Contemporary Surgical Management of Colorectal Liver Metastases
Abstract
Simple Summary
Abstract
1. Introduction
History of Liver Resection and the Goals of Resection
2. Perioperative Considerations
2.1. The Role of Early Surgical Evaluation
2.2. Patient Selection and Factors Influencing Outcome
2.2.1. Anatomic Factors
2.2.2. Tumor Biology Factors
| Anatomic | Clinicopathologic | Genomic |
|---|---|---|
| Tumor relation to vessels and bile ducts [31] Maintaining two contiguous segments [31] Vascular inflow, outflow, and biliary drainage [31] Adequate FLR [35,36] Ability to achieve an R0 resection | Disease-free interval [34] Primary tumor nodal status [34] Primary tumor sidedness [37,38] Number of lesions [34] Diameter of maximum lesion [34] CEA level [34] | KRAS [39,40,41] BRAF [42] MMR [43] SMAD-4 [44] FBXW7 [45] TP53-RAS [46,47] |
2.2.3. Genomic Factors
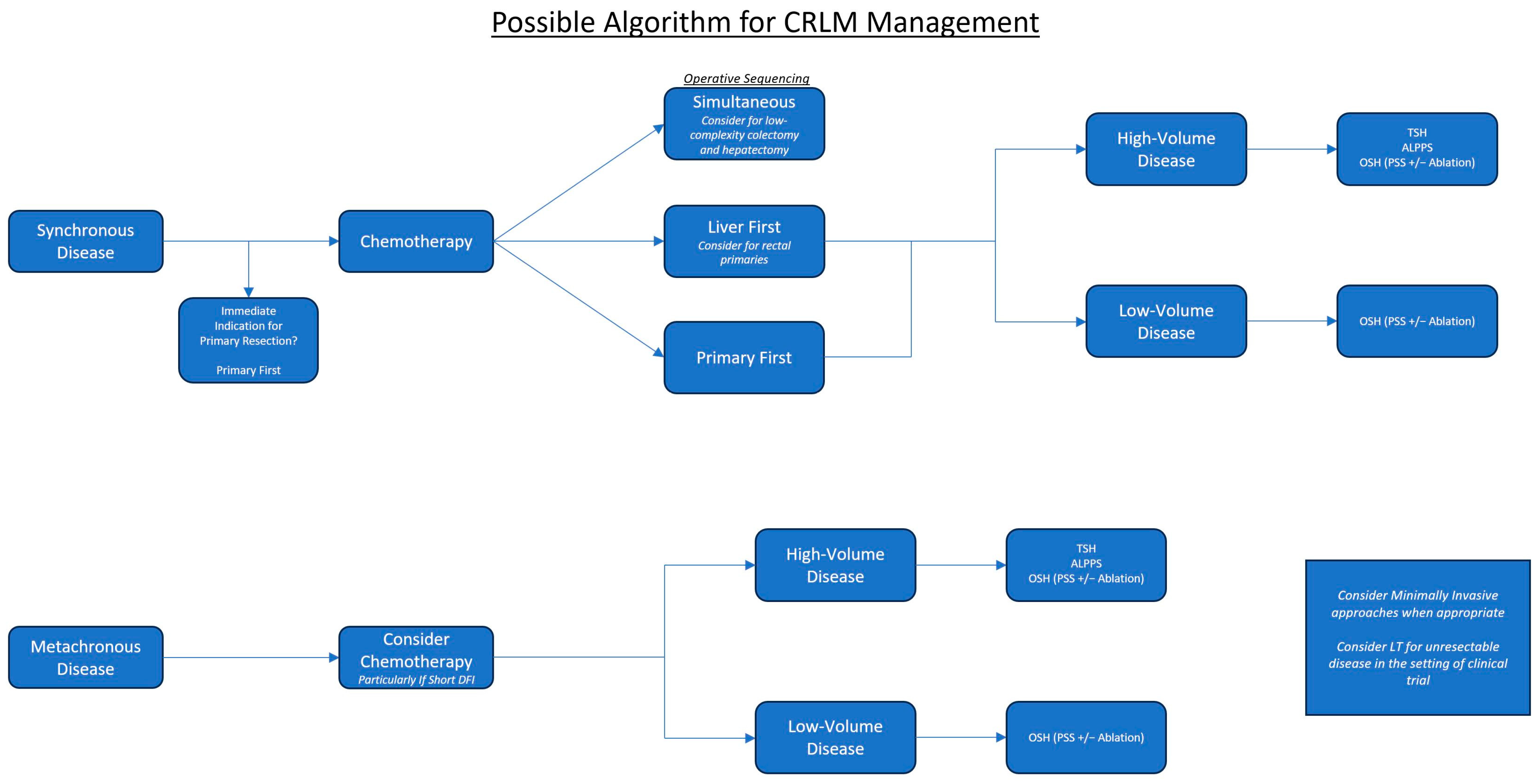
2.3. Operative Sequencing for Synchronous Disease
2.3.1. The Primary-First Approach
2.3.2. The Liver-First Approach
2.3.3. The Simultaneous Approach
2.4. Chemotherapy Sequencing
2.4.1. Adjuvant Therapy
2.4.2. Perioperative Therapy
2.5. Implications of Chemotherapy-Associated Hepatotoxicity
2.5.1. Specific Regimens and Associated Toxicities
2.5.2. Duration of Therapy
2.5.3. Protection against Toxicity
2.6. Disappearing Liver Metastasis
2.6.1. Risk Factors for Developing DLMs
2.6.2. Imaging Studies of CRLM
2.6.3. Contrast-Enhanced CT
2.6.4. Liver MRI
2.6.5. The Role of Intraoperative Imaging
2.6.6. Factors Associated with Complete Pathologic Response
2.6.7. Management Strategies and Outcomes
2.7. Hepatic Arterial Infusion Pump: Unresectable Disease and Conversion Therapy
2.7.1. Conceptual Basis
2.7.2. Trial Data
2.7.3. Biliary Sclerosis
2.7.4. Adoption
3. Operative Considerations
3.1. Portal Vein Embolization, Liver Venous Depletion, and Functional Assessment
3.1.1. Portal Vein Embolization History and Volumetric Requirements
3.1.2. FLR Function Assessment
3.1.3. Liver Venous Depletion
3.1.4. PVE Considerations
3.2. Resection Margin
3.2.1. Historical Perspective of the 1 mm Margin
3.2.2. Vascular R1 versus Parenchymal R1
3.2.3. The Effect of Margin Stratified by Other Prognostic Factors
3.3. The Two-Stage Hepatectomy: A Method to Address Bilobar Disease
3.3.1. Description and Historical Perspectives
3.3.2. Oncologic Outcomes and Failure to Progress
3.4. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy
3.4.1. Description and Early Perioperative Morbidity
3.4.2. Two-Stage Hepatectomy vs. ALPPS
3.5. Parenchymal-Sparing Surgery
Post-Hepatectomy Recurrence following PSS
3.6. Minimally Invasive Liver Resection
3.6.1. Laparoscopic Liver Resections
3.6.2. Robotic Liver Resections
3.7. Ablation
3.7.1. Microwave Ablation and Radiofrequency Ablation
3.7.2. Safety and Efficacy of Ablative Therapy
3.7.3. Limitations
3.8. Liver Transplantation
3.8.1. History of Liver Transplant for Cancer
3.8.2. SECA-I
3.8.3. SECA-II
3.8.4. Interpretation of the Data
3.9. Literature Research Strategy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A.-M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef]
- Tong, G.-J.; Zhang, G.-Y.; Liu, J.; Zheng, Z.-Z.; Chen, Y.; Niu, P.-P.; Xu, X.-T. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J. Clin. Oncol. 2018, 9, 148–161. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.S.; Van Heerden, J.A.; Adson, M.H.; Ilstrup, D.M. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann. Surg. 1984, 199, 502–508. [Google Scholar] [CrossRef]
- Thirion, P.; Wolmark, N.; Haddad, E.; Buyse, M.; Piedbois, P. Survival impact of chemotherapy in patients with colorectal metastases confined to the liver: A re-analysis of 1458 non-operable patients randomised in 22 trials and 4 meta-analyses. Meta-Analysis Group in Cancer. Ann. Oncol. 1999, 10, 1317–1320. [Google Scholar] [CrossRef]
- Scheithauer, W.; Rosen, H.; Kornek, G.V.; Sebesta, C.; Depisch, D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993, 306, 752–755. [Google Scholar] [CrossRef]
- Foster, J.H. Survival after liver resection for secondary tumors. Am. J. Surg. 1978, 135, 389–394. [Google Scholar] [CrossRef]
- Adam, R.; Vinet, E. Regional treatment of metastasis: Surgery of colorectal liver metastases. Ann. Oncol. 2004, 15 (Suppl. S4), iv103–iv106. [Google Scholar] [CrossRef] [PubMed]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Sandström, P.; Røsok, B.I.; Sparrelid, E.; Larsen, P.N.; Larsson, A.L.; Lindell, G.; Schultz, N.A.; Bjørnbeth, B.A.; Isaksson, B.; Rizell, M.; et al. ALPPS Improves Resectability Compared with Conventional Two-stage Hepatectomy in Patients with Advanced Colorectal Liver Metastasis: Results from a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann. Surg. 2018, 267, 833–840. [Google Scholar] [CrossRef]
- Ratti, F.; Soldati, C.; Catena, M.; Paganelli, M.; Ferla, G.; Aldrighetti, L. Role of portal vein embolization in liver surgery: Single centre experience in sixty-two patients. Updates Surg. 2010, 62, 153–159. [Google Scholar] [CrossRef]
- Agarwal, P.D.; Phillips, P.; Hillman, L.; Lucey, M.R.; Lee, F.; Mezrich, J.D.; Said, A. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J. Clin. Gastroenterol. 2017, 51, 845–849. [Google Scholar] [CrossRef]
- Hellingman, T.; de Swart, M.; Joosten, J.; Meijerink, M.; de Vries, J.; de Waard, J.; van Zweeden, A.; Zonderhuis, B.; Kazemier, G. The value of a dedicated multidisciplinary expert panel to assess treatment strategy in patients suffering from colorectal cancer liver metastases. Surg. Oncol. 2020, 35, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Oxenberg, J.; Papenfuss, W.; Esemuede, I.; Attwood, K.; Simunovic, M.; Kuvshinoff, B.; Francescutti, V. Multidisciplinary cancer conferences for gastrointestinal malignancies result in measureable treatment changes: A prospective study of 149 consecutive patients. Ann. Surg. Oncol. 2015, 22, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Keen, W.W. Report of a Case of Resection of the Liver for the Removal of a Neoplasm, with a Table of Seventy-six Cases of Resection of the Liver for Hepatic Tumors. Ann. Surg. 1899, 30, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Trimble, I.R.; McGoon, D.C. Resection of primary tumor of colon three years after excision of metastatic tumor. J. Am. Med. Assoc. 1951, 147, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K. Liver Cancer Study Group of Japan Primary liver cancers in Japan. Cancer 1980, 45, 2663–2669. [Google Scholar] [CrossRef]
- Couinaud, C. Le Foie: Études Anatomiques et Chirurgicales; Masson: Paris, France, 1957. [Google Scholar]
- Man, K.; Fan, S.-T.; Ng, I.O.L.; Lo, C.-M.; Liu, C.-L.; Wong, J. Prospective Evaluation of Pringle Maneuver in Hepatectomy for Liver Tumors by a Randomized Study. Ann. Surg. 1997, 226, 704–713. [Google Scholar] [CrossRef] [PubMed]
- A Melendez, J.; Arslan, V.; E Fischer, M.; Wuest, D.; Jarnagin, W.R.; Fong, Y.; Blumgart, L.H. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: Blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J. Am. Coll. Surg. 1998, 187, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Takemura, N.; Ito, K.; Mihara, F. The history of liver surgery: Achievements over the past 50 years. Ann. Gastroenterol. Surg. 2020, 4, 109–117. [Google Scholar] [CrossRef]
- Ribero, D.; Wang, H.; Donadon, M.; Zorzi, D.; Thomas, M.B.; Eng, C.; Chang, D.Z.; Curley, S.A.; Abdalla, E.K.; Ellis, L.M.; et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007, 110, 2761–2767. [Google Scholar] [CrossRef]
- Sato, M.; Tateishi, R.; Yasunaga, H.; Horiguchi, H.; Yoshida, H.; Matsuda, S.; Koike, K. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: A national survey of 54,145 patients. J. Gastroenterol. 2012, 47, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, W.M.; Martel, G.; Mimeault, R.; Fairfull-Smith, R.J.; Auer, R.C.; Balaa, F.K. Evaluating agreement regarding the resectability of colorectal liver metastases: A national case-based survey of hepatic surgeons. HPB 2012, 14, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Krell, R.W.; Reames, B.N.; Hendren, S.; Frankel, T.L.; Pawlik, T.M.; Chung, M.; Kwon, D.; Wong, S.L. Surgical Referral for Colorectal Liver Metastases: A Population-Based Survey. Ann. Surg. Oncol. 2015, 22, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Homayounfar, K.; Bleckmann, A.; Helms, H.; Lordick, F.; Rüschoff, J.; Conradi, L.; Sprenger, T.; Ghadimi, M.; Liersch, T. Discrepancies between medical oncologists and surgeons in assessment of resectability and indication for chemotherapy in patients with colorectal liver metastases. Br. J. Surg. 2014, 101, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Choti, M.A.; Thomas, M.; Wong, S.L.; Eaddy, M.; Pawlik, T.M.; Hirose, K.; Weiss, M.J.; Kish, J.; Green, M.R. Surgical Resection Preferences and Perceptions among Medical Oncologists Treating Liver Metastases from Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Vauthey, J.; Adam, R.; Rees, M.; Berry, D.; Jackson, R.; Grimes, N.; Fenwick, S.W.; Poston, G.J.; Malik, H.Z. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br. J. Surg. 2012, 99, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Meguid, C.; Schulick, R.D.; Schefter, T.E.; Lieu, C.H.; Boniface, M.; Williams, N.; Vogel, J.D.; Gajdos, C.; McCarter, M.; Edil, B.H. The multidisciplinary approach to gi cancer results in change of diagnosis and management of patients. Multidisciplinary care impacts diagnosis and management of patients. Ann. Surg. Oncol. 2016, 23, 3986–3990. [Google Scholar] [CrossRef] [PubMed]
- Charnsangavej, C.; Clary, B.; Fong, Y.; Grothey, A.; Pawlik, T.M.; Choti, M.A. Selection of patients for resection of hepatic colorectal metastases: Expert consensus statement. Ann. Surg. Oncol. 2006, 13, 1261–1268. [Google Scholar] [CrossRef]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Dvorchik, I.; Madariaga, J.R.; Marsh, J.W.; Dodson, F.; Bonham, A.C.; Geller, D.A.; Gayowski, T.J.; Fung, J.J.; Starzl, T.E. Hepatic resection for metastatic colorectal adenocarcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 1999, 189, 291–299. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Adam, R.; Bilchik, A.J.; Jaeck, D.; Vauthey, J.-N.; Mahvi, D. Improving resectability of hepatic colorectal metastases: Expert consensus statement. Ann. Surg. Oncol. 2006, 13, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Tzeng, C.-W.D.; Aloia, T.A.; Curley, S.A.; Zimmitti, G.; Wei, S.H.; Huang, S.Y.; Mahvash, A.; Gupta, S.; Wallace, M.J.; et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann. Surg. Oncol. 2013, 20, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Gasser, E.; Braunwarth, E.; Riedmann, M.; Cardini, B.; Fadinger, N.; Presl, J.; Klieser, E.; Ellmerer, P.; Dupré, A.; Imai, K.; et al. Primary tumour location affects survival after resection of colorectal liver metastases: A two-institutional cohort study with international validation, systematic meta-analysis and a clinical risk score. PLoS ONE 2019, 14, e0217411. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.-W.; Wang, K.; Xing, B.-C. The primary tumor location impacts survival outcome of colorectal liver metastases after hepatic resection: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 1349–1356. [Google Scholar] [CrossRef]
- Margonis, G.A.; Kim, Y.; Spolverato, G.; Ejaz, A.; Gupta, R.; Cosgrove, D.; Anders, R.; Karagkounis, G.; Choti, M.A.; Pawlik, T.M. Association Between Specific Mutations inKRASCodon 12 and Colorectal Liver Metastasis. JAMA Surg. 2015, 150, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Velasco, J.D.; Arvide, E.M.; Wei, S.H.; Vauthey, J.-N. Interactions of multiple gene alterations in colorectal liver metastases. Chin. Clin. Oncol. 2019, 8, 50. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Zimmitti, G.; Kopetz, S.E.; Shindoh, J.; Chen, S.S.; Andreou, A.; Curley, S.A.; Aloia, T.A.; Maru, D.M. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann. Surg. 2013, 258, 619–626; discussion 626–627. [Google Scholar] [CrossRef]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Kim, Y.; Wagner, D.; Sasaki, K.; Beer, A.; Schwarz, C.; Løes, I.M.; Smolle, M.; et al. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver cancer. JAMA Surg. 2018, 153, e180996. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kopetz, S.; Newhook, T.E.; De Bellis, M.; Chun, Y.S.; Tzeng, C.-W.D.; Aloia, T.A.; Vauthey, J.-N. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin. Cancer Res. 2019, 25, 5843–5851. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Newhook, T.E.; Cao, H.S.T.; Tzeng, C.-W.D.; Chun, Y.S.; Aloia, T.A.; Dasari, A.; Kopetz, S.; Vauthey, J.-N. Alteration of FBXW7 is Associated with Worse Survival in Patients Undergoing Resection of Colorectal Liver Metastases. J. Gastrointest. Surg. 2021, 25, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Lillemoe, H.A.; Panettieri, E.; Chun, Y.S.; Tzeng, C.-W.D.; Aloia, T.A.; Kopetz, S.; Vauthey, J.-N. Conditional Recurrence-Free Survival after Resection of Colorectal Liver Metastases: Persistent Deleterious Association with RAS and TP53 Co-Mutation. J. Am. Coll. Surg. 2019, 229, 286–294.e1. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Passot, G.; Yamashita, S.; Nusrat, M.; Katsonis, P.; Loree, J.M.; Conrad, C.; Tzeng, C.-W.D.; Xiao, L.; Aloia, T.A.; et al. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Ann. Surg. 2019, 269, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Ricard, I.; Heinemann, V.; Hegewisch-Becker, S.; Schmiegel, W.; Porschen, R.; Stintzing, S.; Graeven, U.; Arnold, D.; von Weikersthal, L.F.; et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann. Oncol. 2016, 27, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kopetz, S.; Kwong, L.; Xiao, L.; Morris, J.S.; Cao, H.S.T.; Tzeng, C.-W.D.; Chun, Y.S.; Lee, J.E.; Vauthey, J.-N. Genomic Sequencing and Insight into Clinical Heterogeneity and Prognostic Pathway Genes in Patients with Metastatic Colorectal Cancer. J. Am. Coll. Surg. 2021, 233, 272–284.e13. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kopetz, S.; Lillemoe, H.A.; Hwang, H.; Wang, X.; Tzeng, C.-W.D.; Chun, Y.S.; Aloia, T.A.; Vauthey, J.-N. A new surveillance algorithm after resection of colorectal liver metastases based on changes in recurrence risk and RAS mutation status. J. Natl. Compr. Cancer Netw. 2020, 18, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Jones, R.P.; Giuliante, F.; Shindoh, J.; Passot, G.; Chung, M.H.; Song, J.; Li, L.; Dagenborg, V.J.; Fretland, Å.A.; et al. RAS mutation clinical risk score to predict survival after resection of colorectal liver metastases. Ann. Surg. 2019, 269, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Maki, H.; Ayabe, R.I.; Nishioka, Y.; Konishi, T.; Newhook, T.E.; Cao, H.S.T.; Chun, Y.S.; Tzeng, C.-W.D.; You, Y.N.; Vauthey, J.-N. Hepatectomy Before Primary Tumor Resection as Preferred Approach for Synchronous Liver Metastases from Rectal Cancer. Ann. Surg. Oncol. 2023, 30, 5390–5400. [Google Scholar] [CrossRef] [PubMed]
- Mentha, G.; E Majno, P.; Andres, A.; Rubbia-Brandt, L.; Morel, P.; Roth, A.D. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br. J. Surg. 2006, 93, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Toso, C.; Adam, R.; Barroso, E.; Hubert, C.; Capussotti, L.; Gerstel, E.; Roth, A.; Majno, P.E.; Mentha, G. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases: A LiverMetSurvey-based study. Ann. Surg. 2012, 256, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Shubert, C.R.; Habermann, E.B.; Bergquist, J.R.; Thiels, C.A.; Thomsen, K.M.; Kremers, W.K.; Kendrick, M.L.; Cima, R.R.; Nagorney, D.M. A NSQIP review of major morbidity and mortality of synchronous liver resection for colorectal metastasis stratified by extent of liver resection and type of colorectal resection. J. Gastrointest. Surg. 2015, 19, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Telem, D.A.; Chin, E.H.; Nguyen, S.Q.; Divino, C.M. Risk factors for anastomotic leak following colorectal surgery: A case-control study. Arch. Surg. 2010, 145, 371–376; discussion 376. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.; Ludwig, K.A.; Ridolfi, T.J. Low anterior resection syndrome: Current management and future directions. Clin. Colon Rectal Surg. 2016, 29, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Helling, T.S.; Martin, M. Cause of death from liver metastases in colorectal cancer. Ann. Surg. Oncol. 2014, 21, 501–506. [Google Scholar] [CrossRef]
- De Ridder, J.A.; van der Stok, E.P.; Mekenkamp, L.J.; Wiering, B.; Koopman, M.; Punt, C.J.; Verhoef, C.; de Wilt, J.H. Management of liver metastases in colorectal cancer patients: A retrospective case-control study of systemic therapy versus liver resection. Eur. J. Cancer 2016, 59, 13–21. [Google Scholar] [CrossRef]
- Rectal Cancer Version 05.2023, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 2 November 2023).
- Smith, J.J.; Strombom, P.; Chow, O.S.; Roxburgh, C.S.; Lynn, P.; Eaton, A.; Widmar, M.; Ganesh, K.; Yaeger, R.; Cercek, A.; et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients with a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019, 5, e185896. [Google Scholar] [CrossRef]
- Siriwardena, A.K.; Serrablo, A.; Fretland, A.; Wigmore, S.J.; Ramia-Angel, J.M.; Malik, H.Z.; Stättner, S.; Søreide, K.; Zmora, O.; Meijerink, M.; et al. Multisocietal European consensus on the terminology, diagnosis, and management of patients with synchronous colorectal cancer and liver metastases: An E-AHPBA consensus in partnership with ESSO, ESCP, ESGAR, and CIRSE. Br. J. Surg. 2023, 110, 1161–1170. [Google Scholar] [CrossRef]
- Wang, S.-H.; Song, L.; Tang, J.-Y.; Sun, W.-P.; Li, Z. Safety and long-term prognosis of simultaneous versus staged resection in synchronous colorectal cancer with liver metastasis: A systematic review and meta-analysis. Eur. J. Med. Res. 2022, 27, 297. [Google Scholar] [CrossRef]
- Snyder, R.A.; Hao, S.; Irish, W.; Zervos, E.E.; Tuttle-Newhall, J.E.; Parikh, A.A. Thirty-Day Morbidity after Simultaneous Resection of Colorectal Cancer and Colorectal Liver Metastasis: American College of Surgeons NSQIP Analysis. J. Am. Coll. Surg. 2020, 230, 617–627.e9. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sahara, K.; Hyer, J.M.; Diaz, A.; Moris, D.; Bagante, F.; Guglielmi, A.; Ruzzenente, A.; Alexandrescu, S.; Poultsides, G.; et al. Trends and outcomes of simultaneous versus staged resection of synchronous colorectal cancer and colorectal liver metastases. Surgery 2021, 170, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Alaimo, L.; Moazzam, Z.; Woldesenbet, S.; Lima, H.A.; Munir, M.M.; Shaikh, C.F.; Yang, J.; Azap, L.; Katayama, E.; et al. Postoperative morbidity after simultaneous versus staged resection of synchronous colorectal liver metastases: Impact of hepatic tumor burden. Surgery 2024, 175, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “Metro-ticket” Prognostic Tool for Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Kelly, M.; Spolverato, G.; Lê, G.; Mavros, M.; Doyle, F.; Pawlik, T.; Winter, D. Synchronous colorectal liver metastasis: A network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J. Surg. Oncol. 2015, 111, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, K.; Locher, C.; Sabbagh, C.; Ortega-Deballon, P.; Heyd, B.; Bachellier, P.; Métairie, S.; Paye, F.; Bourlier, P.; Adam, R.; et al. Simultaneous Versus Delayed Resection for Initially Resectable Synchronous Colorectal Cancer Liver Metastases: A Prospective, Open-label, Randomized, Controlled Trial. Ann. Surg. 2021, 273, 49–56. [Google Scholar] [CrossRef]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Liu, J.; Xia, Y.; Pan, X.; Yan, Z.; Zhang, L.; Yang, Z.; Wu, Y.; Xue, H.; Bai, S.; Shen, F.; et al. Simultaneous versus staged major hepatectomy (≥3 liver segments) for outcomes of synchronous colorectal liver metastases: A systematic review and meta-analysis. Cancer Rep. 2022, 5, e1617. [Google Scholar] [CrossRef]
- Qadan, M.; D’angelica, M.I. Complex Surgical Strategies to Improve Resectability in Borderline-Resectable Disease. Curr. Color. Cancer Rep. 2015, 11, 369–377. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Pawlik, T.M.; Ribero, D.; Wu, T.-T.; Zorzi, D.; Hoff, P.M.; Xiong, H.Q.; Eng, C.; Lauwers, G.Y.; Mino-Kenudson, M.; et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J. Clin. Oncol. 2006, 24, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Portier, G.; Elias, D.; Bouche, O.; Rougier, P.; Bosset, J.-F.; Saric, J.; Belghiti, J.; Piedbois, P.; Guimbaud, R.; Nordlinger, B.; et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J. Clin. Oncol. 2006, 24, 4976–4982. [Google Scholar] [CrossRef]
- Langer, B.; Bleiberg, H.; Labianca, R.; Shepherd, L. Fluorouracil (FU) plus I-leucovorin (I-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): Results of the ENG (EORTC/NCIC CTG GIVIO) randomized trial. In Proceedings of the ASCO, Orlando, FL, USA, 18–21 May 2002; p. 149a. [Google Scholar]
- Mitry, E.; Fields, A.L.; Bleiberg, H.; Labianca, R.; Portier, G.; Tu, D.; Nitti, D.; Torri, V.; Elias, D.; O’Callaghan, C.; et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. J. Clin. Oncol. 2008, 26, 4906–4911. [Google Scholar] [CrossRef]
- Hasegawa, K.; Saiura, A.; Takayama, T.; Miyagawa, S.; Yamamoto, J.; Ijichi, M.; Teruya, M.; Yoshimi, F.; Kawasaki, S.; Koyama, H.; et al. Adjuvant Oral Uracil-Tegafur with Leucovorin for Colorectal Cancer Liver Metastases: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0162400. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Hohenberger, W.; Thezenas, S.; Navarro, M.; Maurel, J.; Bokemeyer, C.; Shacham-Shmueli, E.; Rivera, F.; Choi, C.K.-K.; Santoro, A. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann. Oncol. 2009, 20, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Shimizu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 3789–3799. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of metastatic colorectal cancer: ASCO guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601–611. [Google Scholar] [CrossRef] [PubMed]
- A Bridgewater, J.; A Pugh, S.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; O Griffiths, G.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- E de Meijer, V.; Kalish, B.T.; Puder, M.; Ijzermans, J.N.M. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br. J. Surg. 2010, 97, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Wilson, C.H.; Burt, A.D.; Manas, D.M.; White, S.A. Chemotherapy-associated liver injury in patients with colorectal liver metastases: A systematic review and meta-analysis. Ann. Surg. Oncol. 2012, 19, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Knoedler, D.M.; Saeian, K.; Wallace, J.R.; Komorowski, R.A. Effects of interventions on intra- and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann. Diagn. Pathol. 2011, 15, 19–24. [Google Scholar] [CrossRef]
- El-Badry, A.M.M.B.; Breitenstein, S.; Jochum, W.; Washington, K.; Paradis, V.; Rubbia-Brandt, L.; Puhan, M.A.; Slankamenac, K.; Graf, R.; Clavien, P.-A. Assessment of hepatic steatosis by expert pathologists: The end of a gold standard. Ann. Surg. 2009, 250, 691–697. [Google Scholar] [CrossRef]
- Ryan, P.; Nanji, S.; Pollett, A.; Moore, M.; Moulton, C.-A.; Gallinger, S.; Guindi, M. Chemotherapy-induced liver injury in metastatic colorectal cancer: Semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am. J. Surg. Pathol. 2010, 34, 784–791. [Google Scholar] [CrossRef]
- Vigano, L.; De Rosa, G.; Toso, C.; Andres, A.; Ferrero, A.; Roth, A.; Sperti, E.; Majno, P.; Rubbia-Brandt, L. Reversibility of chemotherapy-related liver injury. J. Hepatol. 2017, 67, 84–91. [Google Scholar] [CrossRef]
- Nakano, H.; Oussoultzoglou, E.; Rosso, E.; Casnedi, S.; Chenard-Neu, M.-P.; Dufour, P.; Bachellier, P.; Jaeck, D.M. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann. Surg. 2008, 247, 118–124. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Zhao, J.; Bi, X.; Li, Z.; Huang, Z.; Zhang, Y.; Zhou, J.; Zhao, H.; Cai, J. What is the optimal number of neoadjuvant chemotherapy cycles for resectable colorectal liver oligometastases? Ann. Transl. Med. 2021, 9, 7. [Google Scholar] [CrossRef]
- White, R.R.; Schwartz, L.H.; Munoz, J.A.; Raggio, G.; Jarnagin, W.R.; Fong, Y.; D’Angelica, M.I.; Kemeny, N.E. Assessing the optimal duration of chemotherapy in patients with colorectal liver metastases. J. Surg. Oncol. 2008, 97, 601–604. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Lauwers, G.Y.; Wang, H.; E Majno, P.; Tanabe, K.; Zhu, A.X.; Brezault, C.; Soubrane, O.; Abdalla, E.K.; Vauthey, J.; et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology 2010, 56, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.; Eipeldauer, S.; Hacker, S.; Herberger, B.; Tamandl, D.; Dorfmeister, M.; Koelblinger, C.; Gruenberger, B.; Gruenberger, T. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur. J. Surg. Oncol. 2009, 35, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Van Vledder, M.G.; de Jong, M.C.; Pawlik, T.M.; Schulick, R.D.; Diaz, L.A.; Choti, M.A. Disappearing colorectal liver metastases after chemotherapy: Should we be concerned? J. Gastrointest. Surg. 2010, 14, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Tani, K.; Shindoh, J.; Akamatsu, N.; Arita, J.; Kaneko, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Management of disappearing lesions after chemotherapy for colorectal liver metastases: Relation between detectability and residual tumors. J. Surg. Oncol. 2018, 117, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.W.; Fowler, K.J.; Doyle, M.B.; Saad, N.E.; Linehan, D.C.; Chapman, W.C. Colorectal liver metastases: Disappearing lesions in the era of Eovist hepatobiliary magnetic resonance imaging. HPB 2016, 18, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Niekel, M.C.; Bipat, S.; Stoker, J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: A meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010, 257, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Carnaghi, C.; Tronconi, M.C.; Rimassa, L.; Tondulli, L.; Zuradelli, M.; Rodari, M.; Doci, R.; Luttmann, F.; Torzilli, G.; Rubello, D.; et al. Utility of 18F-FDG PET and contrast-enhanced CT scan in the assessment of residual liver metastasis from colorectal cancer following adjuvant chemotherapy. Nucl. Med. Rev. Cent. East Eur. 2007, 10, 12–15. [Google Scholar]
- Choi, S.H.; Kim, S.Y.; Park, S.H.; Kim, K.W.; Lee, J.Y.; Lee, S.S.; Lee, M. Diagnostic performance of CT, gadoxetate disodium-enhanced MRI, and PET/CT for the diagnosis of colorectal liver metastasis: Systematic review and meta-analysis. J. Magn. Reson. Imaging 2018, 47, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, C.S.; Buckens, C.F.M.; van den Bosch, M.A.A.J.; van Leeuwen, M.S.; van Hillegersberg, R.; Verkooijen, H.M. Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: A meta-analysis. Ann. Surg. Oncol. 2012, 19, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Angliviel, B.; Benoist, S.; Penna, C.; El Hajjam, M.; Chagnon, S.; Julié, C.; Beauchet, A.; Rougier, P.; Nordlinger, B. Impact of chemotherapy on the accuracy of computed tomography scan for the evaluation of colorectal liver metastases. Ann. Surg. Oncol. 2009, 16, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takakura, H.; Takeda, K.; Matsuo, K.; Nagano, Y.; Endo, I. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann. Surg. 2009, 250, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Langella, S.; Russolillo, N.; Vigano’, L.; Tesoriere, R.L.; Capussotti, L. Intraoperative detection of disappearing colorectal liver metastases as a predictor of residual disease. J. Gastrointest. Surg. 2012, 16, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Sturesson, C.; Nilsson, J.; Lindell, G.; Andersson, R.G.; Keussen, I. Disappearing liver metastases from colorectal cancer: Impact of modern imaging modalities. HPB 2015, 17, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Macera, A.; Lario, C.; Petracchini, M.; Gallo, T.; Regge, D.; Floriani, I.; Ribero, D.; Capussotti, L.; Cirillo, S. Staging of colorectal liver metastases after preoperative chemotherapy. Diffusion-weighted imaging in combination with Gd-EOB-DTPA MRI sequences increases sensitivity and diagnostic accuracy. Eur. Radiol. 2013, 23, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Arita, J.; Ono, Y.; Takahashi, M.; Inoue, Y.; Takahashi, Y.; Saiura, A. Usefulness of contrast-enhanced intraoperative ultrasound in identifying disappearing liver metastases from colorectal carcinoma after chemotherapy. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), S390–S397. [Google Scholar] [CrossRef]
- Arita, J.; Ono, Y.; Takahashi, M.; Inoue, Y.; Takahashi, Y.; Matsueda, K.; Saiura, A. Routine Preoperative Liver-specific Magnetic Resonance Imaging Does Not Exclude the Necessity of Contrast-enhanced Intraoperative Ultrasound in Hepatic Resection for Colorectal Liver Metastasis. Ann. Surg. 2015, 262, 1086–1091. [Google Scholar] [CrossRef]
- Auer, R.C.; White, R.R.; Kemeny, N.E.; Schwartz, L.H.; Shia, J.; Blumgart, L.H.; DeMatteo, R.P.; Fong, Y.; Jarnagin, W.R.; D’Angelica, M.I. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer 2010, 116, 1502–1509. [Google Scholar] [CrossRef]
- Song, K.D.; Kim, Y.K.; Kim, H.C.; Huh, J.W.; Park, Y.S.; Park, J.O.; Kim, S.T. Disappearing or residual tiny (≤5 mm) colorectal liver metastases after chemotherapy on gadoxetic acid-enhanced liver MRI and diffusion-weighted imaging: Is local treatment required? Eur. Radiol. 2017, 27, 3088–3096. [Google Scholar] [CrossRef]
- Muaddi, H.; Silva, S.; Choi, W.J.; Coburn, N.; Hallet, J.; Law, C.; Cheung, H.; Karanicolas, P.J. When is a Ghost Really Gone? A Systematic Review and Meta-analysis of the Accuracy of Imaging Modalities to Predict Complete Pathological Response of Colorectal Cancer Liver Metastases After Chemotherapy. Ann. Surg. Oncol. 2021, 28, 6805–6813. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.B.; Aloia, T.A.; Loyer, E.; Pawlik, T.M.; Taouli, B.; Vauthey, J.-N. Selection for hepatic resection of colorectal liver metastases: Expert consensus statement. HPB 2013, 15, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Oba, A.; Mise, Y.; Ito, H.; Hiratsuka, M.; Inoue, Y.; Ishizawa, T.; Arita, J.; Matsueda, K.; Takahashi, Y.; Saiura, A. Clinical implications of disappearing colorectal liver metastases have changed in the era of hepatocyte-specific MRI and contrast-enhanced intraoperative ultrasonography. HPB 2018, 20, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Melstrom, L.G.; Warner, S.G.; Wong, P.; Sun, V.; Raoof, M.; Singh, G.; Chavin, K.D.; Fong, Y.; Adam, R.; Hugh, T.J. Management of disappearing colorectal liver metastases: An international survey. HPB 2021, 23, 506–511. [Google Scholar] [CrossRef]
- Kuhlmann, K.; Tufo, A.; Kok, N.; Gordon-Weeks, A.; Poston, G.; Nieto, R.D.; Jones, R.; Fenwick, S.; Malik, H. Disappearing colorectal liver metastases in the era of state-of-the-art triple-modality diagnostic imaging. Eur. J. Surg. Oncol. 2023, 49, 1016–1022. [Google Scholar] [CrossRef]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.J.; Larson, D.W.; Grothey, A.; Vauthey, J.-N.; Nagorney, D.M.; McWilliams, R.R. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef]
- Breedis, C.; Young, G. The blood supply of neoplasms in the liver. Am. J. Pathol. 1954, 30, 969–977. [Google Scholar]
- Ensminger, W.D.; Rosowsky, A.; Raso, V.; Levin, D.C.; Glode, M.; Come, S.; Steele, G.; Frei, E. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2′-deoxyuridine and 5-fluorouracil. Cancer Res. 1978, 38, 3784–3792. [Google Scholar]
- D’Angelica, M.I.; Correa-Gallego, C.; Paty, P.B.; Cercek, A.; Gewirtz, A.N.B.; Chou, J.F.; Capanu, M.; Kingham, T.P.; Fong, Y.; DeMatteo, R.P.; et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: Conversion to resection and long-term outcomes. Ann. Surg. 2015, 261, 353–360. [Google Scholar] [CrossRef]
- Pak, L.M.; Kemeny, N.E.; Capanu, M.; Chou, J.F.; Boucher, T.; Cercek, A.; Balachandran, V.P.; Kingham, T.P.; Allen, P.J.; DeMatteo, R.P.; et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J. Surg. Oncol. 2018, 117, 634–643. [Google Scholar] [CrossRef]
- O’Leary, M.P.; Wang, C.; Sandhu, J.; Malhotra, G.K.; Thornblade, L.W.; Lau, C.; Melstrom, L.G.; Fong, Y.; Singh, G.; Fakih, M.G. Salvage regional therapy using hepatic artery infusion pump in unresectable chemotherapy resistant colorectal liver metastases. Am. J. Surg. 2022, 223, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Hohn, D.; Melnick, J.; Stagg, R.; Altman, D.; Friedman, M.; Ignoffo, R.; Ferrell, L.; Lewis, B. Biliary sclerosis in patients receiving hepatic arterial infusions of floxuridine. J. Clin. Oncol. 1985, 3, 98–102. [Google Scholar] [CrossRef]
- Ito, K.; Ito, H.; Kemeny, N.E.; Gonen, M.; Allen, P.J.; Paty, P.B.; Fong, Y.; DeMatteo, R.P.; Blumgart, L.H.; Jarnagin, W.R.; et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: Incidence, clinical features, and risk factors. Ann. Surg. Oncol. 2012, 19, 1609–1617. [Google Scholar] [CrossRef]
- Kemeny, N.; Seiter, K.; Niedzwiecki, D.; Chapman, D.; Sigurdson, E.; Cohen, A.; Botet, J.; Oderman, P.; Murray, P. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer 1992, 69, 327–334. [Google Scholar] [CrossRef]
- Elijah, J.; Schepers, A.J.; Krauss, J.C.; McDevitt, R.L. Evaluation of biliary toxicity in patients with hepatic artery infusion pumps. J. Oncol. Pharm. Pract. 2023, 29, 1915–1920. [Google Scholar] [CrossRef]
- Kemeny, M.M.; Battifora, H.; Blayney, D.W.; Cecchi, G.; Goldberg, D.A.; Leong, L.A.; Margolin, K.A.; Terz, J.J. Sclerosing cholangitis after continuous hepatic artery infusion of FUDR. Ann. Surg. 1985, 202, 176–181. [Google Scholar] [CrossRef]
- Zaidi, M.Y.; Nussbaum, D.P.; Hsu, S.D.; Strickler, J.H.; Uronis, H.E.; Zani, S.; Allen, P.J.; Lidsky, M.E. Hepatic artery infusion for unresectable colorectal cancer liver metastases: Palliation and conversion. Surgery 2023, 174, 428–430. [Google Scholar] [CrossRef]
- Takayasu, K.; Muramatsu, Y.; Shima, Y.; Moriyama, N.; Yamada, T.; Makuuchi, M. Hepatic lobar atrophy following obstruction of the ipsilateral portal vein from hilar cholangiocarcinoma. Radiology 1986, 160, 389–393. [Google Scholar] [CrossRef]
- Makuuchi, M.; Thai, B.L.; Takayasu, K.; Takayama, T.; Kosuge, T.; Gunvén, P.; Yamazaki, S.; Hasegawa, H.; Ozaki, H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: A preliminary report. Surgery 1990, 107, 521–527. [Google Scholar]
- Azoulay, D.; Castaing, D.; Krissat, J.; Smail, A.; Hargreaves, G.M.; Lemoine, A.; Emile, J.-F.; Bismuth, H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann. Surg. 2000, 232, 665–672. [Google Scholar] [CrossRef]
- Hemming, A.W.M.; Reed, A.I.; Howard, R.J.; Fujita, S.; Hochwald, S.N.; Caridi, J.G.; Hawkins, I.F.; Vauthey, J.-N. Preoperative portal vein embolization for extended hepatectomy. Ann. Surg. 2003, 237, 686–691, discussion 691. [Google Scholar] [CrossRef]
- De Graaf, W.; van Lienden, K.P.; Dinant, S.; Roelofs, J.J.T.H.; Busch, O.R.C.; Gouma, D.J.; Bennink, R.J.; van Gulik, T.M. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J. Gastrointest. Surg. 2010, 14, 369–378. [Google Scholar] [CrossRef]
- De Graaf, W.; van Lienden, K.P.; van Gulik, T.M.; Bennink, R.J. (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J. Nucl. Med. 2010, 51, 229–236. [Google Scholar] [CrossRef]
- Shindoh, J.; Truty, M.J.; Aloia, T.A.; Curley, S.A.; Zimmitti, G.; Huang, S.Y.; Mahvash, A.; Gupta, S.; Wallace, M.J.; Vauthey, J.-N. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: Toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J. Am. Coll. Surg. 2013, 216, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, B.; Gallon, A.; Cauchy, F.; Pereira, B.; Gagnière, J.; Lambert, C.; Yoh, T.; Boyer, L.; Pezet, D.; Buc, E.; et al. Combined biembolization induces higher hypertrophy than portal vein embolization before major liver resection. HPB 2020, 22, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Heil, J.; Korenblik, R.; Heid, F.; Detry, O.; Dili, A.; Metrakos, P.; Heise, D.; van der Leij, C.; van Dam, R.; Schadde, E. Increased Resectability after Simultaneous Portal and Hepatic Vein Embolization (PVE/HVE) Compared to PVE Alone in Patients with Small FLRs—A DRAGON GROUP Analysis. HPB 2021, 23, S715–S716. [Google Scholar] [CrossRef]
- Pamecha, V.; Levene, A.; Grillo, F.; Woodward, N.; Dhillon, A.; Davidson, B.R. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br. J. Cancer 2009, 100, 617–622. [Google Scholar] [CrossRef]
- Kokudo, N.; Tada, K.; Seki, M.; Ohta, H.; Azekura, K.; Ueno, M.; Ohta, K.; Yamaguchi, T.; Matsubara, T.; Takahashi, T.; et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology 2001, 34, 267–272. [Google Scholar] [CrossRef]
- Cady, B.; Jenkins, R.L.; Steele, G.D.J.; Lewis, W.D.; Stone, M.D.; McDermott, W.V.; Jessup, J.M.; Bothe, A.; Lalor, P.; Lovett, E.J.; et al. Surgical margin in hepatic resection for colorectal metastasis: A critical and improvable determinant of outcome. Ann. Surg. 1998, 227, 566–571. [Google Scholar] [CrossRef]
- Ekberg, H.; Tranberg, K.; Andersson, R.; Lundstedt, C.; Hägerstrand, I.; Ranstam, J.; Bengmark, S. Determinants of survival in liver resection for colorectal secondaries. Br. J. Surg. 1986, 73, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Steele, G.; Bleday, R.; Mayer, R.J.; Lindblad, A.; Petrelli, N.; Weaver, D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J. Clin. Oncol. 1991, 9, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, G.M.; Curley, S.A.; Hohn, D.C.; Roh, M.S. Improved survival after resection of colorectal liver metastases. Eur. J. Surg. Oncol. 1995, 21, 696. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann. Surg. 2005, 241, 715–722; discussion 722–724. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Miki, Y.; Sugai, S.; Yanagisawa, A.; Kato, Y.; Sakamoto, Y.; Yamamoto, J.; Yamaguchi, T.; Muto, T.; Makuuchi, M. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: Minimum surgical margins for successful resection. Arch. Surg. 2002, 137, 833–840. [Google Scholar] [CrossRef]
- Sadot, E.; Koerkamp, B.G.; Leal, J.N.; Shia, J.; Gonen, M.; Allen, P.J.; DeMatteo, R.P.; Kingham, T.P.; Kemeny, N.; Blumgart, L.H.; et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: Surgical technique or biologic surrogate? Ann. Surg. 2015, 262, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Margonis, G.A.; Amini, N.; Andreatos, N.; Yuan, C.; Damaskos, C.; Antoniou, E.; Garmpis, N.; Buettner, S.; Barbon, C.; et al. The Prognostic Value of Varying Definitions of Positive Resection Margin in Patients with Colorectal Cancer Liver Metastases. J. Gastrointest. Surg. 2018, 22, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- De Haas, R.J.; Wicherts, D.A.; Flores, E.; Azoulay, D.; Castaing, D.; Adam, R. R1 resection by necessity for colorectal liver metastases: Is it still a contraindication to surgery? Ann. Surg. 2008, 248, 626–637. [Google Scholar] [CrossRef]
- Viganò, L.; Procopio, F.; Cimino, M.M.; Donadon, M.; Gatti, A.; Costa, G.; Del Fabbro, D.; Torzilli, G. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann. Surg. Oncol. 2016, 23, 1352–1360. [Google Scholar] [CrossRef]
- Ayez, N.; Lalmahomed, Z.S.; Eggermont, A.M.M.; Ijzermans, J.N.M.; de Jonge, J.; van Montfort, K.; Verhoef, C. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann. Surg. Oncol. 2012, 19, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Adam, J.; Denost, Q.; Smith, D.; Saric, J.; Chiche, L. Significance of R1 resection for advanced colorectal liver metastases in the era of modern effective chemotherapy. Mol. Med. 2016, 40, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Aloia, T.A.M.; Brouquet, A.; Dickson, P.V.; Zimmitti, G.; Maru, D.M.; Kopetz, S.; Loyer, E.M.; Curley, S.A.M.; Abdalla, E.K.M.; et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann. Surg. 2013, 257, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Truant, S.; Séquier, C.; Leteurtre, E.; Boleslawski, E.; Elamrani, M.; Huet, G.; Duhamel, A.; Hebbar, M.; Pruvot, F. Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB 2015, 17, 176–184. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Brudvik, K.W.; Mise, Y.; Chung, M.H.; Chun, Y.S.; Kopetz, S.E.; Passot, G.; Conrad, C.; Maru, D.M.; Aloia, T.A.; Vauthey, J.-N. RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann. Surg. Oncol. 2016, 23, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Sasaki, K.; Ijzermans, J.N.M.; van Vugt, J.L.A.; Pawlik, T.M.; Choti, M.A.M.; Cameron, J.L.; He, J.; et al. Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann. Surg. 2017, 266, 641–649. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.-W.; Yan, X.-L.; Li, J.; Wang, K.; Xing, B.-C. Sub-millimeter surgical margin is acceptable in patients with good tumor biology after liver resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2019, 45, 1551–1558. [Google Scholar] [CrossRef]
- Bismuth, H.; Adam, R.; Lévi, F.; Farabos, C.; Waechter, F.; Castaing, D.; Majno, P.; Engerran, L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann. Surg. 1996, 224, 509–520; discussion 520. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Laurent, A.; Azoulay, D.; Castaing, D.; Bismuth, H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann. Surg. 2000, 232, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Kianmanesh, R.; Farges, O.; Abdalla, E.K.; Sauvanet, A.; Ruszniewski, P.; Belghiti, J. Right portal vein ligation: A new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J. Am. Coll. Surg. 2003, 197, 164–170. [Google Scholar] [CrossRef]
- Jaeck, D.; Oussoultzoglou, E.; Rosso, E.; Greget, M.; Weber, J.-C.; Bachellier, P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann. Surg. 2004, 240, 1037–1049; discussion 1049. [Google Scholar] [CrossRef]
- Wicherts, D.A.; Miller, R.; de Haas, R.J.; Bitsakou, G.; Vibert, E.; Veilhan, L.-A.; Azoulay, D.; Bismuth, H.; Castaing, D.; Adam, R. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann. Surg. 2008, 248, 994–1005. [Google Scholar] [CrossRef]
- Lam, V.W.; Laurence, J.M.; Johnston, E.; Hollands, M.J.; Pleass, H.C.; Richardson, A.J. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB 2013, 15, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.I.; Gholami, S.; Kim, B.J.; Margonis, G.A.; Ethun, C.G.; Tsai, S.; Christians, K.K.; Clarke, C.; Mogal, H.; Maithel, S.K.; et al. Two-Stage Hepatectomy for Bilateral Colorectal Liver Metastases: A Multi-institutional Analysis. Ann. Surg. Oncol. 2021, 28, 1457–1465. [Google Scholar] [CrossRef]
- Schlitt, H.J.; Hackl, C.; Lang, S.A. ‘In-Situ Split’ Liver Resection/ALPPS—Historical Development and Current Practice. Visc. Med. 2017, 33, 408–412. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Hörbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef]
- Schadde, E.; Ardiles, V.; Robles-Campos, R.; Malago, M.; Machado, M.; Hernandez-Alejandro, R.; Soubrane, O.; Schnitzbauer, A.A.; Raptis, D.; Tschuor, C.; et al. Early survival and safety of ALPPS: First report of the International ALPPS Registry. Ann. Surg. 2014, 260, 829–836; discussion 836. [Google Scholar] [CrossRef]
- Oldhafer, K.J.; Stavrou, G.A.; van Gulik, T.M. ALPPS—Where Do We Stand, Where Do We Go? Eight Recommendations from the First International Expert Meeting. Ann. Surg. 2016, 263, 839–841. [Google Scholar] [CrossRef]
- Linecker, M.; Björnsson, B.; Stavrou, G.A.; Oldhafer, K.J.; Lurje, G.; Neumann, U.; Adam, R.; Pruvot, F.-R.; Topp, S.A.; Li, J.; et al. Risk adjustment in ALPPS is associated with a dramatic decrease in early mortality and morbidity. Ann. Surg. 2017, 266, 779–786. [Google Scholar] [CrossRef]
- Petrowsky, H.; Linecker, M.; Raptis, D.A.; Kuemmerli, C.; Fritsch, R.; Kirimker, O.E.; Balci, D.; Ratti, F.; Aldrighetti, L.; Voskanyan, S.; et al. First Long-term Oncologic Results of the ALPPS Procedure in a Large Cohort of Patients with Colorectal Liver Metastases. Ann. Surg. 2020, 272, 793–800. [Google Scholar] [CrossRef]
- Robles, R.; Parrilla, P.; López-Conesa, A.; Brusadin, R.; de la Peña, J.; Fuster, M.; A García-López, J.; Hernández, E. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br. J. Surg. 2014, 101, 1129–1134; discussion 1134. [Google Scholar] [CrossRef]
- Cillo, U.; Gringeri, E.; Feltracco, P.; Bassi, D.; D’amico, F.E.; Polacco, M.; Boetto, R. Totally Laparoscopic Microwave Ablation and Portal Vein Ligation for Staged Hepatectomy: A New Minimally Invasive Two-Stage Hepatectomy. Ann. Surg. Oncol. 2015, 22, 2787–2788. [Google Scholar] [CrossRef]
- Edmondson, M.J.; Sodergren, M.H.; Pucher, P.H.; Darzi, A.; Li, J.; Petrowsky, H.; Campos, R.R.; Serrablo, A.; Jiao, L.R. Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): Many routes to the summit. Surgery 2016, 159, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ewald, F.; Gulati, A.; Nashan, B. Associating liver partition and portal vein ligation for staged hepatectomy: From technical evolution to oncological benefit. World J. Gastrointest. Surg. 2016, 8, 124–133. [Google Scholar] [CrossRef] [PubMed]
- De Santibañes, E.; Alvarez, F.A.; Ardiles, V.; Pekolj, J.; de Santibañes, M. Inverting the ALPPS paradigm by minimizing first stage impact: The Mini-ALPPS technique. Langenbeck’s Arch. Surg. 2016, 401, 557–563. [Google Scholar] [CrossRef]
- Machado, M.A.C.; Makdissi, F.F.; Surjan, R.C. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann. Surg. 2012, 256, e13; author reply e16-9. [Google Scholar] [CrossRef] [PubMed]
- Pekolj, J.; Alvarez, F.A.; Biagiola, D.; Villegas, L.; Ardiles, V.; de Santibañes, E. Totally Laparoscopic Mini-ALPPS Using a Novel Approach of Laparoscopic-Assisted Transmesenteric Portal Vein Embolization. J. Laparoendosc. Adv. Surg. Technol. 2018, 28, 1229–1233. [Google Scholar] [CrossRef]
- Truant, S.; El Amrani, M.; Baillet, C.; Ploquin, A.; Lecolle, K.; Ernst, O.; Hebbar, M.; Huglo, D.; Pruvot, F.-R. Laparoscopic partial ALPPS: Much better than ALPPS! Ann. Hepatol. 2019, 18, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Imai, K.; Benitez, C.C.; Allard, M.; Vibert, E.; Cunha, A.S.; Cherqui, D.; Baba, H.; Castaing, D. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br. J. Surg. 2016, 103, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Van Lienden, K.P.; van den Esschert, J.W.; De Graaf, W.; Bipat, S.; Lameris, J.S.; Van Gulik, T.M.; Van Delden, O.M. Portal vein embolization before liver resection: A systematic review. Cardiovasc. Interv. Radiol. 2013, 36, 25–34. [Google Scholar] [CrossRef]
- Ali, A.; Ahle, M.; Björnsson, B.; Sandström, P. Portal vein embolization with N-butyl cyanoacrylate glue is superior to other materials: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 5464–5478. [Google Scholar] [CrossRef]
- Regimbeau, J.M.; Cosse, C.; Kaiser, G.; Hubert, C.; Laurent, C.; Lapointe, R.; Isoniemi, H.; Adam, R. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: A LiverMetSurvey analysis. HPB 2017, 19, 396–405. [Google Scholar] [CrossRef]
- Kokudo, N.; Tada, K.; Seki, M.; Ohta, H.; Azekura, K.; Ueno, M.; Matsubara, T.; Takahashi, T.; Nakajima, T.; Muto, T. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am. J. Surg. 2001, 181, 153–159. [Google Scholar] [CrossRef]
- Torzilli, G.; Del Fabbro, D.; Palmisano, A.; Donadon, M.; Bianchi, P.; Roncalli, M.; Balzarini, L.; Montorsi, M. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J. Gastrointest. Surg. 2005, 9, 1148–1153; discussion 1153. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Montorsi, M.; Del Fabbro, D.; Palmisano, A.; Donadon, M.; Makuuchi, M. Ultrasonographically guided surgical approach to liver tumours involving the hepatic veins close to the caval confluence. Br. J. Surg. 2006, 93, 1238–1246. [Google Scholar] [CrossRef]
- Gold, J.S.; Are, C.; Kornprat, P.; Jarnagin, W.R.; Gönen, M.; Fong, Y.; DeMatteo, R.P.; Blumgart, L.H.; D’Angelica, M. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: Trends in treatment over time in 440 patients. Ann. Surg. 2008, 247, 109–117. [Google Scholar] [CrossRef]
- Van der Pool, A.E.M.; Lalmahomed, Z.S.; de Wilt, J.H.W.; Eggermont, A.M.M.; Ijzermans, J.M.N.; Verhoef, C. Local treatment for recurrent colorectal hepatic metastases after partial hepatectomy. J. Gastrointest. Surg. 2009, 13, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Mise, Y.; Aloia, T.A.; Brudvik, K.W.; Schwarz, L.; Vauthey, J.-N.; Conrad, C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann. Surg. 2016, 263, 146–152. [Google Scholar] [CrossRef]
- Torzilli, G.; Viganò, L.; Cimino, M.; Imai, K.; Vibert, E.; Donadon, M.; Mansour, D.; Castaing, D.; Adam, R. Is Enhanced One-Stage Hepatectomy a Safe and Feasible Alternative to the Two-Stage Hepatectomy in the Setting of Multiple Bilobar Colorectal Liver Metastases? A Comparative Analysis between Two Pioneering Centers. Dig. Surg. 2018, 35, 323–332. [Google Scholar] [CrossRef]
- Torzilli, G.; Serenari, M.; Viganò, L.; Cimino, M.; Benini, C.; Massani, M.; Ettorre, G.M.; Cescon, M.; Ferrero, A.; Cillo, U.; et al. Outcomes of enhanced one-stage ultrasound-guided hepatectomy for bilobar colorectal liver metastases compared to those of ALPPS: A multicenter case-match analysis. HPB 2019, 21, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Robles-Campos, R.; Lopez-Lopez, V.; Brusadin, R.; Lopez-Conesa, A.; Gil-Vazquez, P.J.; Navarro-Barrios, Á.; Parrilla, P. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): A prospective randomized controlled trial. Surg. Endosc. 2019, 33, 3926–3936. [Google Scholar] [CrossRef]
- Fretland, Å.A.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kazaryan, A.M.; Kristiansen, R.; Fagerland, M.W.; Hausken, J.; Tønnessen, T.I.; Abildgaard, A.; Barkhatov, L.; et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases The OSLO-COMET Randomized Controlled Trial. Ann. Surg. 2018, 267, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? HepatoBiliary Surg. Nutr. 2016, 5, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Fretland, Å.A.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Aghayan, D.L.; Kazaryan, A.M.; Barkhatov, L.; Kristiansen, R.; Fagerland, M.W.; Edwin, B.; Andersen, M.H. Quality of life from a randomized trial of laparoscopic or open liver resection for colorectal liver metastases. Br. J. Surg. 2019, 106, 1372–1380. [Google Scholar] [CrossRef]
- Aghayan, D.L.; Kazaryan, A.M.; Dagenborg, V.J.; Røsok, B.I.; Fagerland, M.M.W.; Bjørnelv, G.M.W.; Kristiansen, R.; Flatmark, K.; Fretland, A.; Edwin, B.; et al. Long-Term Oncologic Outcomes After Laparoscopic Versus Open Resection for Colorectal Liver Metastases: A Randomized Trial. Ann. Intern. Med. 2021, 174, 175–182. [Google Scholar] [CrossRef]
- Kingham, T.P.; Leung, U.; Kuk, D.; Gönen, M.; D’angelica, M.I.; Allen, P.J.; DeMatteo, R.P.; Laudone, V.P.; Jarnagin, W.R.; Fong, Y. Robotic Liver Resection: A Case-Matched Comparison. Mol. Med. 2016, 40, 1422–1428. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Giannis, D.; Esagian, S.M.; Economopoulos, K.P.; Tohme, S.; Geller, D.A. Laparoscopic versus robotic major hepatectomy: A systematic review and meta-analysis. Surg. Endosc. 2021, 35, 524–535. [Google Scholar] [CrossRef]
- Masetti, M.; Fallani, G.; Ratti, F.; Ferrero, A.; Giuliante, F.; Cillo, U.; Guglielmi, A.; Ettorre, G.M.; Torzilli, G.; Vincenti, L.; et al. Minimally invasive treatment of colorectal liver metastases: Does robotic surgery provide any technical advantages over laparoscopy? A multicenter analysis from the IGoMILS (Italian Group of Minimally Invasive Liver Surgery) registry. Updates Surg. 2022, 74, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Tranchart, H.; Diop, P.S.; Lainas, P.; Pourcher, G.; Catherine, L.; Franco, D.; Dagher, I. Laparoscopic major hepatectomy can be safely performed with colorectal surgery for synchronous colorectal liver metastasis. HPB 2011, 13, 46–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Goswami, J.; Han, K.; Chidi, A.P.; Geller, D.A.; Reddy, S.; Gleisner, A.; Tsung, A. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J. Gastrointest. Surg. 2015, 19, 2199–2206. [Google Scholar] [CrossRef]
- Vreeland, T.J.; Collings, A.T.; Ozair, A.; Adams, A.M.; Dirks, R.; Kushner, B.S.; Sucandy, I.; Morrell, D.; Whiteside, J.; Ansari, M.T.; et al. SAGES/AHPBA guidelines for the use of minimally invasive surgery for the surgical treatment of colorectal liver metastases (CRLM). Surg. Endosc. 2023, 37, 2508–2516. [Google Scholar] [CrossRef]
- Livraghi, T.; Meloni, F.; Solbiati, L.; Zanus, G.; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: Results of a multicenter study. Cardiovasc. Interv. Radiol. 2012, 35, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Groeschl, R.T.; Pilgrim, C.H.C.; Hanna, E.M.; Simo, K.A.; Swan, R.Z.; Sindram, D.; Martinie, J.B.; Iannitti, D.A.; Bloomston, M.; Schmidt, C.; et al. Microwave Ablation for Hepatic Malignancies. Ann. Surg. 2014, 259, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Karagkounis, G.; McIntyre, S.M.; Wang, T.; Chou, J.F.; Nasar, N.; Gonen, M.; Balachandran, V.P.; Wei, A.C.; Soares, K.C.; Drebin, J.A.; et al. Rates and Patterns of Recurrence After Microwave Ablation of Colorectal Liver Metastases: A Per Lesion Analysis of 416 Tumors in the Era of 2.45 GHz Generators. Ann. Surg. Oncol. 2023, 30, 6571–6578. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. JNCI J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef]
- Meijerink, M.R.; Puijk, R.S.; Van Tilborg, A.A.J.M.; Henningsen, K.H.; Fernandez, L.G.; Neyt, M.; Heymans, J.; Frankema, J.S.; De Jong, K.P.; Richel, D.J.; et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2018, 41, 1189–1204. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Wan, X.; Li, Y.; Li, B.; Zhang, Y.; Yuan, Y.; Zheng, Y. Efficacy and safety of thermal ablation in patients with liver metastases. Eur. J. Gastroenterol. Hepatol. 2013, 25, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Foss, A.; Adam, R.; Dueland, S. Liver transplantation for colorectal liver metastases: Revisiting the concept. Transpl. Int. 2010, 23, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Hagness, M.; Foss, A.; Line, P.-D.; Scholz, T.; Jørgensen, P.F.; Fosby, B.; Boberg, K.M.; Mathisen, Ø.; Gladhaug, I.P.; Egge, T.S.; et al. Liver Transplantation for Nonresectable Liver Metastases from Colorectal Cancer. Ann. Surg. 2013, 257, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Solheim, J.M.; Dueland, S.; Line, P.-D.; Hagness, M. Transplantation for Nonresectable Colorectal Liver Metastases: Long-Term Follow-Up of the First Prospective Pilot Study. Ann. Surg. 2023, 278, 239–245. [Google Scholar] [CrossRef]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjørnbeth, B.A.; Hagness, M.; Line, P.-D. Survival Following Liver Transplantation for Patients with Nonresectable Liver-only Colorectal Metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef]
| Fong Clinical Risk Score (0–5) | RAS Mutational Risk Score (0–3) |
|---|---|
| Largest tumor > 5 cm Disease-free interval between primary and diagnosis of CRLM of <12 months Number of metastases > 1 Preoperative CEA > 200 ug/L Node-positive primary | Largest tumor > 5 cm KRAS Mutational Status Node-positive primary |
| Tumor Biology | RAS Status | Response to Chemotherapy (>50%) | 5-Year OS | p-Value | |
|---|---|---|---|---|---|
| R0 | R1 (<1 mm) | ||||
| Good | wt | Yes | 66.4% | 65.2% | 0.884 |
| Bad | mt | Yes | 58.5% | 48.7% | 0.043 |
| wt | No | 24.6% | 11.1% | 0.024 | |
| mt | No | 19.5% | 0% | 0.022 | |
| Oslo Score (0–4) | Fong Clinical Risk Score (0–5) |
|---|---|
| Largest tumor > 5.5 cm Less than 2-year interval between primary resection and LT Progressive disease at the time of LT Preoperative CEA > 80 ug/L | Largest tumor > 5 cm Disease-free interval between primary and diagnosis of CRLM of <12 months Number of metastases > 1 Preoperative CEA > 200 ug/L Node-positive primary |
| SECA-I | SECA-II |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, P.; Sacks, G.D. Contemporary Surgical Management of Colorectal Liver Metastases. Cancers 2024, 16, 941. https://doi.org/10.3390/cancers16050941
Chandra P, Sacks GD. Contemporary Surgical Management of Colorectal Liver Metastases. Cancers. 2024; 16(5):941. https://doi.org/10.3390/cancers16050941
Chicago/Turabian StyleChandra, Pratik, and Greg D. Sacks. 2024. "Contemporary Surgical Management of Colorectal Liver Metastases" Cancers 16, no. 5: 941. https://doi.org/10.3390/cancers16050941
APA StyleChandra, P., & Sacks, G. D. (2024). Contemporary Surgical Management of Colorectal Liver Metastases. Cancers, 16(5), 941. https://doi.org/10.3390/cancers16050941





