Targeting the Cell Cycle, RRM2 and NF-κB for the Treatment of Breast Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Treatment
2.2. Establishment of the Palbociclib-Resistant Cell Lines
2.3. IC50 Determination
2.4. Western Blot Analysis
2.5. Real Time-Quantitative Polymeric Chain Reaction (RT-qPCR) Analysis
2.6. Cell Cycle Analysis
2.7. Cellular ROS Measurement
2.8. NF-κB Activity Assay
2.9. Xenograft Studies
2.10. Statistical Analysis
3. Results
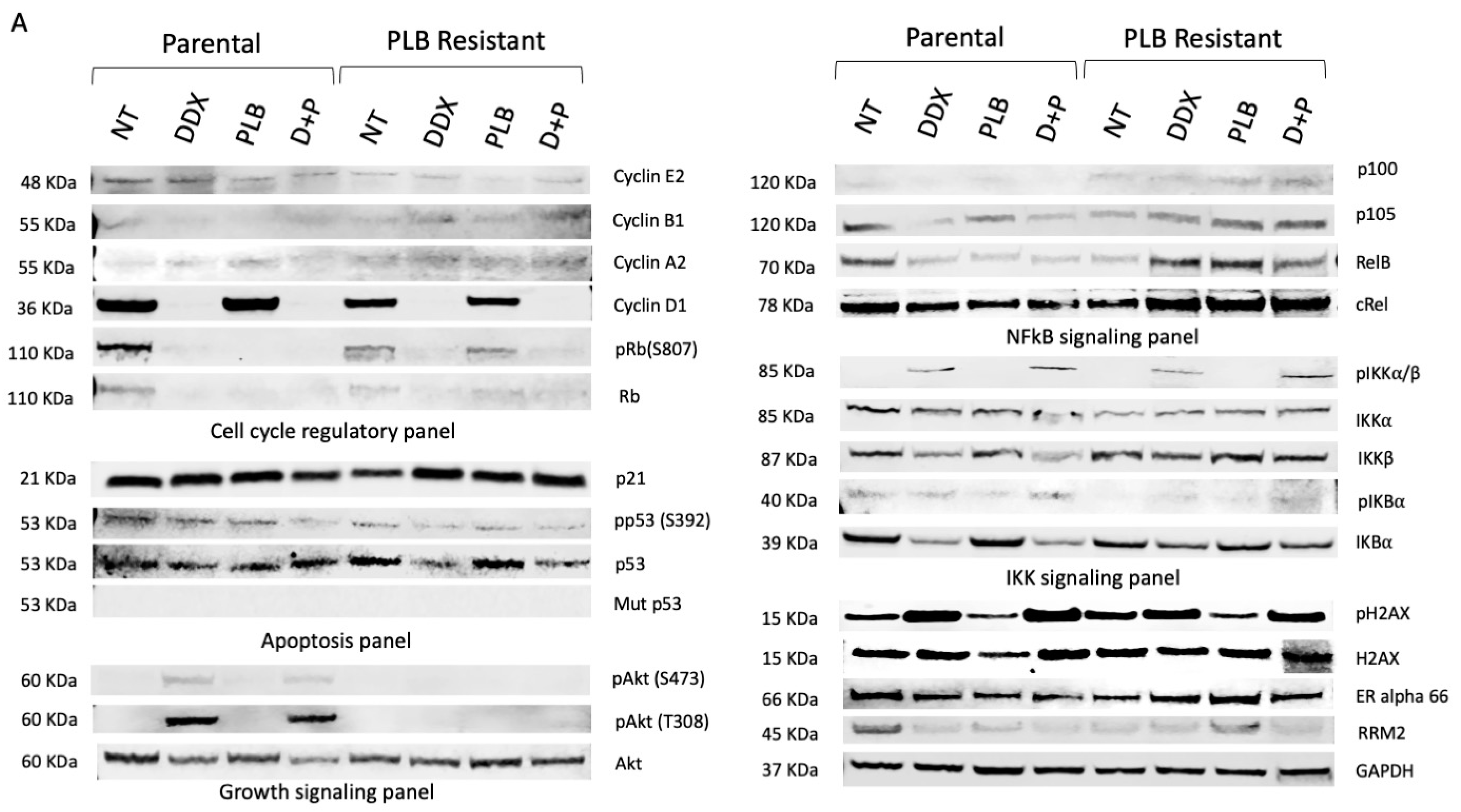
3.1. Resistance to Palbociclib (PLB) Alters Expression of Proteins Involved in Cell Growth and Cell Cycle Regulatory Pathways
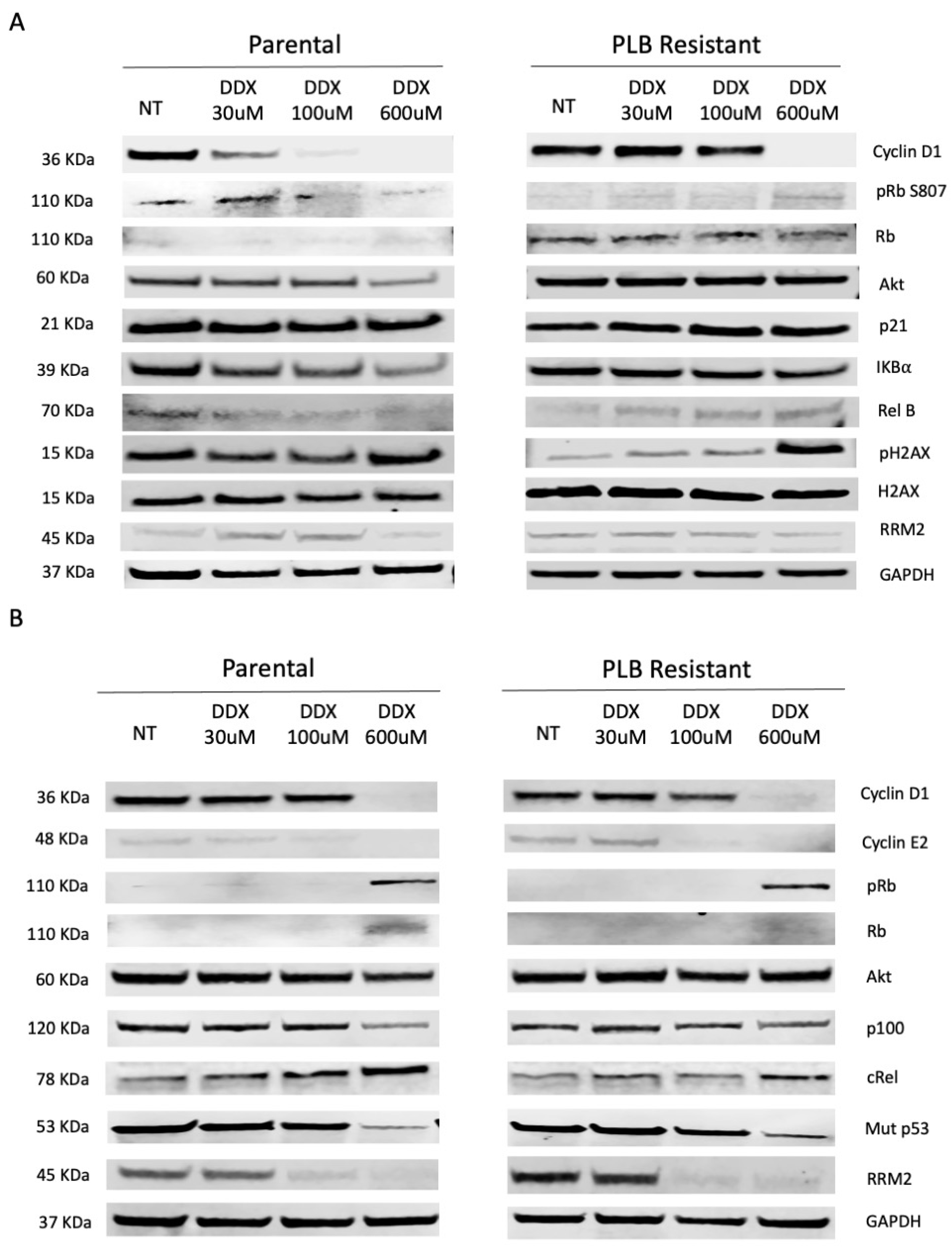
3.2. Inhibition of Ribonucleotide Reductase Alters Cell Cycle Regulatory and NF-κB Pathway Expression in a Dose-Dependent Manner
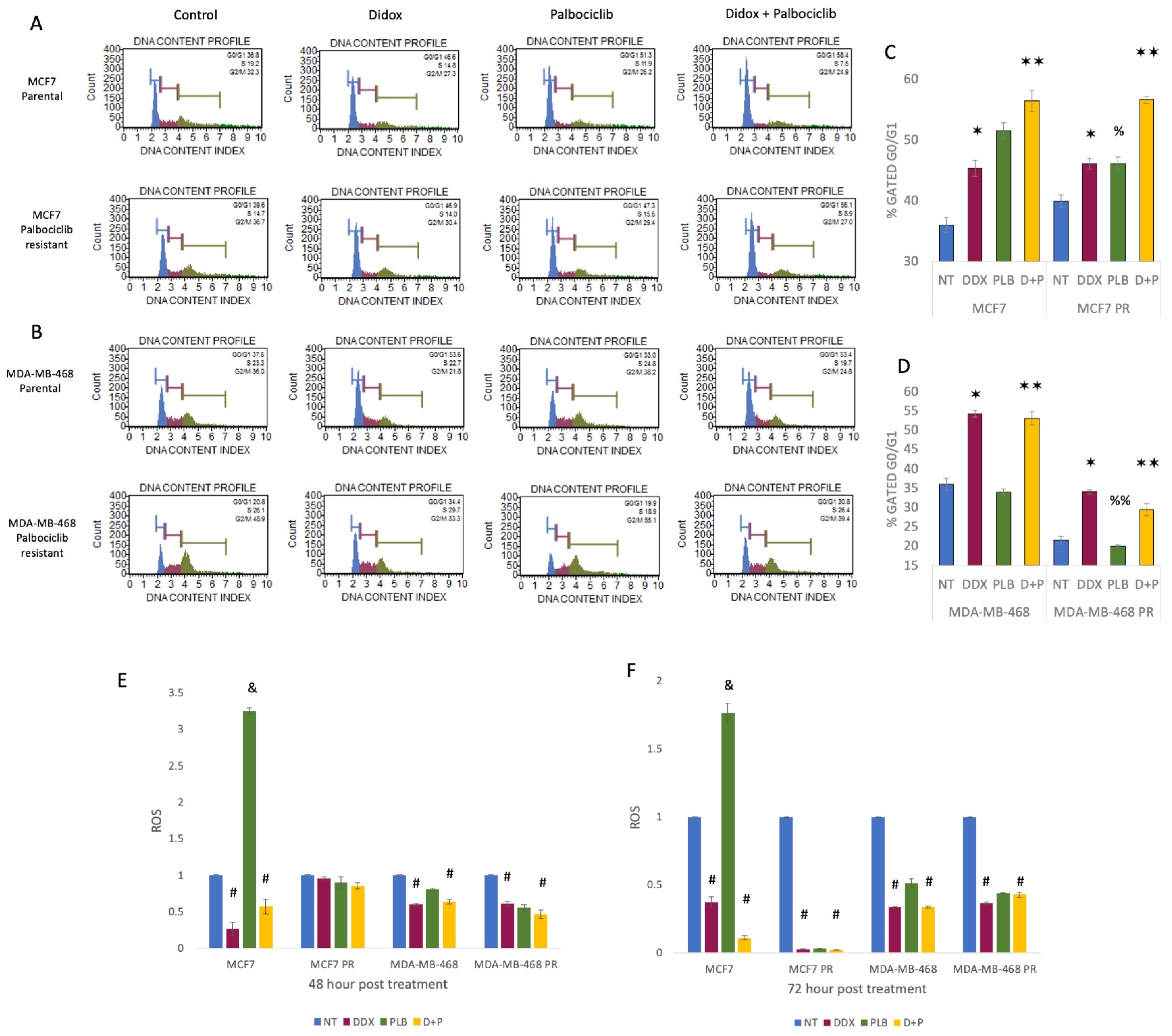
3.3. Inhibition of Ribonucleotide Reductase Causes Cell Cycle Arrest at G1 Phase and ROS Reduction
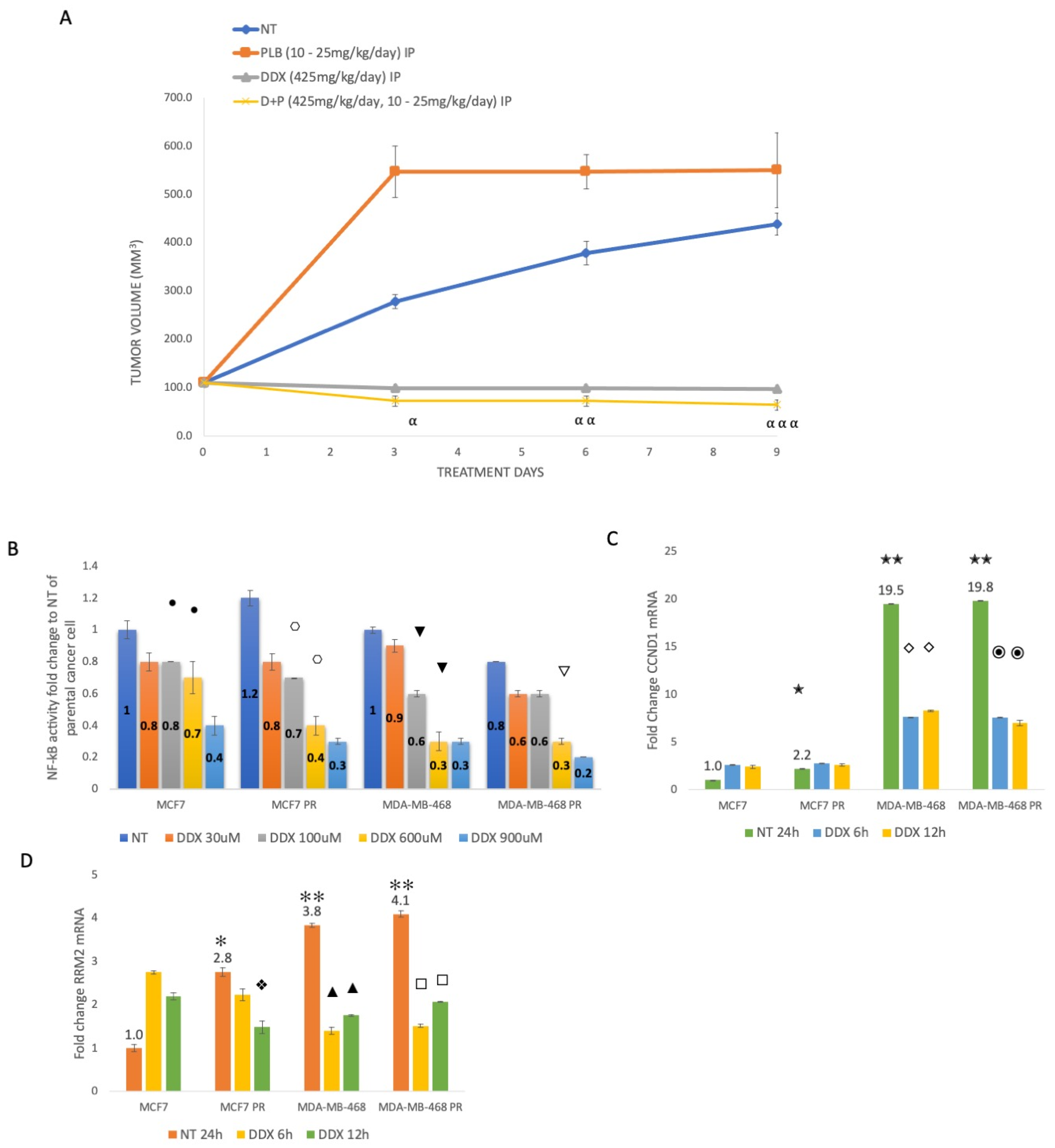
3.4. Inhibition of Ribonucleotide Reductase Reduces ER+ Palbociclib-Resistant Tumor Growth and Decreases NF-κB Activation, Whereas Palbociclib Resistance Increases CCND1 and RRM2 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tong, J.; Tan, X.; Song, X.; Gao, M.; Risnik, D.; Hao, S.; Ermine, K.; Wang, P.; Li, H.; Huang, Y.; et al. CDK4/6 Inhibition Suppresses p73 Phosphorylation and Activates DR5 to Potentiate Chemotherapy and Immune Checkpoint Blockade. Cancer Res. 2022, 82, 1340–1352. [Google Scholar] [CrossRef]
- Tien, A.H.; Sadar, M.D. Cyclin-dependent Kinase 4/6 Inhibitor Palbociclib in Combination with Ralaniten Analogs for the Treatment of Androgen Receptor–positive Prostate and Breast Cancers. Mol. Cancer Ther. 2022, 21, 294–309. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- De Angelis, C.; Fu, X.; Cataldo, M.L.; Nardone, A.; Pereira, R.; Veeraraghavan, J.; Nanda, S.; Qin, L.; Sethunath, V.; Wang, T.; et al. Activation of the IFN Signaling Pathway is Associated with Resistance to CDK4/6 Inhibitors and Immune Checkpoint Activation in ER-Positive Breast Cancer. Clin. Cancer Res. 2021, 27, 4870–4882. [Google Scholar] [CrossRef]
- Fry, D.W.; Harvey, P.J.; Keller, P.R.; Elliott, W.L.; Meade, M.; Trachet, E.; Albassam, M.; Zheng, X.; Leopold, W.R.; Pryer, N.K.; et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004, 3, 1427–1438. [Google Scholar] [CrossRef]
- Chirila, C.; Mitra, D.; Colosia, A.; Ling, C.; Odom, D.; Iyer, S.; Kaye, J.A. Comparison of palbociclib in combination with letrozole or fulvestrant with endocrine therapies for advanced/metastatic breast cancer: Network meta-analysis. Curr. Med. Res. Opin. 2017, 33, 1457–1466. [Google Scholar] [CrossRef]
- Papadimitriou, M.C.; Pazaiti, A.; Iliakopoulos, K.; Markouli, M.; Michalaki, V.; Papadimitriou, C.A. Resistance to CDK4/6 inhibition, Mechanisms and strategies to overcome a therapeutic problem in the treatment of hormone receptor-positive metastatic breast cancer. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2022, 1869, 119346. [Google Scholar] [CrossRef]
- Pandey, K.; An, H.J.; Kim, S.K.; Lee, S.A.; Kim, S.; Lim, S.M.; Kim, G.M.; Sohn, J.; Moon, Y.W. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188. [Google Scholar] [CrossRef]
- Elford, H.L.; Freese, M.; Passamani, E.; Morris, H.P. Ribonucleotide Reductase and Cell Proliferation. J. Biol. Chem. 1970, 245, 5228–5233. [Google Scholar] [CrossRef]
- Lozano, G.; Elledge, S.J. p53 sends nucleotides to repair DNA. Nature 2000, 404, 24–25. [Google Scholar] [CrossRef]
- Mazzu, Y.Z.; Armenia, J.; Chakraborty, G.; Yoshikawa, Y.; Coggins, S.A.; Nandakumar, S.; Gerke, T.A.; Pomerantz, M.M.; Qiu, X.; Zhao, H.; et al. A Novel Mechanism Driving Poor-Prognosis Prostate Cancer, Overexpression of the DNA Repair Gene, Ribonucleotide Reductase Small Subunit M2 (RRM2). Clin. Cancer Res. 2019, 25, 4480–4492. [Google Scholar] [CrossRef]
- Shah, K.N.; Mehta, K.R.; Peterson, D.; Evangelista, M.; Livesey, J.C.; Faridi, J.S. AKT-Induced Tamoxifen Resistance Is Overturned by RRM2 Inhibition. Mol. Cancer Res. 2014, 12, 394–407. [Google Scholar] [CrossRef]
- Shah, K.N.; Wilson, E.A.; Malla, R.; Elford, H.L.; Faridi, J.S. Targeting Ribonucleotide Reductase M2 and NF-κB Activation with Didox to Circumvent Tamoxifen Resistance in Breast Cancer. Mol. Cancer Ther. 2015, 14, 2411–2421. [Google Scholar] [CrossRef]
- Wilson, E.A.; Sultana, N.; Shah, K.N.; Elford, H.L.; Faridi, J.S. Molecular Targeting of RRM2, NF-κB, and Mutant TP53 for the Treatment of Triple-Negative Breast Cancer. Mol. Cancer Ther. 2021, 20, 655–664. [Google Scholar] [CrossRef]
- Du, S.M. The SNHG16/miR-30a axis promotes breast cancer cell proliferation and invasion by regulating RRM2. Neo 2020, 67, 567–575. [Google Scholar] [CrossRef]
- Gandhi, M.; Groß, M.; Holler, J.M.; Coggins, S.A.; Patil, N.; Leupold, J.H.; Munschauer, M.; Schenone, M.; Hartigan, C.R.; Allgayer, H.; et al. The lncRNA lincNMR regulates nucleotide metabolism via a YBX1—RRM2 axis in cancer. Nat. Commun. 2020, 11, 3214. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Y.; Wang, T. High RRM2 expression has poor prognosis in specific types of breast cancer. Batra SK, editor. PLoS ONE 2022, 17, e0265195. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, L.; Zang, Y.; Li, G.; Wang, F. RRM2 elicits the metastatic potential of breast cancer cells by regulating cell invasion, migration and VEGF expression via the PI3K/AKT signaling. Oncol. Lett. 2020, 19, 3349–3355. Available online: http://www.spandidos-publications.com/10.3892/ol.2020.11428 (accessed on 17 October 2022). [CrossRef]
- Chen, W.; Yang, L.; Xu, L.; Cheng, L.; Qian, Q.; Sun, L.; Zhu, Y. Bioinformatics analysis revealing prognostic significance of RRM2 gene in breast cancer. Biosci. Rep. 2019, 39, BSR20182062. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Warden, C.D.; Huang, Y.; Loera, S.; Xue, L.; Zhang, S.; Chu, P.; Zheng, S.; Yen, Y. Prognostic and therapeutic significance of ribonucleotide reductase small subunit M2 in estrogen-negative breast cancers. BMC Cancer 2014, 14, 664. [Google Scholar] [CrossRef]
- Huff, S.E.; Winter, J.M.; Dealwis, C.G. Inhibitors of the Cancer Target Ribonucleotide Reductase, Past and Present. Biomolecules 2022, 12, 815. [Google Scholar] [CrossRef]
- Elford, H.L.; Wampler, G.L.; van’t Riet, B. New ribonucleotide reductase inhibitors with antineoplastic activity. Cancer Res. 1979, 39, 844–851. [Google Scholar]
- Elford, H.L.; Van’t Riet, B.; Wampler, G.L.; Lin, A.L.; Elford, R.M. Regulation of ribonucleotide reductase in mammalian cells by chemotherapeutic agents. Adv. Enzym. Regul. 1981, 19, 151–168. [Google Scholar] [CrossRef]
- Ling, J.; Kumar, R. Crosstalk between ΝΦκΒ and glucocorticoid signaling: A potential target of breast cancer therapy. Cancer Lett. 2012, 322, 119–126. [Google Scholar] [CrossRef]
- Wang, W.; Nag, S.; Zhang, R. Targeting the NFkB Signaling Pathways for Breast Cancer Prevention and Therapy. CMC 2014, 22, 264–289. [Google Scholar] [CrossRef]
- Gilmore, T.D. Multiple Myeloma: Lusting for NF-κB. Cancer Cell 2007, 12, 95–97. [Google Scholar] [CrossRef]
- Chaturvedi, M.M.; Sung, B.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene 2011, 30, 1615–1630. [Google Scholar] [CrossRef]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.-W. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Perkins, N.D.; Felzien, L.K.; Betts, J.C.; Leung, K.; Beach, D.H.; Nabel, G.J. Regulation of NF-κB by Cyclin-Dependent Kinases Associated with the p300 Coactivator. Science 1997, 275, 523–527. [Google Scholar] [CrossRef]
- Hinz, M.; Krappmann, D.; Eichten, A.; Heder, A.; Scheidereit, C.; Strauss, M. NF-κB Function in Growth Control, Regulation of Cyclin D1 Expression and G 0 /G 1 -to-S-Phase Transition. Mol. Cell. Biol. 1999, 19, 2690–2698. [Google Scholar] [CrossRef]
- He, J.; Wei, Q.; Jiang, R.; Luan, T.; He, S.; Lu, R.; Xu, H.; Ran, J.; Li, J.; Chen, D. The Core-Targeted RRM2 Gene of Berberine Hydrochloride Promotes Breast Cancer Cell Migration and Invasion via the Epithelial–Mesenchymal Transition. Pharmaceuticals 2022, 16, 42. [Google Scholar] [CrossRef]
- Abdin, S.M.; Tolba, M.F.; Zaher, D.M.; Omar, H.A. Nuclear factor-κB signaling inhibitors revert multidrug-resistance in breast cancer cells. Chem.-Biol. Interact. 2021, 340, 109450. [Google Scholar] [CrossRef]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef]
- Park, Y.H. The nuclear factor-kappa B pathway and response to treatment in breast cancer. Pharmacogenomics 2017, 18, 1697–1709. [Google Scholar] [CrossRef]
- Barnes, D.M.; Gillett, C.E. Cyclin D1 in Breast Cancer. Breast Cancer Res. Treat. 1998, 52, 1–15. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, J.; Li, Y.; Shi, Q.; Jin, L.; Li, S.; Zhu, M.; Wang, Q.; Wong, L.L.; Yang, W.; et al. Overexpressed Cyclin D1 and CDK4 proteins are responsible for the resistance to CDK4/6 inhibitor in breast cancer that can be reversed by PI3K/mTOR inhibitors. Sci. China Life Sci. 2023, 66, 94–109. [Google Scholar] [CrossRef]
- Wang, G.; Gormley, M.; Qiao, J.; Zhao, Q.; Wang, M.; Di Sante, G.; Deng, S.; Dong, L.; Pestell, T.; Ju, X.; et al. Cyclin D1-mediated microRNA expression signature predicts breast cancer outcome. Theranostics 2018, 8, 2251–2263. [Google Scholar] [CrossRef]
- Velasco-Velázquez, M.A.; Li, Z.; Casimiro, M.; Loro, E.; Homsi, N.; Pestell, R.G. Examining the role of cyclin D1 in breast cancer. Future Oncol. 2011, 7, 753–765. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Karakas, C.; Doostan, I.; Chen, X.; Bui, T.; Yi, M.; Raghavendra, A.S.; Zhao, Y.; Bashour, S.I.; Ibrahim, N.K.; et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat. Commun. 2017, 8, 15916. [Google Scholar] [CrossRef]
- Chen, X.; Lowe, M.; Keyomarsi, K. UCN-01-mediated G1 arrest in normal but not tumor breast cells is pRb-dependent and p53-independent. Oncogene 1999, 18, 5691–5702. [Google Scholar] [CrossRef]
- Gray-Bablin, J.; Zalvide, J.; Fox, M.P.; Knickerbocker, C.J.; DeCaprio, J.A.; Keyomarsi, K. Cyclin E, a redundant cyclin in breast cancer. Proc. Natl. Acad. Sci. USA 1996, 93, 15215–15220. [Google Scholar] [CrossRef]
- Gallaugher, L.D.; Henry, J.C.; Kearns, P.N.; Elford, H.L.; Bergdall, V.K.; Cardounel, A.J. Ribonucleotide reductase inhibitors reduce atherosclerosis in a double-injury rabbit model. Comp. Med. 2009, 59, 567–572. [Google Scholar]
- Mayhew, C.N.; Phillips, J.D.; Greenberg, R.N.; Birch, N.J.; Elford, H.L.; Gallicchio, V.S. In vivo and in vitro comparison of the short-term hematopoietic toxicity between hydroxyurea and trimidox or didox, novel ribonucleotide reductase inhibitors with potential anti-HIV-1 activity. Stem Cells 1999, 17, 345–356. [Google Scholar] [CrossRef]
- Mayhew, C.N.; Sumpter, R.; Inayat, M.; Cibull, M.; Phillips, J.D.; Elford, H.L.; Gallicchio, V.S. Combination of inhibitors of lymphocyte activation (hydroxyurea, trimidox, and didox) and reverse transcriptase (didanosine) suppresses development of murine retrovirus-induced lymphoproliferative disease. Antivir. Res. 2005, 65, 13–22. [Google Scholar] [CrossRef]
- Carmichael, J.; Cantwell, B.; Mannix, K.; Veale, D.; Elford, H.; Blackie, R.; Kerr, D.; Kaye, S.; Harris, A. A phase I and pharmacokinetic study of didox administered by 36 hour infusion. The Cancer Research Campaign Phase I/II Clinical Trials Committee. Br. J. Cancer 1990, 61, 447–450. [Google Scholar] [CrossRef]
- Veale, D.; Carmichael, J.; Cantwell, B.; Elford, H.; Blackie, R.; Kerr, D.; Kaye, S.; Harris, A. A phase 1 and pharmacokinetic study of didox: A ribonucleotide reductase inhibitor. Br. J. Cancer 1988, 58, 70–72. [Google Scholar] [CrossRef]
- Rubens, R.D.; Kaye, S.B.; Soukop, M.; Williams, C.J.; Brampton, M.H.; Harris, A.L. Phase II trial of didox in advanced breast cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Br. J. Cancer 1991, 64, 1187–1188. [Google Scholar] [CrossRef][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, N.; Elford, H.L.; Faridi, J.S. Targeting the Cell Cycle, RRM2 and NF-κB for the Treatment of Breast Cancers. Cancers 2024, 16, 975. https://doi.org/10.3390/cancers16050975
Sultana N, Elford HL, Faridi JS. Targeting the Cell Cycle, RRM2 and NF-κB for the Treatment of Breast Cancers. Cancers. 2024; 16(5):975. https://doi.org/10.3390/cancers16050975
Chicago/Turabian StyleSultana, Nahid, Howard L. Elford, and Jesika S. Faridi. 2024. "Targeting the Cell Cycle, RRM2 and NF-κB for the Treatment of Breast Cancers" Cancers 16, no. 5: 975. https://doi.org/10.3390/cancers16050975
APA StyleSultana, N., Elford, H. L., & Faridi, J. S. (2024). Targeting the Cell Cycle, RRM2 and NF-κB for the Treatment of Breast Cancers. Cancers, 16(5), 975. https://doi.org/10.3390/cancers16050975






