Clinical Outcome after Surgical Treatment of Sacral Chordomas: A Single-Center Retrospective Cohort of 27 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patients
2.3. Surgical Procedure
2.4. Risk Factors
2.5. Statistical Analysis
3. Results
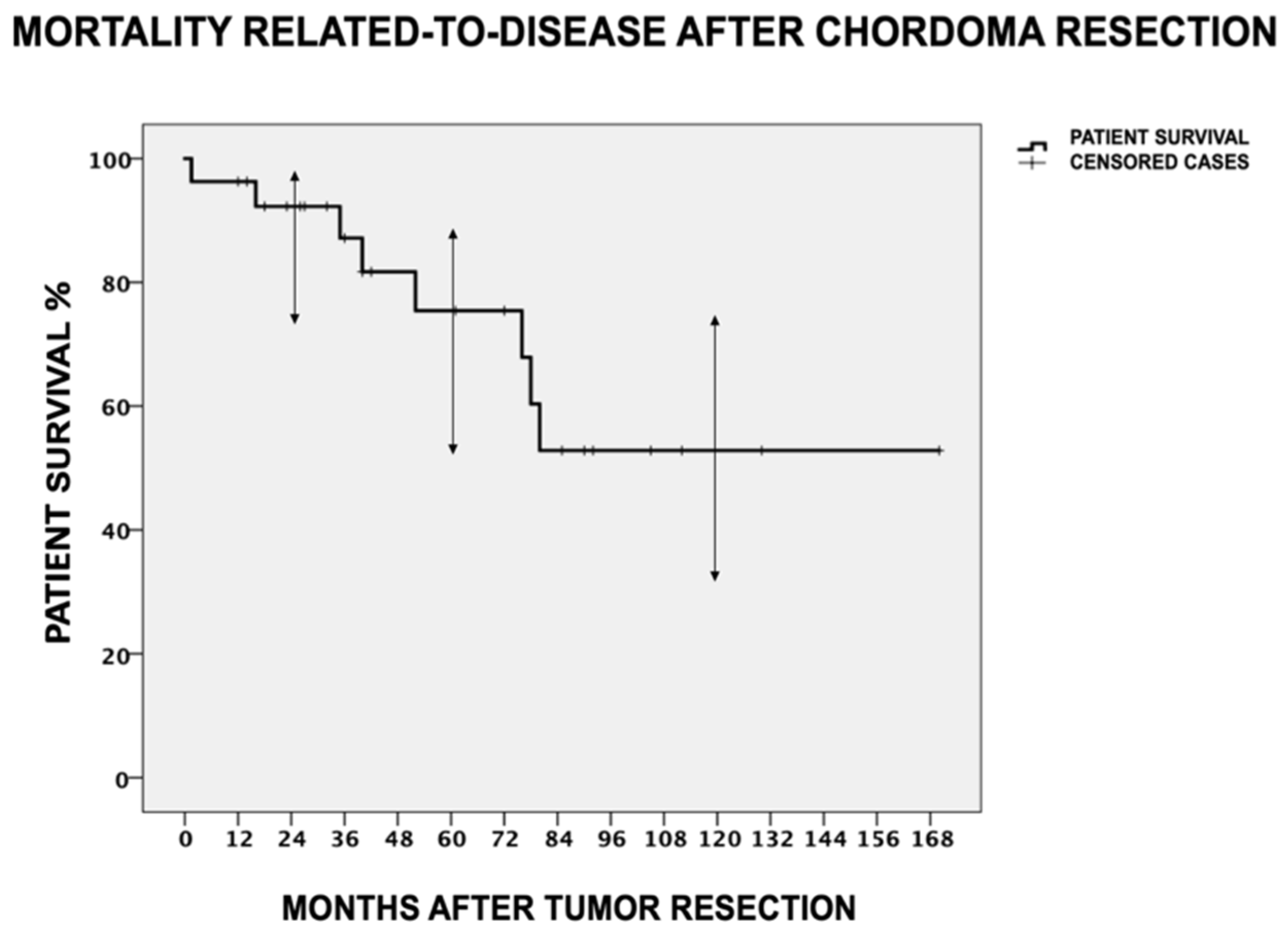
3.1. Disease Mortality
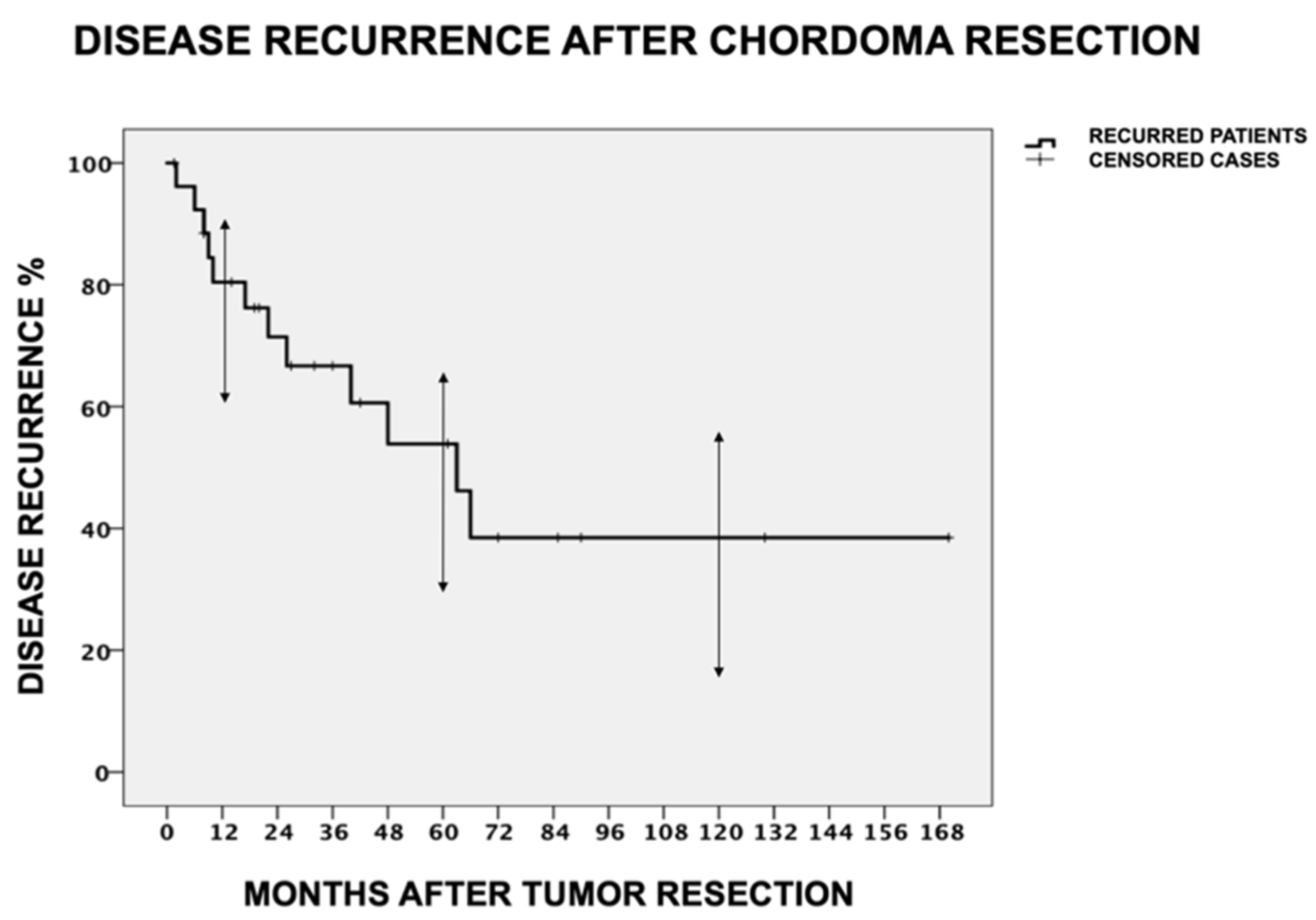
3.2. Disease Recurrence
3.3. Wound-Related Complications (WRCs)
3.4. Final Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| BMI | Body Mass Index |
| CCI | Charlson Comorbidity Index |
| VRAM | Vertical rectus abdominis myocutaneous flap |
| LDR | Local disease recurrence |
| DDR | Distant disease recurrence |
| DSS | Disease-specific survival |
| DFS | Disease-free survival |
| WRCs | Wound-related complications |
| SD | Standard deviation |
| VAC | Vacuum-assisted secondary wound closure treatment |
References
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma—Current Understanding and Modern Treatment Paradigms. JCM 2021, 10, 1054. [Google Scholar] [CrossRef]
- Garofalo, F.; di Summa, P.G.; Christoforidis, D.; Pracht, M.; Laudato, P.; Cherix, S.; Bouchaab, H.; Raffoul, W.; Demartines, N.; Matter, M. Multidisciplinary approach of lumbo-sacral chordoma: From oncological treatment to reconstructive surgery. J. Surg. Oncol. 2015, 112, 544–554. [Google Scholar] [CrossRef]
- Kerekes, D.; Goodwin, C.R.; Ahmed, A.K.; Verlaan, J.J.; Bettegowda, C.; Abu-Bonsrah, N.; Sciubba, D.M. Local and Distant Recurrence in Resected Sacral Chordomas: A Systematic Review and Pooled Cohort Analysis. Glob. Spine J. 2019, 9, 191–201. [Google Scholar] [CrossRef]
- Dial, B.L.; Kerr, D.L.; Lazarides, A.L.; Catanzano, A.A.; Green, C.L.; Risoli, T., Jr.; Blazer, D.G.; Goodwin, R.C.; Brigman, B.E.; Eward, W.C.; et al. The Role of Radiotherapy for Chordoma Patients Managed with Surgery: Analysis of the National Cancer Database. Spine 2020, 45, E742–E751. [Google Scholar] [CrossRef]
- Houdek, M.T.; Wellings, E.P.; Moran, S.L.; Bakri, K.; Dozois, E.J.; Mathis, K.L.; Yaszemski, M.J.; Sim, F.H.; Rose, P.S. Outcome of Sacropelvic Resection and Reconstruction Based on a Novel Classification System. J. Bone Joint Surg. Am. 2020, 102, 1956–1965. [Google Scholar] [CrossRef]
- Fujiwara, T.; Tsuda, Y.; Stevenson, J.; Parry, M.; Jeys, L. Sacral chordoma: Do the width of surgical margin and the use of photon/proton radiotherapy affect local disease control? Int. Orthop. (SICOT) 2020, 44, 381–389. [Google Scholar] [CrossRef]
- Zuckerman, S.L.; Lee, S.H.; Chang, G.J.; Walsh, G.L.; Mehran, R.J.; Gokaslan, Z.L.; Rao, G.; Tatsui, C.E.; Rhines, L.D. Outcomes of Surgery for Sacral Chordoma and Impact of Complications: A Report of 50 Consecutive Patients with Long-Term Follow-Up. Glob. Spine J. 2021, 11, 740–750. [Google Scholar] [CrossRef]
- Ailon, T.; Torabi, R.; Fisher, C.G.; Rhines, L.D.; Clarke, M.J.; Bettegowda, C.; Boriani, S.; Yamada, Y.J.; Kawahara, N.; Varga, P.P.; et al. Management of Locally Recurrent Chordoma of the Mobile Spine and Sacrum: A Systematic Review. Spine 2016, 41, S193–S198. [Google Scholar] [CrossRef]
- Hindi, N.; Casali, P.G.; Morosi, C.; Messina, A.; Palassini, E.; Pilotti, S.; Tamborini, E.; Radaelli, S.; Gronchi, A.; Stacchiotti, S. Imatinib in advanced chordoma: A retrospective case series analysis. Eur. J. Cancer 2015, 51, 2609–2614. [Google Scholar] [CrossRef]
- Konieczkowski, D.J.; DeLaney, T.F.; Yamada, Y.J. Radiation Strategies for Spine Chordoma. Neurosurg. Clin. N. Am. 2020, 31, 263–288. [Google Scholar] [CrossRef]
- Ji, T.; Guo, W.; Yang, R.; Tang, X.; Wang, Y.; Huang, L. What Are the Conditional Survival and Functional Outcomes After Surgical Treatment of 115 Patients with Sacral Chordoma? Clin. Orthop. Relat. Res. 2017, 475, 620–630. [Google Scholar] [CrossRef]
- Schwab, J.H.; Healey, J.H.; Rose, P.; Casas-Ganem, J.; Boland, P.J. The Surgical Management of Sacral Chordomas. Spine 2009, 34, 2700–2704. [Google Scholar] [CrossRef]
- Wright, C.H.; Wright, J.; Cioffi, G.; Hdeib, A.; Kasliwal, M.K.; Kruchko, C.; Barnholtz-Sloan, J.S.; Sloan, A.E. Association of cancer center type with treatment patterns and overall survival for patients with sacral and spinal chordomas: An analysis of the National Cancer Database from 2004 to 2015. J. Neurosurg. Spine 2020, 32, 311–320. [Google Scholar] [CrossRef]
- Radaelli, S.; Stacchiotti, S.; Ruggieri, P.; Donati, D.; Casali, P.G.; Palmerini, E.; Collini, P.; Gambarotti, M.; Porcu, L.; Boriani, S.; et al. Sacral Chordoma: Long-term Outcome of a Large Series of Patients Surgically Treated at Two Reference Centers. Spine 2016, 41, 1049–1057. [Google Scholar] [CrossRef]
- Dubory, A.; Missenard, G.; Lambert, B.; Court, C. “En bloc” resection of sacral chordomas by combined anterior and posterior surgical approach: A monocentric retrospective review about 29 cases. Eur. Spine J. 2014, 23, 1940–1948. [Google Scholar] [CrossRef]
- Van Wulfften Palthe, O.D.; Tromp, I.; Ferreira, A.; Fiore, A.; Bramer, J.A.; van Dijk, N.C.; DeLaney, T.F.; Schwab, J.H.; Hornicek, F.J. Sacral chordoma: A clinical review of 101 cases with 30-year experience in a single institution. Spine J. 2019, 19, 869–879. [Google Scholar] [CrossRef]
- Fuchs, B. Operative Management of Sacral Chordoma. J. Bone Joint Surg. Am. 2005, 87, 2211. [Google Scholar]
- Houdek, M.T.; Rose, P.S.; Hevesi, M.; Schwab, J.H.; Griffin, A.M.; Healey, J.H.; Petersen, I.A.; DeLaney, T.F.; Chung, P.W.; Yaszemski, M.J.; et al. Low dose radiotherapy is associated with local complications but not disease control in sacral chordoma. J. Surg. Oncol. 2019, 119, 856–863. [Google Scholar] [CrossRef]
- Lee, I.J.; Lee, R.J.; Fahim, D.K. Prognostic Factors and Survival Outcome in Patients with Chordoma in the United States: A Population-Based Analysis. World Neurosurg. 2017, 104, 346–355. [Google Scholar] [CrossRef]
- Chen, K.W.; Yang, H.L.; Lu, J.; Liu, J.Y.; Chen, X.Q. Prognostic factors of sacral chordoma after surgical therapy: A study of 36 patients. Spinal Cord. 2010, 48, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Sewell, M.D.; Tan, K.A.; Hanna, S.A.; Williams, R.; Pollock, R.; Skinner, J.; Briggs, T.W. Prognostic Factors in the Operative Management of Sacral Chordomas. World Neurosurg. 2015, 84, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Nunna, R.S.; Nie, J.; Ansari, D.; Chaudhry, N.S.; Mehta, A.I. Incidence, Management, and Outcomes of Adult Spinal Chordoma Patients in the United States. Glob. Spine J. 2023, 13, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.P.; Szövérfi, Z.; Fisher, C.G.; Boriani, S.; Gokaslan, Z.L.; Dekutoski, M.B.; Chou, D.; Quraishi, N.A.; Reynolds, J.J.; Luzzati, A.; et al. Surgical treatment of sacral chordoma: Prognostic variables for local recurrence and overall survival. Eur. Spine J. 2015, 24, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kou, C.; Bai, W.; Yu, W.; Zhu, B.; Zhang, M.; Hua, W.; Li, Y.; Duan, R.; Yin, F. Comparison of Wide Margin and Inadequate Margin for Recurrence in Sacral Chordoma: A Meta-Analysis. Spine 2020, 45, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, S.L.; Amini, B.; Lee, S.H.; Rao, G.; Tatsui, C.E.; Rhines, L.D. Predictive Value of Preoperative Magnetic Resonance Imaging Findings for Survival and Local Recurrence in Patients Undergoing En Bloc Resection of Sacral Chordomas. Neurosurgery 2019, 85, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Chen, D.; Sang, C.M.; Zheng, X.Q.; Lin, J.L.; Lin, Y.; Ni, W.F.; Wang, X.Y.; Li, Y.M.; Wu, A.M. Nomogram for Individualized Prediction and Prognostic Factors for Survival in Patients with Primary Spinal Chordoma: A Population-Based Longitudinal Cohort Study. World Neurosurg. 2019, 128, e603–e614. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Liu, W.; Xu, H.; Niu, X. The clinical outcome of recurrent sacral chordoma with further surgical treatment. Medicine 2018, 97, e13730. [Google Scholar] [CrossRef]
- Ji, J.; Hemminki, K. Incidence of multiple primary malignancies among patients with bone cancers in Sweden. J. Cancer Res. Clin. Oncol. 2006, 132, 529–535. [Google Scholar] [CrossRef]
- e Silva, B.M.; Conceição Maia Martins, S.; Voltan Garofo, K.; Eduardo Hideo Hanasilo, C.; Etchebehere, M. Analysis of morbidity and mortality in patients with primary bone tumors who underwent sacrectomy: A systematic review. J. Bone Oncol. 2022, 35, 100445. [Google Scholar] [CrossRef]
- Chen, K.W.; Yang, H.L.; Lu, J.; Wang, G.L.; Ji, Y.M.; Bao, Z.H.; Wu, G.Z.; Gu, Y.; Sun, Z.Y.; Zhu, R.F. Risk Factors for Postoperative Wound Infections of Sacral Chordoma After Surgical Excision. J. Spinal Disord. Tech. 2011, 24, 230–234. [Google Scholar] [CrossRef]
- Houdek, M.T.; Bakri, K.; Tibbo, M.E.; Wagner, E.R.; Rose, P.S.; Sim, F.H.; Moran, S.L. Outcome and Complications following Vertical Rectus Abdominis Myocutaneous Flap Surgery to Reconstruct Sacrectomy Defects. Plast. Reconstr. Surg. 2018, 142, 1327–1335. [Google Scholar] [CrossRef]
- Ruggieri, P.; Angelini, A.; Pala, E.; Mercuri, M. Infections in Surgery of Primary Tumors of the Sacrum. Spine 2012, 37, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, W.; Qu, H.; Yang, R.; Tang, X.; Yan, T.; Tang, S.; Yang, Y.; Ji, T.; Dong, S. Experience with wound complications after surgery for sacral tumors. Eur Spine J. 2013, 22, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, R.; Bose, J.C.; Muthusamy, V.; Natarajan, M.; Kunjithapatham, D. Staged sacrectomy—An adaptive approach: Clinical article. SPI 2009, 11, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Hevesi, M.; Griffin, A.M.; Yaszemski, M.J.; Sim, F.H.; Ferguson, P.C.; Rose, P.S.; Wunder, J.S. Can the ACS-NSQIP surgical risk calculator predict postoperative complications in patients undergoing sacral tumor resection for chordoma? J. Surg. Oncol. 2020, 121, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Deskoulidi, P.; Stavrianos, S.D.; Mastorakos, D.; Kontogeorgakos, V.A.; Savvidou, O.; Chrysikos, D.; Samolis, A.; Pappas, N.; Troupis, T.; Papagelopoulos, P.J.; et al. Anatomical Considerations and Plastic Surgery Reconstruction Options of Sacral Chordoma Resection. Cureus [Internet]. 2023. Available online: https://www.cureus.com/articles/148646-anatomical-considerations-and-plastic-surgery-reconstruction-options-of-sacral-chordoma-resection (accessed on 3 November 2023).
- Zhang, Y.; Chen, W.G.; Yang, S.Z.; Qiu, H.; Sun, J.; Hu, X.; Liao, T.Q.; Yang, L.; Liu, Y.G.; Chu, T.W. Preliminary investigation of bilateral internal iliac artery ligation and anterior tumor separation through laparoscopy before posterior resection of a giant sacral tumor. Surg. Oncol. 2020, 34, 24–30. [Google Scholar] [CrossRef]
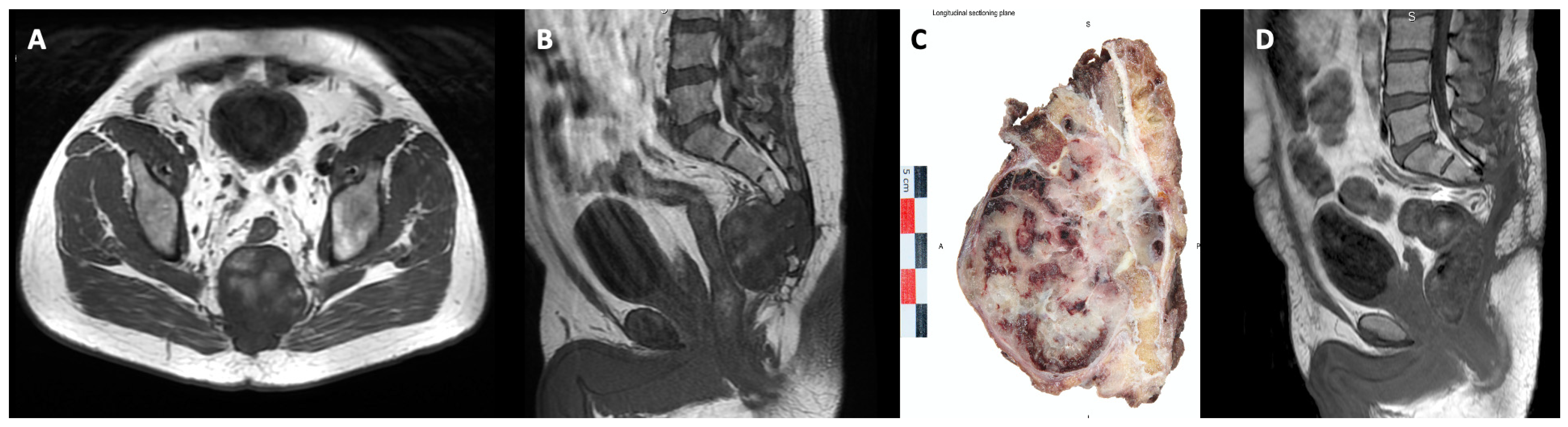


| Survivors (N = 15 Patients) | Recurred (N = 12 Patients) | Significance (Univariate) | Significance (COX) | Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|
| Survival (months) | 55 ± 47.1 [2:170] | 61.7 ± 33.4 [14:112] | p = 0.411 | - | - |
| Age (years) | 60.7 ± 14.7 [36:87] | 64.9 ± 6.8 [57:78] | p = 0.667 | - | - |
| BMI (kg/m2) | 28.2 ± 4.4 [22:35] | 29.8 ± 5.1 [21:36] | p = 0.236 | - | - |
| Gender (M/F) | 10 (67%)/5 (33%) | 7 (58%)/5 (42%) | p = 0.541 | - | - |
| CCI | 5.2 ± 2 [2:9] | 5.4 ± 1.1 [4:7] | p = 0.732 | - | - |
| Primary/recurrent tumor | 15 (100%)/0 (0%) | 9 (75%)/3 (25%) | p = 0.001 * | p = 0.457 | 0.255 (0.007–9.335) |
| Second primary tumor (yes/no) | 2 (13%)/13 (87%) | 2 (17%)/10 (83%) | p = 0.358 | - | - |
| Tumor size (cm3) | 500.5 ± 456.9 [5:1440] | 787.2 ± 839.5 [34:3050] | p = 0.038 * | p = 0.321 | 1.001 (0.999–1.003) |
| Dorsal soft tissue involvement (yes/no) | 7 (47%)/8 (53%) | 9 (75%)/3 (25%) | p = 0.022 * | p = 0.518 | 0.438 (0.036–5.355) |
| Level of sacrectomy | 9 (60%) S1–S2/ 4 (27%) S2–S3/ 2 (13%) S3–S4 | 8 (67%) S1–S2/ 3 (25%) S2–S3/ 1 (8%) S3–S4 | p = 0.854 | - | - |
| Surgical approach (combined/posterior) | 9 (60%)/6 (40%) | 5 (42%)/7 (58%) | p = 0.645 | - | - |
| Surgical margins (N > 2 mm/N < 2 mm/P) | 14 (93%)/ 1 (7%)/ 0 (0%) | 2 (17%)/ 7 (58%)/ 3 (25%) | p < 0.001 * | p = 0.005 a | 10.179 (2.034–50.941) |
| Preoperative radiotherapy (yes/no) | 1 (7%)/14 (93%) | 3 (25%)/9 (75%) | p = 0.173 | - | - |
| Uncomplicated (N = 15 Patients) | Complicated (N = 12 Patients) | Significance (Univariate) | Significance (COX) | Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|
| Age (years) | 64.1 ± 13.5 [36:87] | 60.6 ± 9.6 [38:75] | p = 0.702 | - | - |
| BMI (kg/m2) | 28.3 ± 4.2 [22:34] | 29.5 ± 5.3 [20:35] | p = 0.273 | - | - |
| Gender (M/F) | 9 (60%)/6 (40%) | 8 (67%)/4 (33%) | p = 0.829 | - | - |
| CCI | 5.2 ± 1.8 [2:9] | 5.4 ± 1.5 [3:8] | p = 0.584 | - | - |
| Primary/recurrent tumor | 15 (100%)/0 (0%) | 9 (75%)/3 (25%) | p = 0.114 | - | - |
| Tumor size (cm3) | 329 ± 310.7 [5:950] | 1102.7 ± 796 [385:3050] | p = 0.016 * | p = 0.608 | 0.001 (0.998–1.001) |
| Dorsal soft tissue involvement (yes/no) | 7 (47%)/8 (53%) | 9 (75%)/3 (25%) | p = 0.081 | - | - |
| Maximum specimen area (cm2) | 172 ± 96.6 [70:380] | 297.9 ± 77.8 [220:425] | p = 0.013 * | p = 0.874 | 0.002 (0.977–1.027) |
| Level of sacrectomy | 7 (47%) S1–S2/5 (33%) S2–S3/ 3 (20%) S3–S4 | 10 (83%) S1–S2/2 (17%) S2–S3/ 0 (0%) S3–S4 | p = 0.053 | - | - |
| Duration of surgery (min) | 364 ± 164.3 [150:665] | 535 ± 231 [370:830] | p = 0.012 * | p = 0.841 | −0.026 (0.755–1.257) |
| Iliac vessel ligation (yes/no) | 4 (27%)/11 (73%) | 10 (83%)/2 (17%) | p = 0.003 * | p = 0.574 | 1.058 (0.72–115.14) |
| Colostomy (yes/no) | 3 (20%)/12 (80%) | 5 (42%)/7 (58%) | p = 0.209 | - | - |
| Flap (VRAM/Gluteal/No) | 1 (7%)/2 (13%)/ 12 (80%) | 4 (33%)/3 (25%)/ 5 (42%) | p = 0.039 * | p = 0.924 | 0.149 (0.054–24.85) |
| Preoperative serum albumin (G/DL) | 3.9 ± 0.7 [2.8:4.7] | 4.2 ± 0.8 [3:4.9] | p = 0.215 | - | - |
| Postoperative serum albumin (G/DL) | 2.8 ± 0.2 [2.4:3.2] | 2.2 ± 0.3 [1.7:2.7] | p = 0.001 * | p = 0.122 | −2.472 (0.004–1.929) |
| Preoperative radiotherapy (yes/no) | 1 (7%)/14 (93%) | 3 (25%)/9 (75%) | p = 0.155 | - | - |
| Study | N | 5 y DSS | 5 y DFS | Risk Factors for Disease Progression | Treatment | WRCs |
|---|---|---|---|---|---|---|
| Fujiwara et al., 2019 [7] | 48 | 88% | 46% | RM, aRT, tumor size | Surgical ± aRT | - |
| Ji T et al., 2017 [12] | 115 | 81% | 52% | RM, recurrent tumor | Surgical ± aRT | - |
| Schwab et al., 2009 [13] | 42 | 77% | 56% | Prior and intralesional resections | Surgical ± aRT | 45% |
| Radaelli et al., 2016 [15] | 99 | 92% | 62% | RM, tumor size | Surgical ± aRT | - |
| Van Wulfften et al., 2018 [17] | 101 | 65–79% | 64–86% | aRT, tumor size | Surgical ± aRT | 40% |
| Fuchs et al., 2005 [18] | 52 | 74% | 59% | RM, age | Surgical ± aRT | 33% |
| Houdek et al., 2019 [19] | 193 | 75% | 71% | RM, RT dose, tumor size, excision level | Surgical ± aRT | 32% |
| Kayani et al., 2015 [22] | 58 | 62% | 52% | RM, tumor size, differentiation, aRT, infiltration of sacroiliac joint/muscles | Surgical ± aRT | 17% |
| Varga et al., 2015 [24] | 167 | ≈70% | ≈65% | Motor deficit, age, RM, recurrent tumor | Surgical ± aRT | - |
| Current study | 27 | 75% | 54% | RM | Surgical ± aRT | 66.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goumenos, S.; Kakouratos, G.; Trikoupis, I.; Gavriil, P.; Gerasimidis, P.; Soultanis, K.; Patapis, P.; Kontogeorgakos, V.; Papagelopoulos, P. Clinical Outcome after Surgical Treatment of Sacral Chordomas: A Single-Center Retrospective Cohort of 27 Patients. Cancers 2024, 16, 973. https://doi.org/10.3390/cancers16050973
Goumenos S, Kakouratos G, Trikoupis I, Gavriil P, Gerasimidis P, Soultanis K, Patapis P, Kontogeorgakos V, Papagelopoulos P. Clinical Outcome after Surgical Treatment of Sacral Chordomas: A Single-Center Retrospective Cohort of 27 Patients. Cancers. 2024; 16(5):973. https://doi.org/10.3390/cancers16050973
Chicago/Turabian StyleGoumenos, Stavros, Georgios Kakouratos, Ioannis Trikoupis, Panagiotis Gavriil, Pavlos Gerasimidis, Konstantinos Soultanis, Pavlos Patapis, Vasileios Kontogeorgakos, and Panayiotis Papagelopoulos. 2024. "Clinical Outcome after Surgical Treatment of Sacral Chordomas: A Single-Center Retrospective Cohort of 27 Patients" Cancers 16, no. 5: 973. https://doi.org/10.3390/cancers16050973
APA StyleGoumenos, S., Kakouratos, G., Trikoupis, I., Gavriil, P., Gerasimidis, P., Soultanis, K., Patapis, P., Kontogeorgakos, V., & Papagelopoulos, P. (2024). Clinical Outcome after Surgical Treatment of Sacral Chordomas: A Single-Center Retrospective Cohort of 27 Patients. Cancers, 16(5), 973. https://doi.org/10.3390/cancers16050973







