre-Rangil, J. (2024). Could Periodontitis Increase the Risk of Suffering from Pancreatic Cancer?—A Systematic Review. Cancers, 16(7), 1257. https://doi.org/10.3390/cancers16071257






