CA125 Kinetics as a Potential Biomarker for Peritoneal Metastasis Progression following Taxane-Plus-Ramucirumab Administration in Patients with Advanced Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
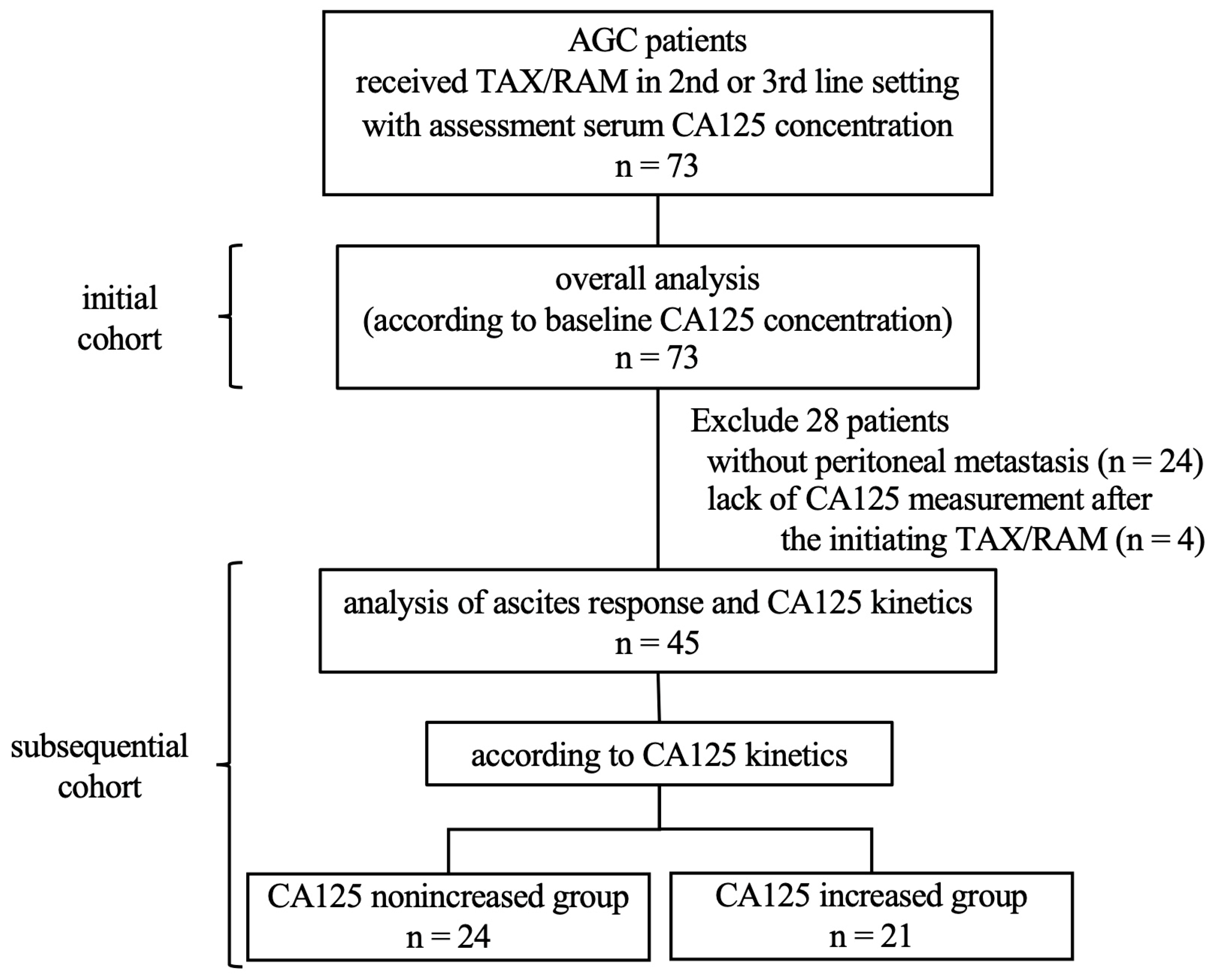
2. Materials and Methods
2.1. Patients and Treatment
2.2. Evaluation of the Ascites Burden and CA125 Kinetics
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
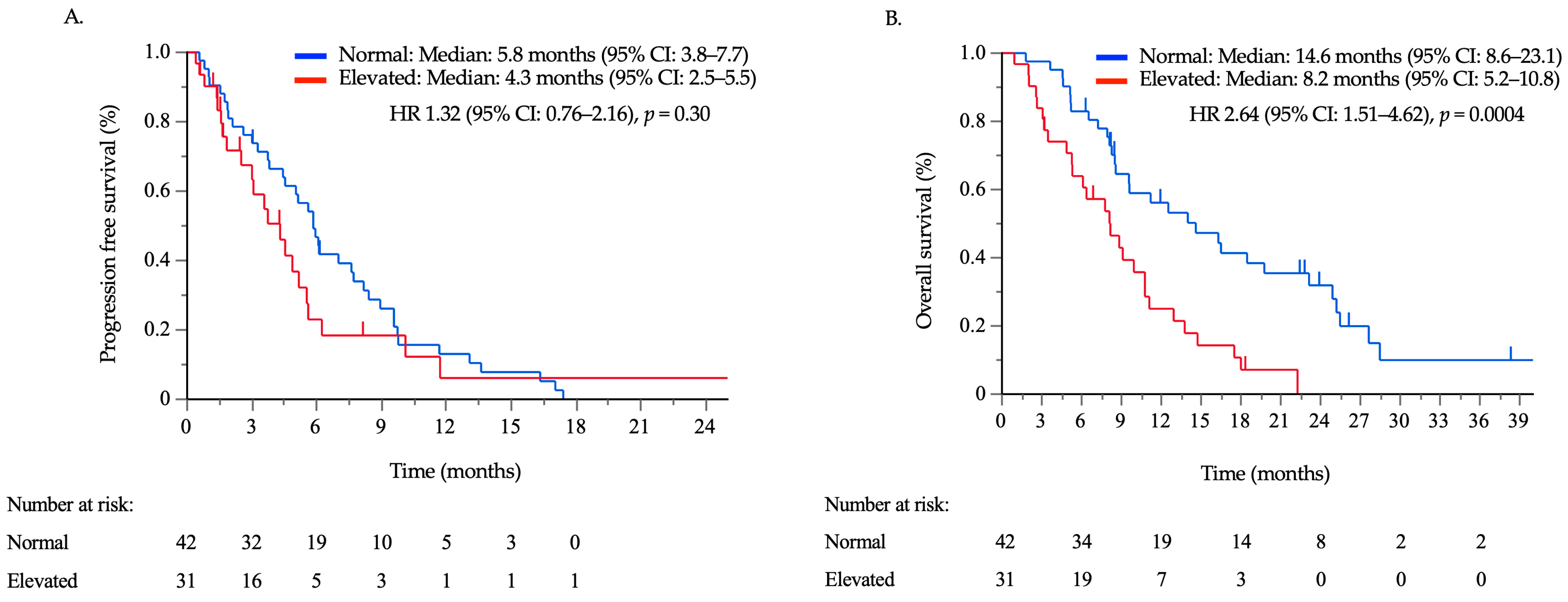
3.2. Treatment Outcomes according to the Serum CA125 Concentration
3.3. Ascites Response and Survival Outcomes According to CA125 Kinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1437–1448. [Google Scholar] [CrossRef]
- Bang, Y.J.; Ruiz, E.Y.; Van Cutsem, E.; Lee, K.W.; Wyrwicz, L.; Schenker, M.; Alsina, M.; Ryu, M.H.; Chung, H.C.; Evesque, L.; et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann. Oncol. 2018, 29, 2052–2060. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Yamada, Y.; Higuchi, K.; Nishikawa, K.; Gotoh, M.; Fuse, N.; Sugimoto, N.; Nishina, T.; Amagai, K.; Chin, K.; Niwa, Y.; et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann. Oncol. 2015, 26, 141–148. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Niclauss, N.; Gütgemann, I.; Dohmen, J.; Kalff, J.C.; Lingohr, P. Novel biomarkers of gastric adenocarcinoma: Current research and future perspectives. Cancers 2021, 13, 5660. [Google Scholar] [CrossRef]
- Takashima, A.; Iizumi, S.; Boku, N. Survival after failure of first-line chemotherapy in advanced gastric cancer patients: Differences between Japan and the rest of the world. Jpn. J. Clin. Oncol. 2017, 47, 583–589. [Google Scholar] [CrossRef]
- Iizumi, S.; Takashima, A.; Sakamaki, K.; Morita, S.; Boku, N. Survival impact of post-progression chemotherapy in advanced gastric cancer: Systematic review and meta-analysis. Cancer Chemother. Pharmacol. 2018, 81, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Hironaka, S.; Tanizawa, Y.; Cai, Z.; Piao, Y.; Boku, N. Treatment pattern for advanced gastric cancer in Japan and factors associated with sequential treatment: A retrospective administrative claims database study. Adv. Ther. 2022, 39, 296–313. [Google Scholar] [CrossRef]
- Ando, T.; Hosokawa, A.; Sakumura, M.; Motoo, I.; Kajiura, S.; Hirano, K.; Miwa, T.; Yokota, T.; Nakada, N.; Ueda, Y.; et al. Factors, including clinical trial eligibility, associated with induction of third-line treatment for advanced gastric cancer. Oncology 2023, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Kim, S.Y.; Hong Lee, M.; Yoo, M.W.; Bang, H.Y.; Lee, K.Y.; Yoon, S.Y. Comparative analysis of the efficacy and safety of chemotherapy with oxaliplatin plus fluorouracil/leucovorin between elderly patients over 65 years and younger patients with advanced gastric cancer. Gastric Cancer 2012, 15, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, S.; Nakajima, T.E.; Nakamura, K.; Takashima, A.; Kato, K.; Hamaguchi, T.; Yamada, Y.; Shimada, Y. First-line fluorouracil-based chemotherapy for patients with severe peritoneal disseminated gastric cancer. Gastric Cancer 2012, 15, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Kadowaki, S.; Asayama, M.; Ooki, A.; Yamada, T.; Yoshii, T.; Yamaguchi, K. First-line bolus 5-fluorouracil plus leucovorin for peritoneally disseminated gastric cancer with massive ascites or inadequate oral intake. Int. J. Clin. Oncol. 2018, 23, 275–280. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kawazoe, A.; Shimada, K.; Fukuoka, S.; Kuboki, Y.; Bando, H.; Kojima, T.; Ohtsu, A.; Yoshino, T.; Doi, T.; et al. A retrospective study of the safety and efficacy of paclitaxel plus ramucirumab in patients with advanced or recurrent gastric cancer with ascites. BMC Cancer 2018, 18, 120. [Google Scholar] [CrossRef]
- Hasegawa, H.; Fujitani, K.; Nakazuru, S.; Hirao, M.; Yamamoto, K.; Mita, E.; Tsujinaka, T. Optimal treatment change criteria for advanced gastric cancer with non-measurable peritoneal metastasis: Symptom/tumor marker-based versus CT-based. Anticancer Res. 2014, 34, 5169–5174. [Google Scholar]
- Maeda, H.; Kobayashi, M.; Sakamoto, J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J. Gastroenterol. 2015, 21, 10936–10947. [Google Scholar] [CrossRef]
- Yamao, T.; Kai, S.; Kazami, A.; Koizumi, K.; Handa, T.; Takemoto, N.; Maruyama, M. Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Jpn. J. Clin. Oncol. 1999, 29, 550–555. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, K.W.; Kim, Y.J.; Oh, D.Y.; Kim, J.H.; Im, S.A.; Lee, J.S. Chemotherapy-induced transient CEA and CA19-9 surges in patients with metastatic or recurrent gastric cancer. Acta Oncol. 2009, 48, 385–390. [Google Scholar] [CrossRef]
- Rustin, G.J.; Vergote, I.; Eisenhauer, E.; Pujade-Lauraine, E.; Quinn, M.; Thigpen, T.; du Bois, A.; Kristensen, G.; Jakobsen, A.; Sagae, S.; et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int. J. Gynecol. Cancer 2011, 21, 419–423. [Google Scholar] [CrossRef]
- Emoto, S.; Ishigami, H.; Yamashita, H.; Yamaguchi, H.; Kaisaki, S.; Kitayama, J. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer 2012, 15, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.E.; Yamaguchi, K.; Boku, N.; Hyodo, I.; Mizusawa, J.; Hara, H.; Nishina, T.; Sakamoto, T.; Shitara, K.; Shinozaki, K.; et al. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer 2020, 23, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Muro, K.; Tomasek, J.; Van Cutsem, E.; Cho, J.Y.; Oh, S.C.; Safran, H.; Bodoky, G.; Chau, I.; Shimada, Y.; et al. Prognostic factor analysis of overall survival in gastric cancer from two Phase III studies of second-line ramucirumab (REGARD and RAINBOW) using pooled patient data. J. Gastric Cancer 2017, 17, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Takahari, D.; Mizusawa, J.; Koizumi, W.; Hyodo, I.; Boku, N. Validation of the JCOG prognostic index in advanced gastric cancer using individual patient data from the SPIRITS and G-SOX trials. Gastric Cancer 2017, 20, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the Phase II KEYNOTE-158 study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Byström, P.; Berglund, A.; Nygren, P.; Wernroth, L.; Johansson, B.; Larsson, A.; Einarsson, R.; Glimelius, B. An explorative study on the clinical utility of baseline and serial serum tumour marker measurements in advanced upper gastrointestinal cancer. Oncol. Rep. 2010, 24, 1645–1652. [Google Scholar] [CrossRef]
- Lin, J.P.; Lin, J.X.; Ma, Y.B.; Xie, J.W.; Yan, S.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Ma, X.F.; Cao, L.L.; et al. Prognostic significance of pre- and post-operative tumour markers for patients with gastric cancer. Br. J. Cancer 2020, 123, 418–425. [Google Scholar] [CrossRef]
- Kochi, M.; Fujii, M.; Kanamori, N.; Kaiga, T.; Kawakami, T.; Aizaki, K.; Kasahara, M.; Mochizuki, F.; Kasakura, Y.; Yamagata, M. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer 2000, 3, 177–186. [Google Scholar] [CrossRef]
- Takahashi, Y.; Takeuchi, T.; Sakamoto, J.; Touge, T.; Mai, M.; Ohkura, H.; Kodaira, S.; Okajima, K.; Nakazato, H.; Tumor Marker Committee. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: A prospective clinical study. Gastric Cancer 2003, 6, 142–145. [Google Scholar] [CrossRef]
- Whiting, J.; Sano, T.; Saka, M.; Fukagawa, T.; Katai, H.; Sasako, M. Follow-up of gastric cancer: A review. Gastric Cancer 2006, 9, 74–81. [Google Scholar] [CrossRef]
- Lin, J.X.; Wang, W.; Lin, J.P.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.; et al. Preoperative tumor markers independently predict survival in Stage III gastric cancer patients: Should we include tumor markers in AJCC staging? Ann. Surg. Oncol. 2018, 25, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Esmail, A.; Muhsen, I.; Salah, H.; Abdelrahim, M. Recent trends and advancements in the diagnosis and management of gastric cancer. Cancers 2022, 14, 5615. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Kinami, S.; Ninomiya, I.; Kitagawa, H.; Fushida, S.; Nishimura, G.; Kayahara, M.; Shimizu, K.; Ohta, T.; Miwa, K. Diagnostic laparoscopy, serum CA125, and peritoneal metastasis in gastric cancer. Endoscopy 2002, 34, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Bafna, S.; Kaur, S.; Batra, S.K. Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010, 29, 2893–2904. [Google Scholar] [CrossRef]
- Dennis, J.L.; Hvidsten, T.R.; Wit, E.C.; Komorowski, J.; Bell, A.K.; Downie, I.; Mooney, J.; Verbeke, C.; Bellamy, C.; Keith, W.N.; et al. Markers of adenocarcinoma characteristic of the site of origin: Development of a diagnostic algorithm. Clin. Cancer Res. 2005, 11, 3766–3772. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Amroun, K.; Chaltiel, R.; Reyal, F.; Kianmanesh, R.; Savoye, A.M.; Perrier, M.; Djerada, Z.; Bouché, O. Dynamic prediction of resectability for patients with advanced ovarian cancer undergoing neo-adjuvant chemotherapy: Application of joint model for longitudinal CA-125 levels. Cancers 2022, 15, 231. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, X.Q.; Ren, X.G. Third-line chemotherapy in advanced gastric cancer: A systematic review and meta-analysis. Medicine 2017, 96, e6884. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Tabernero, J.; Diez, M. Chemorefractory gastric cancer: The evolving terrain of third-line therapy and beyond. Cancers 2022, 14, 1408. [Google Scholar] [CrossRef] [PubMed]
- Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; Yeh, K.H.; et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 2021, 24, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Q.; Wang, H.; Zhuo, W.; Ding, Y.; Lu, J.; Wu, G.; Xu, N.; Teng, L. Predicting peritoneal dissemination of gastric cancer in the era of precision medicine: Molecular characterization and biomarkers. Cancers 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, Y. Complications in advanced or recurrent gastric cancer patients with peritoneal metastasis during and after palliative systemic chemotherapy. Mol. Clin. Oncol. 2015, 3, 539–542. [Google Scholar] [CrossRef]
- International, B.R. Retracted: Levels and significance of tumor markers and cytokines in serum and peritoneal lavage fluid of patients with peritoneal metastasis of gastric cancer. Biomed Res. Int. 2023, 2023, 9892635. [Google Scholar] [CrossRef]
- Muro, K.; Jen, M.H.; Cheng, R. Is ramucirumab and paclitaxel therapy beneficial for second-line treatment of metastatic gastric or junctional adenocarcinoma for patients with ascites? Analysis of RAINBOW phase 3 trial data. Cancer Manag. Res. 2019, 11, 2261–2267. [Google Scholar] [CrossRef]



| Normal | Elevated | p-Value | ||
|---|---|---|---|---|
| Number of Patients | 42 | 31 | ||
| Age (years) | Median (range) | 68 (37–86) | 65 (34–81) | 0.41 |
| Sex | Male/female | 31/11 | 28/3 | 0.077 |
| ECOG PS | 0 | 17 (40.4) | 5 (16.1) | 0.0029 |
| 1 | 25 (59.6) | 20 (64.5) | ||
| ≥2 | 0 (0) | 6 (19.4) | ||
| Treatment line | 2/≥3 | 35/7 | 24/7 | 0.53 |
| Metastatic organ (MO) | Liver | 17 (40.4) | 14 (45.2) | 0.69 |
| Lung | 5 (11.9) | 2 (6.5) | 0.43 | |
| Lymph node | 23 (54.8) | 19 (61.3) | 0.58 | |
| Peritoneum | 24 (57.1) | 25 (80.6) | 0.035 | |
| Number of MOs | Median (range) | 2 (1–4) | 2 (1–5) | 0.18 |
| Peritoneal nodule | Yes | 17 (40.4) | 15 (48.4) | 0.50 |
| No | 25 (59.6) | 16 (51.6) | ||
| Ascites | None | 25 (59.6) | 8 (25.8) | 0.025 |
| Mild | 10 (23.8) | 11 (35.5) | ||
| Moderate | 3 (7.1) | 4 (12.9) | ||
| Severe | 4 (9.5) | 8 (25.8) | ||
| Histopathologic type * | Intestinal | 18 (43.9) | 15 (48.4) | 0.71 |
| Diffuse | 23 (56.1) | 16 (51.6) | ||
| HER2 status | Positive | 8 (19.0) | 10 (32.3) | 0.19 |
| Negative | 33 (78.6) | 20 (64.5) | ||
| Unknown | 1 (2.4) | 1 (3.2) | ||
| Resection of the primary site | Yes | 14 (33.3) | 8 (25.8) | 0.61 |
| No | 28 (66.7) | 23 (74.2) | ||
| Duration of the first line * | ≥6 months | 27 (64.3) | 13 (43.3) | 0.096 |
| <6 months | 15 (35.7) | 17 (56.7) | ||
| Neutrophil count (/µL) | Median (range) | 3158 (1575–9416) | 3310 (1690–11,995) | 0.42 |
| Lymphocyte count (/µL) | 1308 (361–3347) | 1170 (370–2490) | 0.20 | |
| NLR | 2.3 (0.6–8.7) | 3.3 (1.1–12.9) | 0.076 | |
| AST (U/L) | 22 (10–138) | 28 (10–123) | 0.11 | |
| ALP (U/L) | 292 (121–1368) | 390 (155–3840) | 0.050 | |
| LDH (U/L) | 212 (136–1811) | 246 (132–1172) | 0.065 | |
| CEA (ng/mL) | 4.9 (1.2–1549) | 11 (0.5–4925.3) | 0.071 | |
| CA19-9 (U/mL) | 27.3 (0.1–2587) | 122 (0.1–175,497) | 0.0027 | |
| n | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| ECOG PS | 0 (ref)/≥1 | 22/51 | 1.78 | 0.99–3.18 | 0.050 | 1.48 | 0.82–2.66 | 0.20 |
| Peritoneal metastases | No/Yes | 53/20 | 2.60 | 1.38–4.90 | 0.0031 | 1.93 | 0.97–3.85 | 0.061 |
| Ascites | No/Yes | 33/40 | 1.83 | 1.07–3.15 | 0.028 | |||
| Peritoneal nodule | No/Yes | 41/32 | 2.44 | 1.40–4.24 | 0.0016 | |||
| Histopathologic type * | Intestinal/diffuse | 33/39 | 1.55 | 0.92–2.62 | 0.098 | |||
| Resection of the primary site | Yes/No | 22/51 | 1.16 | 0.66–2.04 | 0.61 | |||
| Duration of the first line * | ≥6/<6 months | 40/32 | 1.44 | 0.85–2.46 | 0.18 | |||
| Neutrophil count | Normal/elevated | 64/9 | 1.28 | 0.57–2.84 | 0.55 | |||
| Lymphocyte count | Normal/reduced | 25/48 | 1.80 | 0.57–2.84 | 0.044 | 1.26 | 0.68–2.33 | 0.46 |
| NLR | <2.6/≥2.6 (median) | 37/36 | 1.27 | 0.76–2.14 | 0.36 | |||
| AST | Normal/elevated | 48/25 | 0.42 | 0.72–2.19 | 0.42 | |||
| ALP | Normal/elevated | 39/34 | 1.19 | 0.70–2.02 | 0.53 | |||
| LDH | Normal/elevated | 35/38 | 0.98 | 0.59–1.64 | 0.94 | |||
| CA125 | Normal/elevated | 42/31 | 2.64 | 1.51–4.62 | 0.0007 | 1.94 | 1.07–3.52 | 0.030 |
| CEA | Normal/elevated | 25/48 | 1.54 | 0.89–2.68 | 0.12 | |||
| CA19-9 | Normal/elevated | 38/35 | 1.28 | 0.76–2.15 | 0.35 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, A.; Yuki, S.; Ando, T.; Hosokawa, A.; Nakada, N.; Kito, Y.; Motoo, I.; Ito, K.; Sakumura, M.; Nakayama, Y.; et al. CA125 Kinetics as a Potential Biomarker for Peritoneal Metastasis Progression following Taxane-Plus-Ramucirumab Administration in Patients with Advanced Gastric Cancer. Cancers 2024, 16, 871. https://doi.org/10.3390/cancers16050871
Ueda A, Yuki S, Ando T, Hosokawa A, Nakada N, Kito Y, Motoo I, Ito K, Sakumura M, Nakayama Y, et al. CA125 Kinetics as a Potential Biomarker for Peritoneal Metastasis Progression following Taxane-Plus-Ramucirumab Administration in Patients with Advanced Gastric Cancer. Cancers. 2024; 16(5):871. https://doi.org/10.3390/cancers16050871
Chicago/Turabian StyleUeda, Akira, Satoshi Yuki, Takayuki Ando, Ayumu Hosokawa, Naokatsu Nakada, Yosuke Kito, Iori Motoo, Ken Ito, Miho Sakumura, Yurika Nakayama, and et al. 2024. "CA125 Kinetics as a Potential Biomarker for Peritoneal Metastasis Progression following Taxane-Plus-Ramucirumab Administration in Patients with Advanced Gastric Cancer" Cancers 16, no. 5: 871. https://doi.org/10.3390/cancers16050871
APA StyleUeda, A., Yuki, S., Ando, T., Hosokawa, A., Nakada, N., Kito, Y., Motoo, I., Ito, K., Sakumura, M., Nakayama, Y., Ueda, Y., Kajiura, S., Nakashima, K., Harada, K., Kawamoto, Y., Komatsu, Y., & Yasuda, I. (2024). CA125 Kinetics as a Potential Biomarker for Peritoneal Metastasis Progression following Taxane-Plus-Ramucirumab Administration in Patients with Advanced Gastric Cancer. Cancers, 16(5), 871. https://doi.org/10.3390/cancers16050871






